Indexed In
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 17, Issue 4
Gestational Anemia: The Factors Associated and the Outcomes in the Mother and the Infant
Tebbani Fouzia*, Oulamara Hayet and Agli AbdenacerReceived: 20-Jun-2020 Published: 22-Jul-2020, DOI: 10.35248/2090-7214.20.17.352
Abstract
Background: Maternal anemia is considered a risk factor for pregnancy, because it is hazardous to both mother and fetus. Our study aimed to determine in each trimester of pregnancy the factors associated with anemia and the outcomes in the mother and infant in a cohort of Algerian pregnant women.
Methods: We conducted a prospective and longitudinal cohort study of 300 women from December 2013 to July 2016. All consenting women attending antenatal consultation and had undergone complete blood count were included in the study. Sociodemographic characteristics and individual’s obstetrical history were collected. Factors associated to maternal anemia were investigated during each trimester of pregnancy. Data on delivery term, delivery mode and birth outcomes were collected. We performed a bivariate analysis using the Chi-square test for proportions, Student’s t-test, or one-way ANOVA for continuous variables and multiple comparisons.
Results: The rate of gestational anemia was 58.0%. The average concentration of hemoglobin, hematocrit, blood volume and platelets were lower in anemic pregnant women. Factors associated with anemia were: lower hemoglobin levels, lower BMI in the 3rd trimester, inadequate gestational weight gain, insufficient caloric intake in the 1st and 3rd trimesters and non supplementation with iron. Maternal anemia increased the risk of low birth weight in early and late pregnancy and the risk of overrun term in mid and late pregnancy.
Conclusion: Various antropomethric, haematological and nutritional factors affected gestational anemia. Gestational anemia was associated with increased risks of maternal and fetal complications. Community-based interventions should be enhanced considering the identified associated factors.
Keywords
Pregnancy; Anemia; Cohort study; Associated factors; Adverse outcomes.
Introduction
Anemia is a condition characterized as a low level of hemoglobin in the blood, as evidenced by a reduced quality or quantity of red blood cells which decreases oxygen-carrying capacity to tissues [1, 2]. It occurs at all age groups, but is more prevalent in pregnant women [3]. Gestational anemia is defined as a hemoglobin less than 11.0 g/dl in the first and third trimesters of pregnancy and less than 10.5 g/dl in the second trimester and it is one of the most common problems in obstetrics [2].
Around 40% of women begin their pregnancy with low or absent iron stores (serum ferritin <30 mg/l) and up to 90% have iron stores of <500 mg (serum ferritin <70 mg/l) worldwide, which is insufficient to meet the increased iron needs during pregnancy and postpartum [4]. In Africa the prevalence of anemia among pregnant women was 57.1% [2] which is associated with adverse health outcomes for both mother and infant, like maternal mortality, perinatal mortality, growth restriction and low birth weight [5, 6]. Some other countries report prevalence of 16.8 %, 22.1 %, 24.4 % 32.8 %, 41.6 % and 100 % respectively in Iran, Uganda, the United Kingdom, Ethiopia, India and Turkey [7, 8].
Gestational anemia can be explained primarily by a diet low in iron. Its origin may be due to several factors including single or combined deficiency of nutrients such as iron, folic acid and vitamin B12 [9]. Nutritional deficiencies are still a common problem during pregnancy causing anemia.
Maternal anemia is considered a risk factor for pregnancy, because it is hazardous to both mother and fetus, and is associated with increased risk of maternal-fetal morbidity, as well as the nutritional status of child [10]. Current knowledge indicates that iron deficiency anemia in pregnancy is a risk factor for preterm delivery and subsequent low birth weight, and possibly for inferior neonatal health [11,12]. Deficiency can have serious consequences for the mother in case of major bleeding during delivery [13]. However, information on the incidence of anemia, the factors associated and the outcomes in the mother and in the fetus, especially in Algeria, are insufficient.
The aim of this study was to determine the frequency of anemia in pregnant women in the first, second and third trimesters of pregnancy. Then to determine hematological, anthropometric and nutritional associated factors of gestational anemia. We also examined trimester specific maternal and fetal consequences of maternal anemia during pregnancy. Detailed information about maternal characteristics and trimester specific maternal and fetal adaptations might identify groups at risk and specific effects of low hemoglobin levels during pregnancy. This might provide further insight in the suggested associations of maternal hemoglobin levels with the risk of pregnancy complications.
Materials and Methods
Type, duration and period of the study
We conducted a prospective and longitudinal cohort study. We followed for 9 months a cohort of Algerian pregnant women attending antenatal clinics in early pregnancy. They were recruited at three different sites; at maternities, antenatal centers and private gynecologists in Constantine (Algeria), from December 2013 to July 2016. The participants were recruited and followed up longitudinally, once at the end of each trimester of pregnancy. The trimesters were defined as first (less than 16 weeks of amenorrhea), second (16 – 28 weeks of amenorrhea) and third (29–41 weeks of amenorrhea) [14].
Study Population
Inclusion and Exclusion Criteria
We included all pregnant women attending antenatal clinics during the study period, presenting for a pregnancy follow-up and agreeing to participate freely in the study. They were eligible for participation if they aged 18 years old and more, were healthy and mentally competent, entered prenatal care early in the first trimester of pregnancy and had a complete blood count (CBC).
We excluded women refusing to participate in the study, women with missing information on pre-pregnancy weight (in order to calculate pre-pregnancy BMI and weight gain), known diabetes, hypertension and anemia before pregnancy.
Pregnant women who met the inclusion criteria were informed of the objectives of the study. They agreed to be part of it until the birth of their babies. Written consent was obtained from all participating mothers.
Sampling and sample size
During the study period, 1231 women came for the first pregnancy consultation. Of these, 703 did not agree to participate in the study, representing 57.1% of the total enrollment.
Of 528 women (42.9% of the total) responding favorably to the survey, we excluded 110 women who presented themselves after the date of starting weight measurement. Also, 52 women were excluded because they had a pre-pregnancy pathology (diabetes, high blood pressure, anemia, or endocrine pathology).
Among pregnant women meeting the inclusion criteria, 366 (69.3%) were selected for the study. Of these women, 26 were excluded because they had a pregnancy stop and 26 dropped out. The sample that was selected for the first trimester of pregnancy consists of 314 women. In the second trimester, 14 women dropped out of the study. The final sample consisted of 300 pregnant women for the analysis of all data, representing a participation rate of 56.8% of women who agreed to participate in the study.
Data Collected
Blood counts formula
The biological parameters of the pregnant women were investigated: hemogram (hemoglobin level, mean corpuscular volume, red blood cells, white blood cells, hematocrit and platelets). Our data were based on blood tests of pregnant women. We defined anaemia levels according to the World Health Organization criteria for anaemia during pregnancy (haemoglobin concentration ≤11 g/dl or haematocrit ≤33%) [15].
Sociodemographic characteristics of the pregnant women
Data were collected using pretested structured questionnaire by face-to-face interview. All women were interviewed about their socio-demographic characteristics. For each pregnant woman we have raised the age, parity, level of education and wealth index. The level of education was divided into three categories according to the level of schooling: a low level (illiterate and primary), a medium level (middle and secondary plus training) and a high level (university).
For the wealth index, we proceeded to calculate a score reflecting the socio-economic level of our subjects. The approach consisted in assigning a score, which reflected the woman's comfort level for each of the variables considered as predictive. The establishment indicators selected were: the overall monthly income of the household, the number of active persons per household that allowed us to define a given economic coverage index by the number of active persons for each person living under the same roof. Also, the type of occupancy (owner or tenant), the occupancy rate per household, which was defined as
the ratio between the size of the household (number of persons) and the number of rooms in the family dwelling and finally possessions owned (TV, freezer, stove, bath heater, air conditioner, microwave, washing machine, internet connection and car). The low wealth index score (WIS) was assigned to pregnant women whose total was less than 10 points; the average WIS for those with total points between 10 and 15 points and the high WIS group represented women who had more than 15 points.
Anthropometric measurements of the pregnant women
Weight and height were measured according to a standard protocol [16, 17]. Pre-pregnancy weight was measured when the pregnant woman consulted at the early first trimester. During pregnancy, weight was measured at the end of each trimester (first, second and third) by using an electronic weighing balance Seca to the nearest 0.1 kg. Height was measured in centimetres using a Seca toise, with a length of 2 m graduated in centimeters and with a precision of 0.1 cm. Pregnant women were asked to maintain an upright and erect posture with their feet together and the back of their heels touching the pole of the anthropometer. The height was measured when the horizontal headpiece was lowered onto the women’s head.
Pre-pregnancy body mass index (BMI) was calculated using a prepregnancy weight and height. Pre-pregnancy BMI was computed as weight (kg) divided by square of measured height (m). We categorized women ’ s pre-pregnancy weight according to the World Health Organization (WHO) standards [17].
Weight gain (in kg) at each prenatal visit (at the end of the first, second and third trimesters) and total weight gain was collected. Weight gain of each pregnancy trimester was calculated by subtracting the previous trimester weight from the current trimester weight. Total weight gain was calculated as the weight of the woman measured at the end of pregnancy minus her starting weight. We used the 2009 IOM guidelines on GWG to categorize women ’ s weight gain as below, within or above recommended [18]. These guidelines have also been adopted by Health Canada [19]. Weight gain assessment used recommendations of the Institute of Medicine (IOM) [18] according to the recommended weight gain ranges and to BMI categories: underweight: 12.5-18.0 Kg; adequate: 11.5-16.0 Kg; overweight: 7.0-11.5 Kg and obese: 7.0-9.0 Kg.
Factors associated with gestational anemia
Gestational anemia was influenced by a wide range of factors suche as haematological factors (hemoglobin level, mean corpuscular volume, red blood cells, white blood cells, hematocrit and platelets), anthropometric factors (pre-pregnancy BMI and GWG) and nutritional factors (the intake of some nutrients such as iron, vitamin B9, B12 and C and the total caloric intake were investigated). So, women have completed a food consumption diary based on a three-day food registration method regarding their habitual feeding repeated during the three trimesters of pregnancy. Food registration was done over three days (two weekdays and one weekend day), in order to increase the accuracy of the results, to ensure that we actually listed the usual diet and to minimize the risks of atypical days.
Consumed foods per day were quantified, estimated using household units and photos, and then converted to nutrient proportions using compiled tables [20]. Information on maternal iron supplement use during pregnancy was obtained at enrolment by questionnaire.
Maternal and birth outcomes
Outcomes of interest were delivery term, delivery mode and birth weight of newborn. Term of delivery was based on the calculated gestational age. Gestational age was the only criterion used to identify the term delivery in weeks of amenorrhea. It was estimated as the difference between the first day of the last menstrual period (LMP) and the date of birth. It was confirmed or corrected from an early ultrasound report at the first consultation.
In case of discrepancy between the estimation by the LMP and the ultrasound, the decision was to use ultrasound evaluation that was conducted by the gynecologist in early pregnancy [21]. A pre-term delivery was defined as any delivery occurring before 37 weeks of amenorrhea (WA) and was considered as a premature delivery. A term delivery was any delivery occurring between 37 and 41 weeks. A post-term delivery occurred when gestational age was greater than or equal to 42 WA.
Data on mode of delivery included two types of delivery; normal vaginal delivery, and cesarean delivery. Birth weight of newborns was obtained from medical records. Birth weight were divided to three groups [22, 23] ; Normal birth weight: [2500 to 4000 g], low birth weight or hypotrophy: <2500 g and large birth weight or macrosomia: ≥ 4000 g.
Statistical analyses
Statistical analyses were performed using Stat View software version5 (Abacus Concepts TM, Berkeley, USA). Categorical type of data was analyzed by descriptive statistics (frequency and percentage) whereas range, mean and standard deviation were used to present continuous variables.
After some descriptive statistics, we performed a bivariate analysis using the Chi-square test for proportions, Student’s ttest, or one-way ANOVA for continuous variables and multiple comparisons. When it comes to comparing two percentages of low numbers, we used the exact test of Fisher in order to determine the factors associated with anemia. P value <0.05 was considered significant.
Results
A total of 300 pregnant women aged 19 to 43 years participated in the study. The mean age was 30.3 ± 5.0 years. Of those, 82.0 % were between 20 and 35 years old and only 18.0 % were over 35 years old. Socio-demographic data of all subjects were presented in Table 1. A percentage of 35.7 % were nulliparous. Mean spacing between pregnancies were 30.9 ± 22.7 months. 32.7% of women had a low wealth index and only 19.0% had a high wealth index. Before pregnancy, 69.4% of women were overweight (of which 30.7 % obese). None of the women was underweight before pregnancy. Mean pre-pregnancy BMI was 27.8 ± 5.1 kg/m2 (Table 1).
| Variable | Mean ± SD or N (%) |
| Maternal age (years) | 30.3 ± 5.0 |
| 20-35 | 246 (82.0 %) |
| > 35 | 54 (18.0 %) |
| Parity | 1.0 ± 1.0 |
| Nuliparity | 107 (35.7 %) |
| Primiparity | 102 (34.0 %) |
| Multiparity | 91 (30.3 %) |
| Spacing between pregnancies (months) | 30.9 ± 22.7 |
| < 6 months | 4 (2.1 %) |
| 7 to 23 months | 64 (33.2 %) |
| > 24 months | 125 (64.7 %) |
| Maternal education | |
| Low | 102 (34.0 %) |
| Average | 87 (29.0 %) |
| High | 111 (37.0 %) |
| Wealth index | |
| Low | 98 (32.7 %) |
| Average | 145 (48.3 %) |
| High | 57 (19.0 %) |
| Pre-pregnancy BMI (kg/m2) | 27.8 ± 5.1 |
| Normal (18.5-24.9) | 92 (30.7 %) |
| Overweight (25-29.9) | 116 (38.7 %) |
| Obese (=30) | 92 (30.7 %) |
| 1st Trimester GWG | 1.1 ± 3.3 |
| 2nd Trimester GWG | 4.6 ± 3.0 |
| 3rd Trimester GWG | 3.3 ± 2.5 |
| Total GWG | 9.0 ± 5.7 |
| Insufficient | 142 (47.3 %) |
| Normal | 75 (25.0 %) |
| Excessive | 83 (27.7 %) |
Data are expressed as mean ± SD or n (%)
BMI: body mass index
GWG: gestational weight gain
Table 1 : Characteristic of women included in the study
The mean overall gestational weight gain was 9.0 ± 5.7 kg. The mean rate of GWG in 1st, 2nd and 3rd trimester was respectively 1.1 ± 3.3 kg, 4.6 ± 3.0 kg and 3.3 ± 2.5 kg (Table 1). The majority of women (75.0 %) had an abnormal weight gain at the end of pregnancy (of which 47.3 % had an insufficient weight gain and 27.7 % had an excessive weight gain) and only 25.0 % of them had an adequate weight gain. According to trimesters of pregnancy, only 15.7 % of women in the 1st trimester, 20.3 % in the 2nd and 25.4 % in the 3rd one had normal weight gain.
The rate of maternal anemia during pregnancy was 58.0%. Depending on the trimesters of pregnancy, 28.0% of women in trimester 1, 32.3% in trimester 2 and 54.2% in trimester 3 were anemic. Materanl anemia was more frequently observed during the third trimester of pregnancy (P < 0.05) (Figure 1).
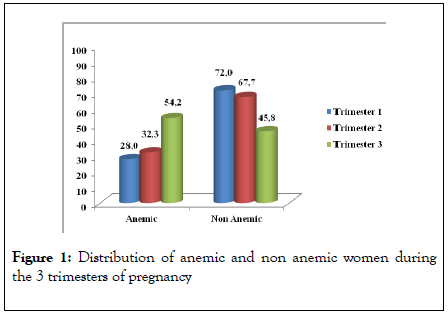
Figure 1 : Distribution of anemic and non anemic women during the 3 trimesters of pregnancy
For haematological factors, the average concentration of hemoglobin (10.4 ± 0.5 vs 12.5 ± 0.9 ; p < 0.0001), hematocrit (33.5 ± 2.5 vs 37.4 ± 3.1 ; p < 0.0001), the average blood volume (214.4 ± 53.0 vs 236.1 ± 61.0 ; p < 0.0001) and platelets (83.4 ± 6.2 vs 86.2 ± 5.1 ; p < 0.0001) were lower in anemic pregnant women compared to non anemic ones (Table 2).
| Biological parameters | Anemic | Non-Anemic | p-value* | Standards$ |
|---|---|---|---|---|
| (N = 174) | (N = 126) | |||
| White blood cells 103/ mm3 | 8.0 ± 2.9 | 8.5 ± 2.2 | 0.06 | 4.0 – 10.0 |
| Red blood cells 106 / mm3 | 3.6 ± 0.4 | 4.4 ± 0.5 | <0.0001 | 4.00 – 6.2 |
| Hemoglobin (Hb) (g/dl) | 10.4 ± 0.5 | 12.5 ± 0.9 | <0.0001 | 11.0 – 17.0 |
| Hb < 11 | 174 (100 %) | 0 (0) | <0.0001 | |
| Hb = 11 | 0 (0) | 126 (100 %) | ||
| Hematocrit (Ht) (%) | 33.5 ± 2.5 | 37.4 ± 3.1 | <0.0001 | 36.0 – 55.0 |
| Platelets 105 /mm3 | 214.4 ± 53.0 | 236.1 ± 61.0 | 0.0012 | 150– 450 |
| Average blood volume (fl) | 83.4 ± 6.2 | 86.2 ± 5.1 | <0.0001 | 85.0 – 100.0 |
Table 2: Haematological factors associated with gestational anemia
In Table 3, we presented anthropometric and nutritional factors associated with gestational anemia in each trimester of pregnancy. Regardless of the trimester of pregnancy, women with inadequate weight gain were more likely to be anemic compared to non anemic women (p < 0.01). Total energy intake in the first (1592.6 ± 769.3 vs 1822.7 ± 705.9 ; p = 0.01) and
third trimesters (2029.1 ± 658.6 vs 2229.9 ± 630.2 ; p = 0.0078) was lower in anemic women compared to non anemic ones. Although not significant, the intakes of iron, vitamin B9 and vitamin C were lower in anemic women. Non supplemented women with iron showed significant association (p < 0.01) with anemia (Table 3).
| Trimester 1 | Trimester 2 | Trimester 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Anemic | Non-anemic | p-value* | Anemic | Non-anemic | p-value* | Anemic | Non-anemic | p-value* | |
| (N = 84) | (N = 216) | (N = 97) | (N = 203) | (N = 162) | (N = 137) | ||||
| Pre-pregnancy BMI (kg/m2) | |||||||||
| Normal (18.5-24.9) | 28 (33.3 %) | 64 (29.6 %) | 32 (33.0 %) | 60 (29.6 %) | 58 (35.8 %) | 34 (24.8 %) | 0.02 | ||
| Overweight (25-29.9) | 33 (39.3 %) | 83 (38.4 %) | 0.7 | 41 (42.3 %) | 75 (36.9 %) | 0.3 | 65 (40.1 %) | 51 (37.2 %) | |
| Obese (=30) | 23 (27.4 %) | 69 (31.9 %) | 24 (24.7 %) | 68 (33.5 %) | 39 (24.1 %) | 52 (38.0 %) | |||
| Trimestrial GWG | |||||||||
| Insufficient | 46 (54.8 %) | 80 (37.0 %) | 43 (44.3 %) | 51 (25.1 %) | 87 (53.7 %) | 51 (37.2 %) | |||
| Adequate | 11 (13.1 %) | 36 (16.7 %) | 0.01 | 13 (13.4 %) | 48 (23.6 %) | 0.0022 | 36 (22.2 %) | 40 (29.2 %) | 0.01 |
| Excessive | 27 (32.1 %) | 100 (46.3 %) | 41 (42.3 %) | 104 (51.2 %) | 39 (24.1 %) | 46 (33.6 %) | |||
| Caloric intake (kcal/d) | 1592.6 ± 769.3 | 1822.7 ± 705.9 | 0.01 | 2086.1 ± 770.4 | 2243.5 ± 647.7 | 0.06 | 2029.1 ± 658.6 | 2229.9 ± 630.2 | 0.0078 |
| Iron intake (mg/d) | 7.8 ± 4.5 | 9.3 ± 7.8 | 0.08 | 10.7 ± 4.2 | 11.0 ± 4.0 | 0.58 | 10.1 ± 3.8 | 10.7 ± 3.9 | 0.14 |
| Vit B9 intake (µg/d) | 184.9 ± 110.1 | 205.2 ± 102.7 | 0.1 | 229.9 ± 104.9 | 246.9 ± 92.7 | 0.16 | 224.2 ± 92.4 | 241.2 ± 95.2 | 0.12 |
| Vit B12 intake (mg/d) | 2.5 ± 4.5 | 3.3 ± 5.0 | 0.2 | 3.7 ± 5.0 | 3.9 ± 5.3 | 0.7 | 3.4 ± 4.6 | 3.6 ± 5.4 | 0.71 |
| Vit C intake (mg/d) | 95.0 ± 87.8 | 105.7 ± 84.6 | 0.3 | 106.1 ± 79.8 | 125.3 ± 76.4 | 0.04 | 114.2 ± 79.8 | 130.2 ± 83.5 | 0.09 |
| Iron supplementation | |||||||||
| Yes | 37 (44.0 %) | 134 (62.0 %) | 0.0047 | 9 (9.3 %) | 85 (41.9 %) | <0.0001 | 7 (4.3 %) | 28 (20.4 %) | <0.0001 |
| No | 47 (56.0 %) | 82 (38.0 %) | 88 (90.7 %) | 118 (58.1 %) | 155 (95.7 %) | 109 (79.6 %) | |||
Table 3: Factors associated with gestational anemia in the three trimesters of pregnancy.
As real consequences, we found that maternal anemia in the first trimester raised significantly the risk of low birth weight (< 2500 g) (23.8 % vs 11.1 % ; p = 0.006). In the second trimester, it was associated with term overrun (≥ 42 weeks of amenorrhea)
(14.4 % vs 5.9 % ; p = 0.01). In the third trimester, maternal anemia raised the risk of low birth weight (16.7 % vs 11.7 % ; p = 0.04) and term overrun (11.7 % vs 5.1 % ; p = 0.055) (Table 4).
| Variable | Trimester 1 | Trimester 2 | Trimester 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anemic | Non-anemic | p-value* | Anemic | Non-anemic | p-value* | Anemic | Non-anemic | p-value* | |
| (N = 84) | (N = 216) | (N = 97) | (N = 203) | (N = 162) | (N = 137) | ||||
| Delivery term | |||||||||
| Preterm | 22 (26.2 %) | 51 (23.6 %) | 0.6 | 16 (16.5 %) | 57 (28.1 %) | 0.01 | 33 (20.4 %) | 39 (28.5 %) | 0.055 |
| Full term | 53 (63.1 %) | 148 (68.5 %) | 67 (69.1 %) | 134 (66.0 %) | 110 (67.9 %) | 91 (66.4 %) | |||
| Overrun | 9 (10.7 %) | 17 (7.9 %) | 14 (14.4 %) | 12 (5.9 %) | 19 (11.7 %) | 7 (5.1 %) | |||
| Delivery type | |||||||||
| Normal | 43 (51.2 %) | 125 (57.9 %) | 0.29 | 47 (48.5 %) | 121 (59.6 %) | 0.06 | 90 (55.6 %) | 78 (56.9 %) | 0.8 |
| Cesarean | 41 (48.8 %) | 91 (42.1 %) | 50 (51.5 %) | 82 (40.4 %) | 72 (44.4 %) | 59 (43.1 %) | |||
| Birth weight | |||||||||
| < 2500g | 20 (23.8 %) | 24 (11.1 %) | 0.006 | 13 (13.4 %) | 31 (15.3 %) | 0.4 | 27 (16.7 %) | 16 (11.7 %) | 0.04 |
| [2500-4000 g[ | 50 (59.5 %) | 130 (60.2 %) | 63 (64.9 %) | 117 (57.6 %) | 103 (63.6 %) | 77 (56.2 %) | |||
| = 4000 g | 14 (16.7 %) | 62 (28.7 %) | 21 (21.6 %) | 55 (27.1 %) | 32 (19.8 %) | 44 (32.1 %) | |||
Table 4: Outcomes of gestational anemia in the mother and the infant in the three trimesters of pregnancy.
Discussion
The study finding revealed that anemia was common in pregnant women: 58.0 % of our patients had gestational anemia. Nowadays, maternal anemia is considered as a public health problem in the world, especially in developing countries. A study in Sidi Bel Abbes (Algeria), [24], found that the prevalence of gestational anemia was 74.0 %. This percentage is higher than that observed in our population. This might be due.
to socio-economic variations, cultural and dietary patterns across regions within the same country. Studies in developing countries on pregnancy-induced anemia revealed a high prevalence, up to more than 50.0% [25]. The WHO considers anemia in pregnant women as a serious public health problem when the prevalence is greater than 40% [26]. The frequency of anemia in this study (58.0 %) was consistent with that expected for developing nations, being classified as severe in epidemiological scale (> 40%). The same was observed in other developing countries, such as in eastern Ethiopia [27] and China [28], which had a frequency of anemic pregnant women of 43.9 % and 58.6 %, respectively, while in developed countries, this prevalence is much lower (≤ 20%) [29].
Women in the second and third trimester were more likely to be anemic than those who were in the first trimester. Our results were similar with other studies which found that anemia is even stronger as the age of pregnancy is advanced [30, 31]. This might be due to the fact that during pregnancy the need for calorie and nutrients are increased to support increased maternal metabolism, blood volume and the delivery of nutrients to the fetus [32] and this demand more increases in the second and third trimesters of pregnancy. In the first trimester there is a marked decrease in the absorption of iron probably because of lower iron requirements and menstruation stops, saving median of 0.56 mg Fe/day (160 mg/pregnancy) [33]. However, in the second trimester iron absorption from a diet of very high iron bioavailability increases by 1.9mg/day and in the last trimester it increases by up to 5.0 mg/day [34]. The results of this study showed that, gestational anemia is still a public health problem that is far from being resolved and that might be caused by the combination of several factors.
For the diagnosis of anemia in populations, hemoglobin and hematocrit were generally used. The importance of these indicators during pregnancy should be highlighted, considering that they were frequently used as a screening test [35, 36]. In our study, the average concentration of hemoglobin (10.4 ± 0.5 vs 12.5 ± 0.9 ; p < 0.0001), hematocrit (33.5 ± 2.5 vs 37.4 ± 3.1 ; p < 0.0001), the average blood volume (214.4 ± 53.0 vs 236.1 ± 61.0 ; p < 0.0001) and platelets (83.4 ± 6.2 vs 86.2 ± 5.1 ; p < 0.0001) were lower in anemic pregnant women compared with non anemic ones. A U-shaped distribution has been shown between hemoglobin concentration and pregnancy complications [37, 38]. This means that the reduction of hemoglobin (less than 11 g/dL) lead to complications such as preterm delivery, intrauterine growth restriction and increased blood pressure Intrauterine growth retardation [38].
In our study, gestational weight gain was a risk factor for maternal anemia. Women with inadequate weight gain were more likely to be anemic compared to non anemic women (p < 0.01). Anemia can be explained primarily by a diet low in iron [39]. Some authors have shown that iron deficiency can lead to insufficient gestational weight gain, thus promoting the onset of anemia during pregnancy [40]. This was confirmed by the very high percentages of iron deficiency in our patients. In fact, between 86.7% and 100% of our subjects had very low intakes of iron during the three trimesters of pregnancy. Women with low weight gain were the most likely to have very low intakes of iron (p = 0.006).
Women with insufficient energy intake in the first (1592.6 ± 769.3 vs 1822.7 ± 705.9 ; p = 0.01) and third trimesters (2029.1 ± 658.6 vs 2229.9 ± 630.2 ; p = 0.0078) had a higher risk of being anemic. Our results were similar to data from the German National Food Consumption Survey, which indicated that the average intake of energy and nutrients in pregnant women was insufficient [41]. Insufficient caloric intake in trimester 1 (T1), could be explained by the high frequencies of nausea (65.9%), vomiting (52.9%) and food aversions (62.7%) observed in our subjects. Also, the fear of having a large baby or gaining too much weight during pregnancy can lead to restrictive caloric behaviors that may explain lower caloric intakes in T3.
Mean intakes of micronutrients were far below the recommended dietary intakes. Women with low intakes of iron, vitamin B9 and vitamin C had a higher risk of being anemic, although not significant. Iron deficiency was the main cause of anemia, most often associated with iron-deficient diets [42]. Studies have shown that micronutrient deficiency was a limiting factor in fetal growth. Some micronutrients were essential for the formation of body tissues, while others were essential for energy metabolism, bone formation and gene transcription [43]. Inadequate intake of minerals and vitamins during pregnancy was associated with adverse maternal and child outcomes such as anemia, neural tube defects, and low birth weight [44, 45].
Non supplemented women with iron showed significant association (p < 0.01) with anemia, regardless of the trimester of pregnancy. Similar to our results, work done by Preziosi et al. (1997) of 197 pregnant women showed that the frequency of anemia decreased after supplementation [46]. A recent study [47] found that most pregnant women were not receiving adequate amounts of iron, despite taking fortified food and supplementation. Even in developed countries, such as the United Kingdom, up to 50% of women during their reproductive age have poor iron residual supplies, and are at risk of developing anemia if they conceive [47].
Routine maternal supplementation is a vital mean in correcting the global problem of iron deficiency and preventing its negative effects [48]. According to Scholl [9], iron supplementation during pregnancy increases the serum iron concentration, depending on the maternal diet, but does not improve iron stores, and it is therefore important to initiate iron supplementation before pregnancy in order to improve the reserves of this mineral.
To conclude on the impact of anemia on pregnancy outcomes, we found that maternal anemia increased the risk of low birth weight (p < 0.05) in early and late pregnancy. Studies have found that maternal anemia may have a negative effect on birth outcomes such as stillbirth, neonatal death, and low birth weight [49]. Maternal anemia early in pregnancy can result in low birth weight. For example, Welsh women who were first diagnosed with anemia (hemoglobin <104 g/L) at 13–24 wk of gestation had a 1.18–1.75-fold higher relative risk of low birth weight and prenatal mortality [50]. A meta-analysis among 44 cohort studies also reported that maternal anaemia in early. pregnancy was associated with an increased risk of low birthweight [11].
Maternal anemia in the 2nd and the 3rd trimesters, had a significant relationship (p < 0.05) with overrun term. Our results were similar to some other studies [51, 52]. In accordance with some sutdies [53, 54], we observed no associations of maternal anaemia with the risk of preterm delivery. May be was due to the fact that only severe anaemia, but not mild anaemia, is associated with an increased risk of preterm delivery.
In this prospective cohort study from early pregnancy onwards, lower hemoglobin levels, lower BMI (in the 3rd trimester), inadequate gestational weight gain (all trimesters), insufficient caloric intake (in the 1st and 3rd trimesters) and non supplementation with iron (all trimesters) were associated with maternal anemia during pregnancy. As a consequence, maternal anaemia was associated with increased risks of adverse maternal and fetal outcomes, such as low birth weight and overrun term.
Conclusions
In this prospective cohort study from early pregnancy onwards, lower hemoglobin levels, lower BMI (in the 3rd trimester), inadequate gestational weight gain (all trimesters), insufficient caloric intake (in the 1st and 3rd trimesters) and non supplementation with iron (all trimesters) were associated with maternal anemia during pregnancy. As a consequence, maternal anaemia was associated with increased risks of adverse maternal and fetal outcomes, such as low birth weight and overrun term.
There is an increasing need for public health strategies to educate the population on the importance of gaining adequate weight during pregnancy. Health care providers should inform women about the appropriate amount of weight to gain during pregnancy according to their pre-pregnancy BMI. There is also a need for a healthy diet and iron supplementation before conception, or at least at the beginning of pregnancy. Mothers should receive appropriate nutritional advice and supplementation at their first point of contact with healthcare professionals to avoid gestational anemia and its adverse outcomes in the mother and the infant.
Acknowledgments
The authors would like to extend their grateful to all pregnant women who participated in this study. The authors are gratefully acknowledged for their support and permission to conduct this study.
Ethical Approval
The authors declare that the work described has not involved experimentation on humans or animals.
Financial Disclosure
All affiliations with, or financial involvement in any entity with a financial interest in, or in competition with, the manuscript’s subject matter are disclosed. This includes stock ownership, employment, consultancies, honoraria, grants, patents and royalties.
Conflict of Interest
The authors have no conflicts of interest.
Funding Details
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed Consent
The authors declare that they obtained a written informed consent from the patients and/or volunteers included in the article and that this report does not contain any personal information that could lead to their identification.
REFERENCES
- Freire WB, Kahn SG, McGuire J, Post GL. Anemia prevention and control: What works; part 1: Program guidance. USAID.2003.
- World Health Organization. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia.2008.
- Who U, UNU. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. Geneva: World Health Organization.2001:1-14.
- Breymann C. Iron deficiency anemia in pregnancy. Expert Rev Obstetr Gynecol.2013;8(6):587-596.
- Passerini L, Casey GJ, Biggs BA, Cong DT, Phu LB, Phuc TQ, et al. Increased birth weight associated with regular pre-pregnancy deworming and weekly iron-folic acid supplementation for Vietnamese women. PLoS Negl Trop Dis.2012;6(4):e1608.
- Rizwan N, Uddin SR, Mumtaz F. Maternal anaemia impact on maternal and perinatal outcome, an observational study at University Hospital of Sindh. Inrt J Medi Scie.2013;3(1):328-331.
- Mardani M, Rezapour S, Ahmadipour S, Mohsenzadeh A, Khalkhali Rad AH, Roosta S, et al. Prevalence of anemia and its risk factors among pregnant women in Khorramabad (Iran) 2010–2014. J Mater Fetal Neonat Med.2017;30(7):826-829.
- Vemulapalli B, Rao KK. Prevalence of anaemia among pregnant women of rural community in Vizianagaram, North Coastal Andhra Pradesh, India. Asian J Med Sci.2014;5(2):21-25.
- Scholl TO. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev.2011;69(suppl_1):S23-29.
- Tolentino K, Friedman JF. An update on anemia in less developed countries. The Am J Trop Med Hygiene.2007;77(1):44-51.
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ.2013;346.
- Center for Reproductive Rights. Maternal Mortality in India. Using International and Constitutional Law to Promote Accountability and Change. New York: CRR.2008.
- Phomaphi J. The world health report. World Health Organization.2005: 45-58.
- Centers for Disease Control. Pregnancy nutrition surveillance nation summary of trends in maternal health indicators.2011.
- Geneva S, World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. 2011.
- Cogill B. Guide to measuring anthropometric indicators. Technical assis- tance project for food and nutrition. Washinghton, DC: Academy for Development and Education.2003: 104.
- Status WP. The use and interpretation of anthropometry. WHO technical report series.1995;854(9).
- Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Weight gain during pregnancy, Reexamining the guidelines. 2009:1-3.
- Health Canada. Prenatal nutrition guidelines for health professionals: gestational weight gain.2010.
- Ciqual T. Composition nutritionnelle des. 2013.
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization; 2016.
- Annual Health Bulletin; Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka. Ministry of health. 2012.
- Wardlaw T, Blanc A, Zupan J, Ahman E. United Nations Children’s Fund and World Health Organization. Low Birthweight: Country, Regional and Global Estimates. 2005.
- Demmouche A, Moulessehoul S. Prevalence of iron deficiency anemia during pregnancy in the wilaya of Sidi Bel Abbes (western Algeria). Antropo.2010; 21:39-48.
- Sato AP, Fujimori E, Szarfarc SC, Borges AL, Tsunechiro MA. Food consumption and iron intake of pregnant and reproductive aged women. Latin Am J Nursing.2010;18(2):247-254.
- Badham J, Zimmermann MB, Kraemer K. The guidebook nutritional anemia. Task Force Sight and Life; 2007.
- Kedir H, Berhane Y, Worku A. Khat chewing and restrictive dietary behaviors are associated with anemia among pregnant women in high prevalence rural communities in eastern Ethiopia. PloS one.2013;8(11):e78601.
- Ma AG, Schouten E, Wang Y, Xu RX, Zheng MC, Li Y, et al. Anemia prevalence among pregnant women and birth weight in five areas in China. Med Principle Pract.2009;18(5):368-372.
- Bener A, Al-Nufal M, Vachhani PJ, Ali AI, Samson N, Saleh NM. Maternal complications and neonatal outcome in Arab women of a fast developing country. J Family Community Med.2013;20(1):27.
- Heng W, Xuencun C, Wenguang W. Nutritional status of gestating Chinese women and its influence upon neonates, with emphasis on iron. Nutr Res.1990;10(5):493-502.
- Allen LH. Nutrition supplementation for the pregnant women. Clin Obstet Gynecol. 1994;37:587-595.
- Hallberg L, Rossander-Hulten L. Iron requirements in menstruating women. Am J Clin Nutr.1991;54(6):1047-1058.
- Clerk CA, Bruce J, Greenwood B, Chandramohan D. The epidemiology of malaria among pregnant women attending antenatal clinics in an area with intense and highly seasonal malaria transmission in northern Ghana. Tropical Med Int Health.2009;14(6):688-695.
- Hallberg L, Hultén L. Iron requirements, iron balance and iron deficiency in menstruating and pregnant women.1996.
- Massucheti L. Prevalência de anemia em gestantes atendidas na rede pública de saúde do município de Florianópolis-SC.2007.
- Dani C, Rossetto S, Castro SM, Wagner SC. Prevalence of anemia and nutritional deficiencies, through different laboratory parameters, in pregnant women treated at two public health services in Rio Grande do Sul. RBAC.2008;40(3):171-175.
- Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstetr Gynecol.2000;96(5):741-748.
- Chang SC, O'Brien KO, Nathanson MS, Mancini J, Witter FR. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr.2003;133(7):2348-2355.
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci.2014;19(2):164.
- Foulhy C. Food for pregnant and lactating women. Midwife vocation.2007;56:8-12.
- Max Rubner Institu. National consumption study II.Federal Research Institute for Nutrition and Food; 2008.
- Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol.1998;148(10):998-1006.
- German Nutrition Society, Austrian Nutrition Society, Swiss Nutrition Association. For the nutrient supply DC.2015.
- Tebbani F, Oulamara H, Agli A. Maternal nutrition and birth weight: role of vitamins and trace elements. JFIV Reprod Med Genet.2017;5:199.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet.2013;382(9890):427-451.
- Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr.1997;66(5):1178-1182.
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci.2014;19(2):164.
- Schaefer RM, Huch R, Krafft A. Current recommendations for the treatment of iron deficiency anemia. Swiss Med J.2007;3(105):874-880.
- Patel A, Prakash AA, Das PK, Gupta S, Pusdekar YV, Hibberd PL. Maternal anemia and underweight as determinants of pregnancy outcomes: cohort study in eastern rural Maharashtra, India. BMJ open.2018;8(8):e021623.
- Shill KB, Karmakar P, Kibria MG, Das A, Rahman MA, Hossain MS, Sattar MM. Prevalence of iron-deficiency anaemia among university students in Noakhali region, Bangladesh. J Health Population Nutr.2014;32(1):103.
- Rahmati S, Delpisheh A, Parizad N, Sayehmiri K. Maternal anemia and pregnancy outcomes: A systematic review and meta-analysis. Int J Pediatr.2016;4(8):3323-3342.
- Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low-and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr.2016;103(2):495-504.
- Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol.2000;17(03):137-146.
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Systematic Rev.2015:7.
Citation: Fouzia T, Hayet O, Abdenacer A (2020) Gestational Anemia: The Factors Associated and the Outcomes in the Mother and the Infant. Clinics Mother Child Health. 17: 352. DOI: 10.35248/2090-7214.20.17.352.
Copyright: © 2020 Fouzia T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

