Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 8
Evaluation of Stirring during Artificial Spawning of Landlocked Fall Chinook Salmon
David Cook, Jill M. Voorhees* and Michael E. BarnesReceived: 26-Aug-2023, Manuscript No. JARD-23-22723; Editor assigned: 28-Aug-2023, Pre QC No. JARD-23-22723 (PQ); Reviewed: 11-Sep-2023, QC No. JARD-23-22723; Revised: 18-Sep-2023, Manuscript No. JARD-23-22723 (R); Published: 25-Sep-2023, DOI: 10.35248/2155-9546.23.14.785
Abstract
Stirring eggs and milt during artificial spawning of salmonids is a well-established practice. However, it has not been objectively evaluated. This experiment compared egg survival of spawns of landlocked fall Chinook salmon (Oncorhynchus tshawytscha) that were either stirred with a turkey (Meleagris gallopavo) tail feather or unstirred during sperm activation. There was no significant difference in egg survival to the eyed-stage of development between the two treatments, with mean percent (SE) survival in the stirred eggs at 44.6% (7.4) compared to 42.9% (5.8) in the unstirred eggs. Mean percent (SE) survival to hatch was also not significantly different for stirred eggs at 40.0% (7.1) compared to unstirred eggs at 39.1% (6.0). Based on the results of this study, stirring landlocked fall Chinook salmon eggs and milt during sperm activation is unnecessary and can be eliminated with no impact to egg survival to hatch.
Keywords
Chinook salmon; Oncorhynchus tshawytscha; Artificial spawning; Stirring
Introduction
Artificial spawning of salmonids has been practiced for many years [1]. The dry method of fertilization, whereby milt is placed on eggs prior to addition of water, is commonly used [1-3]. After water is added, the entire egg/milt/water mass is typically stirred with a feather or fingers [2-7].
In most fish species, sperm is immobile in milt, and becomes active when contacted with an activating solution such as water [1,8]. Once activated, sperm can pass through the egg micropyle for fertilization [9]. Stirring is believed to improve egg fertilization rates and subsequent egg survival by minimizing the swimming distance to the micropyle of the short-lived sperm [3,7,10,11]. Feathers are frequently used for stirring because they are assumed to be gentle instruments with little ability to cause egg damage during stirring [5,6].
Stirring during salmonid spawning is a well-established tradition. However, there have been no controlled studies verifying the need for this technique. Thus, the objective of this study was to evaluate the stirring of eggs and milt during the sperm activation period of landlocked fall Chinook salmon (Oncorhynchus tshawytscha) artificial spawning.
Materials and Methods
Female landlocked fall Chinook salmon were spawned on the 1 November 2022, at Whitlock Spawning Station, Lake Oahe, near Gettysburg, South Dakota, USA. Before females were spawned milt was taken from males by hand expression. Milt was pooled and placed in 50 ml conical centrifuge tubes. The centrifuge tube with milt was closed and placed in a rack on ice until used. Immediately following eggs from 12 females were pneumatically expelled and used in this experiment. The eggs were expelled into a suspended mesh bag to allow the ovarian fluid to drain. These eggs from a single female were then split in two parts with half of the eggs being transferred to another mesh bag.
Each bag was then gently set into a shallow plastic pan (33 cm × 28 cm × 12 cm). Each pan received 25 ml of pooled milt, to approximate milt-to-egg ratio used during normal spawning operations (50 ml of milt per spawn). After placement of the milt on the eggs, 1,200 ml of lake water (10°C, total hardness as CaCo3, 210 mg/L; pH, 7.6; total dissolved solids, 390 mg/L) was poured into each pan. During sperm activation one pan was allowed to sit, while the other pan that included the egg/milt/ water mass was gently stirred with a wild turkey (Meleagris gallopavo) tail feather. Sperm activation was allowed for 30 seconds [11].
After these 30 seconds each mesh bag of fertilized eggs was immediately removed from their respective pans and rinsed in lake water for approximately 10 seconds. Samples containing approximately 20 eggs were removed from each bag and placed into 1 L plastic bags that were full of lake water. The bags containing the eggs were put into a cooler containing a small amount of ice, to maintain a temperature of approximately 10°C, during the four-hour transport to McNenny State Fish Hatchery, in rural Spearfish, South Dakota, USA.
After arrival at the hatchery, 10 eggs from each bag were placed into 9.5 cm diameter Petri dishes, each dish containing approximately 30 ml of well water (11°C, total hardness as CaCO3 =210 mg/L, pH=7.6, total dissolved solids=390 mg/L). The eggs were incubated in a refrigeration unit (Danby model DWC350BLPA, Findlay, Ohio, USA; New Air model AW-180E, Huntington Beach, California, USA) at 11°C using the process described by Neumiller et al. [12]. Water exchanges were done weekly until the eyed stage of egg development at 28 days, after which water was exchanged every three days. Throughout the duration of incubation, eggs mortalities, as indicated by a change of color to white due to yolk coagulation were removed and recorded [1]. Percent survival to the egg eyed stage of development and percent survival to hatch were calculated using the following equations, respectively.
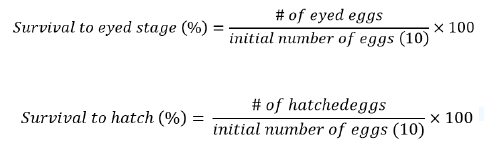
Data were analyzed using the SPSS (24.0) statistical program (IBM, Armonk, New York, USA) using paired-t tests. Significance was predetermined at P<0.05.
Results
There was no significant difference in survival to the eyed stage of egg development between the stirred and unstirred treatments, with percent mean (SE) survival of 44.6% (7.4) and 42.9% (5.8), respectively (Table 1). Similarly, survival to hatch was not significantly different between the treatments, with percent mean (SE) survival at 40.8 % (7.1) for the stirred eggs and 39.1% (6.0) for the unstirred eggs. Of the total of 12 spawns, percent survival to the eyed stage of egg development was higher in five for the stirred treatment and in four for the unstirred treatment. In three of the spawns, percent survival was identical between the treatments. Percent survival to hatch was higher in six spawns in the stirred treatment and five spawns in the unstirred treatment. Percent survival to hatch was identical between the treatments for one spawn.
| Percent eyed-egg (%) | Percent hatch (%) | |||
|---|---|---|---|---|
| Female | Stir | No stir | Stir | No stir |
| 1 | 85 | 65 | 85 | 60 |
| 2 | 80 | 60 | 75 | 55 |
| 3 | 80 | 60 | 60 | 45 |
| 4 | 60 | 70 | 60 | 70 |
| 5 | 40 | 60 | 40 | 60 |
| 6 | 50 | 50 | 50 | 50 |
| 7 | 40 | 35 | 40 | 30 |
| 8 | 35 | 45 | 25 | 45 |
| 9 | 25 | 25 | 20 | 25 |
| 10 | 20 | 15 | 15 | 5 |
| 11 | 10 | 20 | 10 | 20 |
| 12 | 10 | 10 | 10 | 5 |
| Overall mean (SE) | 44.6 (7.4) | 42.9 (5.8) | 40.8 (7.1) | 39.1 (6.0) |
Table 1: Percent survival of eggs to the eyed-stage of development and hatch, from individual female landlocked fall Chinook salmon (Oncorhynchus tshawytscha). The eggs were split and either stirred or not stirred during milt activation in artificial spawning.
Discussion
The results of this study indicate stirring of eggs and milt during landlocked fall Chinook salmon spawning is unnecessary. While not unexpected, these results contradict the established tradition, and common knowledge, of many fish culturists [1,3,5-7]. It must be noted that this is the first study to use controlled experimentation to evaluate these long-held beliefs.
Stirring during sperm activation is believed to be necessary to improve fertilization rates [3,4]. Sperm motility in salmonids is typically very brief and may decrease in as soon as 30 seconds [11,13,14]. By stirring, the newly-activated-and-mobile sperm were believed to be put in closer proximity to the egg micropyle, maximizing the likelihood of fertilization before either micropyle closure in 45 to 60 seconds or sperm motility ceases [15]. In the conditions used in this study, landlocked fall Chinook salmon sperm were able to reach the micropyle without needing any assistance that stirring may have provided.
While stirring with a turkey feather in this study did not improve egg survival, it also did not harm the eggs. Egg sensitivity dramatically increases immediately after fertilization and the lack of stirring-induced mortality appears to indicate that feathers are indeed gentle instruments [16]. None-the-less, stirring is a step of the spawning process that could be eliminated, thereby increasing the efficient use of human labor during artificial spawning of salmonids.
Similar traditions during landlocked fall Chinook salmon spawning in South Dakota, though not as old as stirring gametes during fertilization, have also been challenged and deemed unnecessary. For example, controlled experimentation indicated no benefits from milt residence times with eggs of two minutes or longer. Much shorter residence times, corresponding to actual sperm motility durations of less than 30 seconds, produced similar egg survival [11,17,18]. In addition, using ovarian fluid for sperm activation was also discredited through controlled experimentation [19].
It is unknown if the results of this study with landlocked fall Chinook salmon apply to other Chinook salmon populations or other salmonid species. As controlled experimentation occurs with other species, more conclusive general recommendations can be made. It is also unknown if the results of the small pilot- scale study are representative of the results that would occur at production levels of spawning. However, the ratio of eggs, milt, and water used in this study are typical of those used at production-level spawning of landlocked fall Chinook salmon in South Dakota. Lastly, it must be noted that, although unlikely, the results of this study could have been influenced by the water temperature and unique water chemistry used during artificial spawning [20].
Conclusion
In conclusion, based on the results of this study, stirring landlocked fall Chinook salmon eggs and milt during sperm activation is unnecessary and can be eliminated with no impact to egg survival to hatch. By eliminating stirring, landlocked fall Chinook salmon spawning operations will require less labor and become more efficient. These results may be specific to the landlocked fall Chinook salmon used in this study, making additional controlled experimentation necessary with other populations of both landlocked and oceanic Chinook salmon. In addition, research is needed to determine if stirring is needed during the spawning of other salmonid species, as well as other fish species in general. If indeed stirring can be eliminated during artificial spawning of most fish species, substantial time savings and improvements in efficiency could be realized.
Acknowledgements
We would like to thank the reservoir spawning crew who assisted in the collection of gametes during this experimentation.
Funding
Financially supported by South Dakota Department of Game, Fish and Parks, United States of America.
Conflicts of Interest
There are no conflicts of interest.
References
- Piper RG. Fish hatchery management. (1st edn), U.S. Department of the interior, fish and wildlife service, Washington, D.C. 1982.
- Leach GC. Artificial propagation of brook trout and rainbow trout: With notes on three other species. US government printing office. 1923.
- Leitritz E, Lewis RC. Trout and salmon culture: Hatchery methods. UCANR Publications. 1980.
- Stickney RR. Principles of aquaculture. John Wiley and Sons, Inc. 1994.
- Wilkinson J. Trout Spawn. Globe Gazette. 2001.
- Steuck M. Fall trout spawn in full swing. Iowa DNR news. 2019.
- Fish hatchery management. (1st edn), U.S. Department of the interior, fish and wildlife service, Washington, D.C.
- Rurangwa E, Kime DE, Ollevier F, Nash JP. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 2004;234(1-4):1-28.
- Billard R, Cosson MP. Some problems related to the assessment of sperm motility in freshwater fish. J Exp Zool. 1992;261(2):122-131.
- Ginsburg AS. Sperm-egg association and its relationship to the activation of the egg in salmonid fishes. Development. 1963;11(1):13-33.
[Crossref] [Google Scholar] [PubMed]
- Reese SE, Long AJ, Meyer HA, Barnes ME. Landlocked fall Chinook Salmon sperm motility after short term milt storage. Int J Innov Stud Aquat Biol Fish. 2017;3:9-13.
- Neumiller HK, Blain GA, Barnes ME. Incubation of landlocked fall Chinook Salmon eggs in petri dishes. N Am J Aquac. 2017;79(2):183-186.
- Christen R, GATTI JL, Billard R. Trout sperm motility: The transient movement of trout sperm is related to changes in the concentration of ATP following the activation of the flagellar movement. Eur J Biochem. 1987;166(3):667-671.
[Crossref] [Google Scholar] [PubMed]
- Fitzpatrick JL, Henry JC, Liley NR, Devlin RH. Sperm characteristics and fertilization success of masculinized coho salmon (Oncorhynchus kisutch). Aquaculture. 2005;249(1-4):459-468.
- Rottmann RW, Shireman JV, Chapman FA. Techniques for taking and fertilizing the spawn of fish. Stoneville, Mississippi: Southern regional aquaculture center; 1991.
- Jensen JO, Alderdice DF. Comparison of mechanical shock sensitivity of eggs of five Pacific salmon (Oncorhynchus) species and steelhead trout (Salmo gairdneri). Aquaculture. 1989;78(2):163-181.
- Shannon J, Huysman N, Voorhees JM, Krebs E, Barnes ME. Effect of activated milt residence time on landlocked fall Chinook Salmon egg survival. Open J Appl Sci. 2020;10(4):135-141.
- Stevens J, Voorhees JM, Huysman N, Krebs E, Barnes ME. Effects of two activated milt residence times on landlocked fall Chinook salmon egg survival to the eyed stage of development. N Am J Aquac. 2021;83(3):203-206.
- Wipf M, Barnes ME, Durben DJ. An evaluation of two egg collection and two fertilization techniques during landlocked fall Chinook salmon spawning. N Am J Aquac. 2011;73(3):339-342.
- Finn RN. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquatic Toxicology. 2007;81(4):337-354.
Citation: Cook D, Voorhees JM, Barnes ME (2023) Evaluation of Stirring during Artificial Spawning of Landlocked Fall Chinook Salmon. J Aquac Res Dev. 14:785.
Copyright: © 2023 Cook D, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

