Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2021) Volume 9, Issue 1
Emergency Endovascular Treatment of Recurrent Internal Carotid Artery False Aneurysm Complicated By Carotid-Cutaneous Fistula and Life-Threatening Bleeding
Francesco De Santis*, Roberto Chiappa, Cristina Margot Chaves and Massimiliano MillarelliReceived: 29-Dec-2020 Published: 29-Jan-2021, DOI: 10.35248/2329-6925.9.37.406
Abstract
Background: Carotid pseudoaneurysms can generally be the result of arterial wall degeneration developed after carotid endarterectomy, previous spontaneous carotid dissection, post-traumatic neck injuries, non-vascular procedures and, rarely, infections. The ideal surgical management of extracranial carotid artery false aneurysms is still controversial and treatment should be tailored to the aneurysm’s aetiology, anatomy and presentation.
Case presentation: We describe the case of an 81-year-old man presented with an enlarged pulsatile cervical mass coupled with a small cutaneous fistula, which had developed in roughly 1 year. He had undergone several carotid operations over the last 15 years (endarterectomy, venous-patch-angioplasty for restenosis and bovine-pericardial patch-angioplasty for a pseudoaneurysm).
Methods: A CT-Scan revealed a recurrent pseudoaneurysm. Due to the previous multiple cervicotomies, the aneurysm wall was very closely adjacent to the skin plane; no gas bubbles or cervical fluid collections were evident. The patient rejected treatment at first, but six months later suffered major bleeding from the fistula.
Results: The pseudoaneurysm was emergency excluded with a Viabhan-Stent-graft while the external carotid artery was occluded via vascular-plug. The fistula healed spontaneously in four months. There was no pseudo-aneurysm recurrence, endoleak or any sign of graft infection at 36-month follow-up.
Conclusion: We present a singular case of emergency endovascular treatment of recurrent post-surgical internal carotid artery pseudoaneurysm complicated by a carotid-cutaneous and life-threatening bleeding. In these cases emergency endovascular treatment via covered-stent-graft represents the most effective, definitive or “bridge” management option. Carotid sheath protection via a myo-cutaneous flap could be considered in cases of previous multiple cerivcotomies.
Keywords
Carotid-cutaneous fistula; Emergency endovascular treatment; Life-threatening bleeding; Carotid pseudoaneurysm; Covered stent-graft; Myocutaneous flap
Introduction
Extracranial carotid artery aneurysms (ECAAs) represent an uncommon disease and account for 0.4 to 4% of all peripheral artery aneurysms [1,2].
Atherosclerosis is the most common cause of true aneurysms [1-3]. Conversely, carotid PAs can generally be the result of arterial wall degeneration developed after carotid endarterectomy (CEA), previous spontaneous carotid dissection, post-traumatic neck injuries, non-vascular procedures and, rarely, infections [1,3,4].
We present and discuss a singular case of emergency endovascular treatment (EVT) of a recurrent post-surgical internal carotid artery pseudoaneurysm (ICA-PA) complicated by a carotid-cutaneous fistula (CCF) and life-threatening bleeding.
Case Presentation
An 81-year-old man presented with an enlarged pulsatile cervical mass in the right side of the neck developed 8 years after a bovinepericardial patch carotid angioplasty. The patient had undergone repeated vascular procedures over the past 15 years; ICA endarterectomy with direct arteriotomy closure (15 years earlier); reoperation for ICA restenosis and arterial reconstruction venous patch angioplasty (12 years earlier), and, finally, reoperation for the development of ICA-PA, corrected via bovine-pericardial patch angioplasty (8 years earlier). The cervical mass presented with systolic bruit coupled with a small cutaneous fistula, which had developed over roughly 1 year. A modest amount of serosanguinous drainage came from the fistula (bacteriological exam: St. Epidermidis). No general or local symptoms or signs of infection were present and no underlying fluctuant fluid collections were palpable (Figure 1A). Computed tomography imaging of the neck showed an ICA-PA of approximately 2.5 cm diameter, involving the carotid bifurcation and the internal carotid artery for approximately 2 cm.
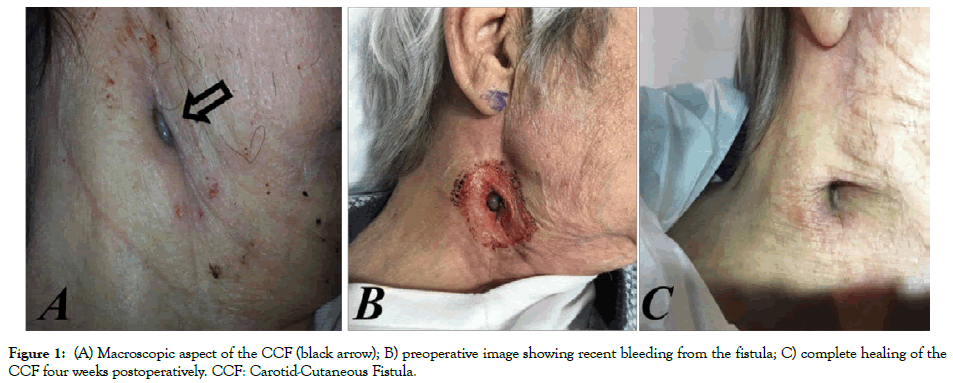
Figure 1: (A) Macroscopic aspect of the CCF (black arrow); B) preoperative image showing recent bleeding from the fistula; C) complete healing of the CCF four weeks postoperatively. CCF: Carotid-Cutaneous Fistula.
Due to the previous multiple cervicotomies, the carotid bifurcation and the PA wall presented closely adjacent to the skin plane where the CCF developed; no gas bubble or cervical fluid collections were evident (Figures 2A-C).
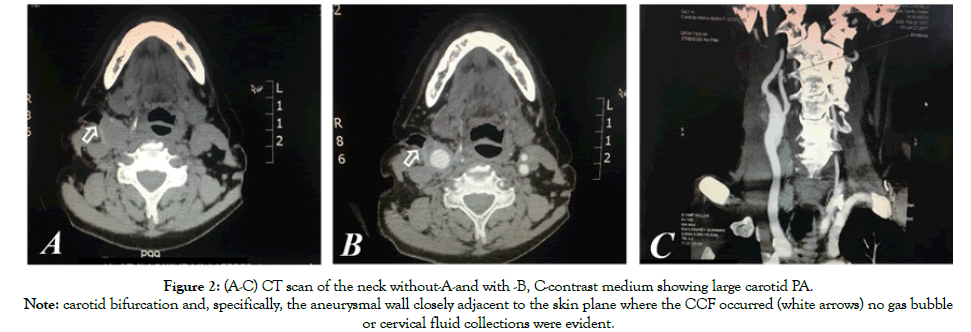
Figure 2: (A-C) CT scan of the neck without-A-and with -B, C-contrast medium showing large carotid PA.
Note: carotid bifurcation and, specifically, the aneurysmal wall closely adjacent to the skin plane where the CCF occurred (white arrows) no gas bubble
or cervical fluid collections were evident.
The patient rejected further surgical treatment and was lost from follow-up control but, six months later, he suffered from major bleeding from the CCF (Figure 1B) and was submitted to emergency endovascular aneurysm exclusion. Through transfemoral approach, after cerebral embolic protection, system deployment (Epifilter Filterwire EZ®), and considering the high risk of Type II endoleak, the external carotid artery was first occluded via 6 mm Amplatzer vascular-plug (AVP; St. Jude Medical, St. Paul, MN). Subsequently, the PA was excluded via a 7 × 10 mm expanded polytetrafluoroethylene (ePTFE) covered Stent-graft (Gore® VIABAHN® Endoprosthesis Flagstaff, AZ, USA) deployment (Figures 3A-3C). The procedure was performed under conscious sedation. The patient remained on iv broadspectrum antibiotics for five days after the procedure and was discharged with dual antiplatelet therapy (Plavix+ASA) and under oral antibiotics for three months. The CCF healed spontaneously over four weeks (Figure 1C). At 36-month follow up, the ICA-PA remained excluded with no signs of endoleak, stent-graft or cervical infection. Furthermore, the patient showed no neurological deficits during the follow up period.
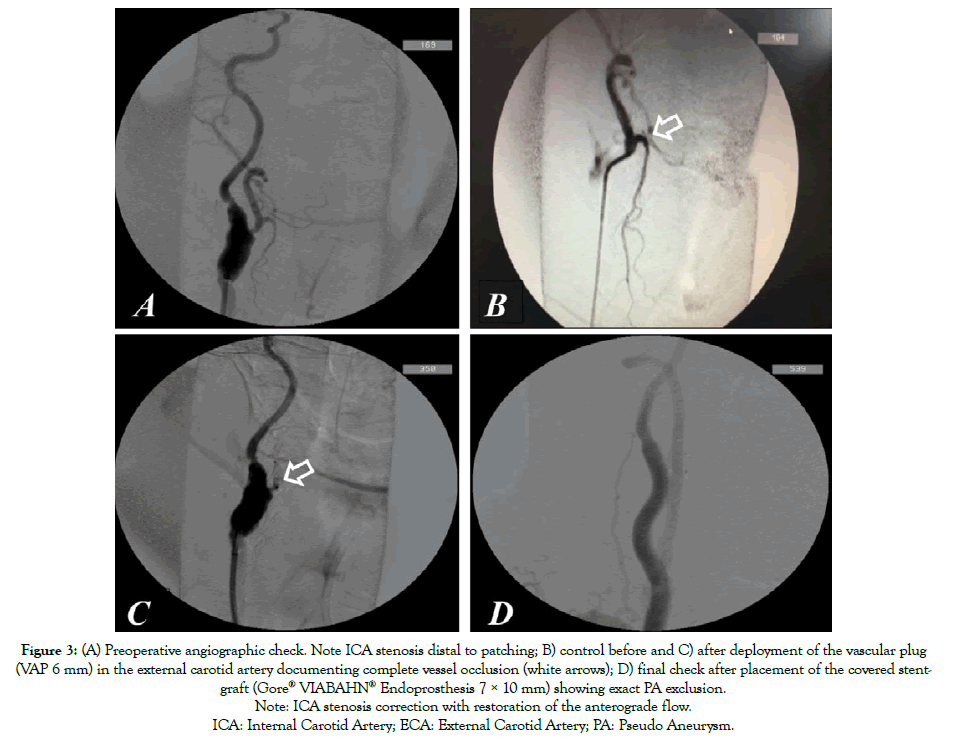
Figure 3: (A) Preoperative angiographic check. Note ICA stenosis distal to patching; B) control before and C) after deployment of the vascular plug
(VAP 6 mm) in the external carotid artery documenting complete vessel occlusion (white arrows); D) final check after placement of the covered stentgraft
(Gore® VIABAHN® Endoprosthesis 7 × 10 mm) showing exact PA exclusion.
Note: ICA stenosis correction with restoration of the anterograde flow.
ICA: Internal Carotid Artery; ECA: External Carotid Artery; PA: Pseudo Aneurysm.
Discussion
Considering the different lengths and methods of follow-up practiced in various vascular centers, it is very difficult to define the real clinical incidence of carotid PAs. In an extensive literature review published in 2019 by Kraemer et Al. which includes 513 ECAAs, 231 (43, 5%) were carotid PAs [1]. In other surgical series, the incidence of carotid PAs, is extremely variable and ranges from 12.5 to 82% of cases [1-5].
The majority of ICA-PAs is related to prior carotid endarterectomies; other possible but much less frequent causes of post carotid endarterectomy PAs are previous spontaneous carotid dissection, post-traumatic neck injuries, non-vascular procedures and rarely, infections [1,4-6].
Infected carotid PAs are in fact uncommon with an incidence as low as 0.025%-0.625% [1-4,7,8].
Regarding non-infected carotid PAs at prior endarterectomy site, their most frequent etiology includes suture failure and deterioration of the arterial wall with an incidence of 24 to 57% of cases in different experiences [1-4,7].
On this point, the largest experience with surgical and medical management of ECAAs (Mayo Clinic System experience published in 2015 by Frankhauser et al.), which includes 141 aneurysms observed in 132 patients over a period of 15 years (1998-2012), 28 (24%) out of 116 PAs were post-surgical [3]. All of the affected arteries in this report were originally patched, probably confirming the relevance of patching closure in the development of postsurgical PAs [3].
It is highly probable that post-surgical PAs may have a longer onset time compared to infect ones [3,6]. In the Mayo Clinic System experience, non-infected post-endarterectomy PAs developed at a mean of 82 months from surgical procedure [3], while PAs related to patch infections seem to appear earlier. In the above reported review, post-surgical patch infections were recorded at a median time of 15 months (interquartile range, 1-55 months) from index CEA to presentation [6]. Even if we cannot exclude with certainty a patch-graft infection due to low virulent staphylococcus epidermidis as a possible cause of PA recurrence in this case, we consider this eventuality highly unlikely because of the very late PA onset time (96 months after the last pericardial patch angioplasty). Furthermore, there was a total absence of general and local signs of an underlying synthetic vascular-graft infection (no evidence of fluid collections or perivascular gas bubble in the cervical region). Instead, we believe that more likely, the previous multiple carotid reconstructions (CEA, venous-patch angioplasty for restenosis and bovine-pericardial patch-angioplasty for PA development) triggered the progressive deterioration of the arterial wall followed by carotid-patch suture failure and, finally, PA reappearance. Specific anatomical conditions (very superficial carotid sheath location, previous multiple cervicotomies), favored the progressive erosion of the subcutaneous tissues followed by the formation of CCF in this particular case; major bleeding from the CCF was most likely related to the ultimate PA breakdown.
Starting from the above reported considerations about the possible etio-pathogenesis of the CCF, we believe that we could have reasonably considered in this case (also in absence of evident signs of vascular infection), the opportunity of carotid sheath protection a myo-cutaneous flap [8].
Indications for intervention in these cases include symptoms, suspected infection and increasing aneurysm size [1-5]. In our challenging case, the post-surgical PA was asymptomatic until lifethreatening hemorrhage from the CCF occurred.
The ideal surgical management of ECAAs (open surgery vs. EVT) is still controversial and treatment should be tailored to the aneurysm’s aetiology, anatomy and presentation [1-3,7,9].
Open surgery is still reported as the most frequent management option for these aneurysms.
In a large literature review published in 2015 by Wellenwerd et al. on ECAAs management including 1,239 patients, 89% of these were submitted to invasive treatment consisting in open surgery in 1,102 aneurysms (94%), EVT in 57 (5%), and hybrid approach in 18 cases (1%) [9].
In the above reported Mayo Clinic System experience review series based on 141 ECAAs, intervention was undertaken in 66 aneurysms (47%), 48 of these (72,7%) were submitted to open surgery and 18 (27,3%), all PAs, received EVT. Precisely, 15 out of 25 (60%) true aneurysms were treated surgically, while 33 out of 116 PAs (29%) underwent open surgery, 18 (15%) endovascular intervention and 65 were managed medically (56%) [3].
In most recent series, however there has been a significant trend for a more general use of EVT in false as well as in true ECAAs [1-3,7,9].
EVT consists of aneurysm exclusion via both bare metal stents and covered stent-grafts, alone or combined with aneurysm sac coiling, and only in particular cases, carotid occlusion [1-4,9].
Aneurysm exclusion via covered stent graft represents a welldocumented management option also in cases of emergency treatment of extra-cranial carotid artery rupture. In 2018, Ho Cheol Choi published a review series of 8 cases of extra cranial carotid artery rupture involving 5 cases of common carotid artery, 2 cervical ICA (n=2) and 1 ICA in the intra-petrous tract, all managed with covered stent-graft [10].
Endovascular aneurysms exclusion can be considered a “definitive” as well as a “bridge management”.
In our case, open surgery was highly contraindicated because of previous multiple carotid operations and in our opinion, PA exclusion with a stent-graft represented the only emergency treatment option. We used a self-expanding Viabhan stent-graft because of its accurate and easy deployment, lower profile and greater flexibility compared to other covered stent grafts. External carotid artery occlusion via vascular plug was indicated by the high risk of PA reperfusion due to Type II endoleak.
The CCF healed spontaneously over four weeks (Figure 1C). At 36-month, follow up the ICA-PA remained excluded with no signs of endoleak or PA recurrence.
Conclusions
In cases of recurrent ICA-PA complicated by cutaneous fistulization and bleeding, endovascular exclusion via covered stent-graft represents the treatment of choice that may be considered, in these cases, both a definitive and “bridge” management option. The risk of pseudoaneurysm recurrence and/or late vascular infection development should always be kept in mind.
The necessity of a better carotid sheath protection (e.g. via a myocutaneous flap) could be considered in selected cases after previous multiple cervicotomy and carotid reconstructions and in presence of very superficial carotid sheath location.
Carotid re-patching after multiple carotid reconstructions should be strictly avoided in general.
Acknowledgment
The authors wish to thank Ms Elena Harwood for the linguistic revision of this paper and the BE Niccolò Vallone for the technical support.
REFERENCES
- Kraemer CJK, Zhou W. Carotid Aneurysm Review. Int J Angiol. 2019;28:017-019.
- El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31:702-712.
- Fankhauser GT, Stone WM, Fowl RJ, O'Donnell ME, Bower TC, Meyer FB, et al. Surgical and medical management of extracranial carotid artery aneurysms. J Vasc Surg. 2015;61:389-393.
- Pulli R, Dorigo W, Innocenti AA. A 20-year experience with surgical management of true and false internal carotid artery aneurysms. Eur J Vasc Endovasc Surg. 2013;45:1-6.
- Attigah N, Külkens S, Zausig N, Hansmann J, Ringleb P, Hakimi M, et al. Surgical therapy of extracranial carotid artery aneurysms: long-term results over a 24-year period. Eur J Vasc Endovasc Surg. 2009;37:127-133.
- Fatima J, Federico VP, BS Scali ST, Feezor RJ, Berceli SA, Giles KA, et al. Management of patch infections after carotid endarterectomy and utility of femoral vein interposition bypass graft. J Vasc Surg. 2019;69:1815-1823.
- Li Z, Chang G, Yao C, Guo L, Liu Y, Wang M, et al. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42:419-426.
- Chakfe N, Diener H, Lejay A, Assadian O, Berard X, Caillon J, et al. A Editor’s Choice – Europena Society fo Vascula Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339-384.
- Welleweerd JC, Den Ruijter HM, Nelissen BGL, Bots ML, Kappelle LJ, Rinkel GJ, et al Management of Extracranial Carotid Artery Aneurysm. Eur J Vasc Endovasc Surg. 2015;50:141-147.
- Choi HC, Park SE, Choi DS, Shin HS, Shin HS, Kim JE, et al. Ruptured extracranial carotid artery: Endovascular treatment with covered stent graft. J Neuroradiol. 2018;45:217-223.
Citation: Santis DF, Chiappa R, Chaves MC, Millarelli M. (2021) Emergency Endovascular Treatment of Recurrent Internal Carotid Artery False Aneurysm Complicated By Carotid-Cutaneous Fistula and Life-Threatening Bleeding. J Vasc Med Surg. 9:406.
Copyright: © 2021 Santis DF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

