Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 16, Issue 3
Efficacy of Velvet Bean (Mucuna pruriens) seeds Ethanolic Extract on Survival, Growth Performance and Sex Reversal of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758)
Mutlen Melvin*, Zango Paul, Nguessie Diffo David Yvan and Tomedi Eyango MinetteReceived: 03-Mar-2025, Manuscript No. JARD-25-28600; Editor assigned: 05-Mar-2025, Pre QC No. JARD-25-28600 (PQ); Reviewed: 19-Mar-2025, QC No. JARD-25-28600; Revised: 26-Mar-2025, Manuscript No. JARD-25-28600 (R); Published: 02-Apr-2025, DOI: 10.35248/2155-9546.25.16.947
Abstract
The present study was conducted to evaluate the efficacy of Mucuna pruriens seeds ethanolic extract on survival, growth performance and masculinization of Oreochromis niloticus juvenile. To this end, 600 O. niloticus juveniles with an average weight of 8.43 ± 0, 52 g were randomly distributed in 20 happas measuring 0.7 × 0.7 × 1 m placed in a 28 m3 tarpaulin tank at a density of 50 juveniles per happa and subjected to natural temperature and light conditions. Offsprings were fed with 5 experimental feeds including 02 controls (negative control (no treatment) and positive control (treatment with 17a – methyltestosterone at 60 mg/kg feed)) and 03 based on M. pruriens seeds ethanolic extract at doses of 0.15; 0.20 and 0.30 g/Kg feeds. After 60 days post-treatment, survival and zootechnical growth parameters were assessed. The study employed the standard acetocarmine squash technique of gonads to analyze the sex reversal percentage. Phytochemical screening revealed the presence of phenolic compounds, flavonoids, alkaloids, tannins saponins and steroids in M. pruriens seeds ethanolic extract. The result showed that total survival rates in all treatments and controls were ranging from 96. 47 ± 2.41% to 82 ± 1.12% (p<0.05). Indeed fishes from group treated at 0.20 g/kg of M. pruriens seeds ethanolic extract obtained the highest survival rates (96,47 ± 2,41%), while the lowest was obtained from fishes from control group (untreated group) with an average value of 82 ± 1.12%. Comparative analysis of the growth characteristics of control group and group treated with different doses of M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) reveals a significantly greater effect (p<0.05) of treatment based on M. pruriens seeds ethanolic extract at 0.20 g/kg and treatment with 17a – methyltestosterone at 60 mg/kg feed compared with the other treatments applied in terms of Average Weight Gain(20,01 ± 6,33 g (MP 0.20) and 21,65 ± 6,24 0 g (MT)), Average Daily Gain (0,35 ± 0,09 g/d (MP 0.20) and 0,36 ± 0,02 g/d (MT)), Specific Growth Rate (6,98 ± 0,06 %/d (MP 0.20) and 6,93 ± 0,35 %/d (MT)) and Average Fish Length (10,22 ± 1,14 cm (MP 0.20) and 10,9 ± 1,03 cm (MT)). A comparative analysis of the sex ratio of treated group with M. pruriens seeds ethanolic extract revealed that the treatment at 0.20 g/kg was the most effective dosage that ensured a maximum male ratio (88.37 ± 1.53%., p<0.05). These findings highlight the potential of M. pruriens seeds ethanolic extract as a viable alternative to synthetic steroid hormones for achieving masculinization in Nile tilapia.
Keywords
Nile tilapia; Mucuna pruriens; Sex reversal; Survival; Growth performance 17a-MT
Introduction
The tilapia Oreochromis niloticus, Linnaeus, 1758, commonly known as “Nile tilapia”, is the most common fish farmed in tropical Africa. A warm-water, farmed fish, it is the mainstay of freshwater fish farming in the world’s intertropical belt [1, 2]. Worldwide, tilapia is the second most farmed and produced group of fish with 3.49 Million tonnes (Mt) well after carp (24 Mt), followed by clarids with 2.97 Mt and salmonids with 2.36 Mt [3-6]. Thanks to its nutritional value, which is rich in essential amino acids and fatty acids of good nutritional quality, tilapia O. niloticus is very edible, with flesh that is much appreciated by consumers, making it a highly commercialized fish [7,8].
Rapid growth rates, high tolerance to low water quality, efficient food conversion, resistance to disease, good consumer acceptance and ease of spawning made tilapia a suitable fish for culture [9]. The fish is reported to sexually mature at a small size of around 6 cm and a young age of around 3 months [10]. However, because of its very high reproduction and precocity, tilapia O. niloticus is exposed to frequent cases of dwarfism and close inbreeding. This could have a negative impact on farm production yields [11,12].
To circumvent these constraints linked to anarchic reproduction and improve yields by producing high-growth individuals, various methods have been developed to produce all-male tilapias, including manual separation of sexes, hybridization, genetic manipulation, and hormonal sex reversal [13]. Among the methods of producing monosex tilapia, hormonal sex reversal is acknowledged as the most efficient and is a commonly used technique that allows for the mass production of all-male tilapia in both small- and large-scale production systems [14,15]. Synthetic steroids such as 17-alpha methyltestosterone, androstenedione or 1-dihydrotestosterone acetate are commonly used to induce sex reversal in tilapia, but because of the potential dangers of such steroids, particularly the impact of treatment residues on the health of aquaculture workers, the environment and biodiversity [16], the use of plants with androgenic properties is a potential alternative worth exploring.
Several medicinal herbs have been reported to possess compounds known as phytoandrogens and phytoestrogens [17,18], which are believed to have functional effects similar to testosterone or estrogens, respectively, in animals. Plant extracts contain phytochemicals capable of inhibiting estrogen biosynthesis and acting as aromatase inhibitors and estrogen receptor antagonists in gonadal germ cells, and can therefore be considered as potential means of sex reversal in fish [19]. Indeed plant extracts contain various bioactive principles such as alkaloids, flavonoids, pigments, phenolics, terpenoids, steroids, essential oils which have been reported to promote various activities such as anti-stress, growth stimulation, appetite stimulation, tonicity and immunostimulant and antimicrobial properties during fish production [20,21].
Reverter et al. [22] and Van Hai [23] indicated that plant extracts containing phytochemicals such as isoflavonoids, flavonoids, and saponins, which possess estrogenic and androgenic properties, could potentially replace synthetic steroid hormones for sex reversal in tilapia.
Mucuna pruriens is a widespread tropical and sub-tropical legume belonging to the order Fabales, family Leguminosae, and subfamily Fabaceae. The plant grows to a height of 3–18 m in bushes, hedges, and forests [24,25]. Different parts of this plant have been traditionally used against several diseases worldwide [26]. The herb, M. pruriens (Linnaeus) has various therapeutic uses and aphrodisiac effects in mammals [27]. It has been reported to possess medicinal values and some works indicated the potential use of M. pruriens as alternative source of protein in fish feed [28-30]. The plant has been found to increase libido in men due to its dopaminergic properties [31,32]. The seeds of M. pruriens are considered as astringent, aphrodisiac, nervine and have a high nutritional value [33,34].
There are reports that M. pruriens seed powder helps in some way against stress, it increases secretion of semen and it acts as a restorative and an invigorating tonic or aphrodisiac in diseases characterized by weakness or loss of sexual power [35]. In another study, it has been reported that M. pruriens seed increased sperm concentration and motility in healthy infertile adults [36]. The seeds of M. pruriens contain tannin, saponin, alkaloid, glycoside, flavonoid, and steroid phytochemicals [37,38]. In particular, the steroids in M. pruriens extracts increased the serum testosterone in animals [39], stimulating androgenic effects, as was observed in rats and fishes. The impact of M. pruriens seeds has been explored in various animal species, revealing influences on reproductive indices such as sexual behaviour, gonad growth and gamete quality in rats. Indeed treatment with ethanolic extracts of M. pruriens seed has resulted a significant and sustained increase in sexual activity, improved mount, intromission and ejaculation, and decreased latencies in normal male rats.
Mukherjee et al. [39] studied the efficacy of M. pruriens seeds and Asparagus racemosus roots for induction of masculinization in tilapia. The result suggested that M. pruriens might be regarded to be more potent for induction of masculinization in Nile tilapia as it produced higher percentage of males compared to A. racemosus. Dietary administration of methanol extract of M. pruriens seeds at a concentration of 0.2 g/kg feed was found to be the best method for production of sex reversed all-male tilapia. However, no study on the efficacy of M. pruriens seeds ethanolic extract over a concentration of 0.2 g/kg feed for induction of masculinization in tilapia has been carried out. Hence the interest of this research works. The objective of the present study was to investigate the potential effect of M. pruriens seeds ethanolic extract on Survival, growth performance and masculinization of O. niloticus.
Materials and Methods
Experimental site
The experimental part was conducted on the production facilities of the Ngomsi Fish Industry of Cameroon Aquaculture farm located in the city of Yaoundé, in the Ahala II quarter, Yaoundé III district, Mfoudi Department, Centre Region of Cameroon, with the following geographical coordinates: 3°48‘and 3°51’ north latitude and 11°29‘and 11°33’ east longitude, with an average altitude of 657 m above sea level.
Acquisition of Oreochromis niloticus hatchling
The broodstock used in these experiments consisted of Thirty sexually mature Nile Tilapia (about 7-9 months old; average weight ± SE=62.37 ± 2.01 g) from the initial stock of the Ngomsi Fish Industry farm. They were stocked (at a ratio of 1 male: 3 females) in a triple happas of 1.3 m³ each, placed in a 28 m3 concrete tank, operating in an open system. The broodstock were fed 3 times a day (with a pelleted feed of 30% CP) and monitored for spawning during 34 days. Freshly hatched O. niloticus fry were collected from the happa and distributed randomly in 12 happas at a rate of 50 larvae/happa according to the treatment. They were subjected to natural conditions of temperature and photoperiod.
Collection of plant material
3 kg of fresh M. pruriens seeds were harvested in their natural habitat near the Ngomsi Fish Industry production farm located in the city of Yaoundé. The collected seeds were cleaned and then dried for 14 days away from the sun, as drying in the sun could cause photoreactions that could alter the molecules of certain active ingredients [40,41]. The seeds were dried under shade and milled into a fine powder; the powder (250 g) was kept in a dry, clean, airtight plastic container at room temperature until usage (Figure 1).
Figure 1: Mucuna pruriens seeds and powder.
200 g of M. pruriens seeds powder were soaked in 1000 ml ethanol at 95% for 24 hours with constant shaking at intervals as described by Musa et al. [42]. It was filtered using Watmann filter paper, the filtrate were concentrated by drying it in the oven under pressure at a temperature of 45°C for 8 hours. The concentrated extract were stored in clean bottle, labeled and then preserved in the refrigerator until when needed. The yield of evaporated dry extract on the initial weight basis was calculated using the following equation: R(%)=(W1 × 100)/W2 where W1: Weight of the extract after evaporation of the solvent; W2: Dry weight of the initial sample. The yields obtained from the extraction process were 20.55%.
Phytochemical characterisation of Mucuna pruriens ethanolic extracts
Phytochemical screening
Phytochemical screening was carried out on the basis of characteristic color tests to identify the main chemical groups. The various phytochemical groups of M. pruriens ethanolic extract were characterized using the techniques described by Akrout et al. [43].
Detection of alkaloids (Buchard reaction)
To 1 mL of each solution, 2 drops of Bourchard’s reagent (iodine-iodide reagent) are added. The observation of a reddish-brown precipitate indicates a positive reaction.
Detection of flavonoids (sodium hydroxide test)
A few drops of a 10% NaOH solution are added to a tube containing 3 mL of the extract solution. A yellow-orange color indicates the presence of flavonoids.
Detection of phenolic compounds (reaction with Ferric Chloride (FeCl3))
A drop of 2% alcoholic ferric chloride solution is added to 2 mL of extract. A more or less dark blue-black or green color indicates a positive reaction.
Detection of tannins (Reaction with 1% ferric chloride)
To 1 ml of extract in a test tube was added 2 ml of water followed by one or two drops of 1% ferric chloride. The appearance of a blue, blue-black or black color indicates the presence of gallic tannins; a green or dark green color indicates the presence of catechic tannins.
Detection of saponins (Foam Index)
0.1 g of extract was dissolved in a test tube containing 10 mL of distilled water. The tube was shaken vigorously lengthwise for 30-45 seconds and then left to stand for 15 minutes. The height of the foam is measured. The persistence of foam more than 1 cm high indicates the presence of saponins.
Detection of steroids (Salkowski test)
5 drops of concentrated H2SO4 were added to 1 mL of extract. A red coloration in each extract indicates the presence of steroids.
Quantitative Determination of Phytochemicals
Quantitative estimation of alkaloids
The assay was performed using the spectrophotometric method described by Sreevidya & Mehrotra [44]. A quantity of 5 mL of extract solution was taken and the pH was maintained between 2 and 2.5 with dilute HCl. 2 mL of Dragendorff’s reagent was added and the precipitate formed was centrifuged. The centrifugate was checked for complete precipitation by adding Dragendorff’s reagent and the centrifuged mixture was decanted completely. The precipitate was washed with alcohol. The filtrate was discarded and the residue was then treated with 2 ml of di-sodium sulphate solution. The brownish-black precipitate formed was then centrifuged. Completion of precipitation was checked by adding 2 drops of disodium sulphate. The residue was dissolved in 2mL of concentrated nitric acid, warming if necessary. This solution was diluted to 10 ml with distilled water. Then 1mL of this diluted solution was taken and 5 mL of thiourea solution was added. The absorbance was measured at 435 nm. The standard curve was made from a stock solution of atropine at 10 mg/L with a range from 0 to 1 mg/ml. Absorbances were read using a spectrophotometer at 435 nm against the white tube prepared under the same conditions by replacing the sample with distilled water. The alkaloid content of the samples was estimated from the linear regression line and expressed in gram equivalents of atropine per 100g of powder.
Quantitative estimation of flavonoids
The method described by Patricia et al. [45] was used for the determination of total flavonoids. In a 25-mL flask, 0.75 mL of 5% (w/v) sodium nitrite (NaNO2) was added to 2.5 mL of extract. 0.75 mL of 10% (w/v) aluminium chloride (AlCl3) was added to the mixture and incubated for 6 minutes in the dark. After incubation, 5 mL of sodium hydroxide (1N NaOH) was added and the volume made up to 25 mL. The mixture was shaken vigorously before being assayed using a UV-visible spectrophotometer. The reading was taken at 510 nm. Trials were carried out in triplicate. Flavonoid content was expressed as milligram quercetin equivalent per gram extract (mg Qc-eq/g extract). Quercetin was used here as the reference standard for quantifying total flavonoid content. The total flavonoid content (concentration) was calculated using the formula: £=������/��; £: Content or concentration (mg.AG/g or mg.Qc/g dry extract); C: concentration of the sample given by the spectrophotometer (mg/mL); V: Volume of the prepared solution (mL); D: dilution factor; m: mass of the extract (g).
Quantitative estimation of phenolic compounds
The method described by Patricia et al. [45] was used to determine total phenolic compounds. A volume of 2.5 mL of diluted (1/10) Folin-Ciocalteu reagent was added to 30 μL of extract. The mixture was kept for 2 minutes in the dark at room temperature, and then 2 mL of sodium carbonate solution (75 g.L-1) was added. The mixture was then placed for 15 minutes in a water bath at 50°C, and then rapidly cooled. Absorbance was measured at 760 nm, using distilled water as the blank. A calibration line was performed with gallic acid at different concentrations. Each analysis was performed in triplicate and the polyphenol concentration was expressed in milligrams per milliliter of gallic acid equivalent extract (mg/mL). Gallic acid was used here as the reference standard for quantifying total polyphenol content; this quantity was expressed in milligrams of gallic acid equivalent per gram of extracts (mg.eq.GA/g extract). Total polyphenol contents (concentrations) were calculated using the formula: £=������/��; £: Content or concentration (mg.GA/g or mg.Qc/g dry extract); C:Concentration of the sample given by the spectrophotometer (mg/mL); V:Volume of the prepared solution (mL); D:Dilution factor; m: mass of the extract (g)
Quantitative estimation of tanins
The tannins are dosed according to the method described by Ba et al. [46]. To 1ml of extract in a test tube are added 5 ml of vanillin reagent at 1% (w/v). The tube is left standing for 30 minutes in the dark and the Optical Density (OD) is read at 415 nm against the white. The amount of tannin in the samples is determined using a standard range established from a stock solution of tannic acid (2 mg/mL) carried out under the same conditions as the test.
Quantitative estimation of saponins
The saponins are dosed according to the method described by Madhu et al. [47]. Test extract were dissolved in 80% methanol, 2 ml of vanilin in ethanol was added, mixed well and the 2 ml of 72% sulphuric acid solution was added, mixed well and heated on a water bath at 60 0C for 10min, absorbance was measured at 544nm against reagent blank. diosgenin is used as a standard material and compared the assay with diosgenin equivalents.
Quantitative estimation of steroids
The Steroids are dosed according to the method described by Madhu et al. [47]. 1ml of test extract of steroid solution was transferred into 10 ml volumetric flasks. Sulphuric acid (4N, 2 ml) and iron (III) chloride (0.5% w/v, 2 ml), were added, followed by potassium hexacyanoferrate (III) solution (0.5% w/v, 0.5 ml). The mixture was heated in a water-bath maintained at 70 ± n 2°C for 30 minutes with occasional shaking and diluted to the mark with distilled water. The absorbance was measured at 780 nm against the reagent blank.
Experimental diets
Five experimental diets corresponding to the different treatments were developed using a commercial feed, Skretting, at 50% crude protein. The crude protein ratio of 50% was based on the protein requirements of O. niloticus fry (30-56%) as recommended by Jauncey [48]. Two experimental diets served as controls for our experiments. One was formulated from the original feed to contain 17-alpha methyltestosterone (TMT). The hormone-treated feed was prepared according to the method of Rothbard et al. [49]. Indeed The hormonal solution was obtained by dissolving 60 mg of hormone (17-alpha methyltestosterone) in 0.7 l of 95% absolute ethanol. The feed was previously reduced to powder and calibrated. Then it was subsequently sprinkled with the hormonal solution at a rate of 60 mg of hormone/kg of feed.
The whole was thus mixed to facilitate the incorporation of the hormonal solution into the feed. The mixture was air-dried in the shade for 24 hours to evaporate the alcohol. After drying, the feed was stored in a cold room at 4°C in a plastic bag to preserve the effectiveness of the hormone [50]. However, the second experimental feed called control (T0) which did not receive the hormonal solution was mixed with absolute ethanol and then left to air dry in the shade for 24 hours just like the other feeds. The other 3 were prepared from a commercial feed Skretting, to contain the ethanolic extract of M. pruriens at doses of 0.15; 0.20 and 0.30 g/Kg of feed.
The preparation of the different extract-based experimental feeds
The preparation of the different extract-based experimental feeds involved impregnating the feeds with different doses of extracts using the feed-extract mixing technique. After initial homogenization, a volume of 250 ml of 95% ethanol per kg of feed was added to ensure better distribution of the extract in the feed. The experimental feeds were then dried on transparent cloths for 48 h to evaporate the ethanol. This process was carried out away from the sun and at room temperature to preserve its effectiveness [51]. Each test feed was stored in hermetically sealed, labeled boxes.
Experimental procedure
Six hundred (600) O. niloticus juveniles with an average weight of 8.43 ± 0, 52 g were placed in 20 happas measuring 0.7 × 0.7 × 1 m placed in a 28 m3 tarpaulin tank (Figure 2) at a density of 50 juveniles per happa and subjected to natural temperature and light conditions. A 50w RS electric aerator was installed in the tank to oxygenate the water. The quantity of feed distributed was set according to the average biomass of fry per week. The fry were fed 30% of their Ichtyo-biomass for the first four weeks of experimentation, 15% the second week, 12% the third week and 10% for the last weeks according to Mareck’s rationing table. The daily ration was divided into 3 meals, from 07:00 to 17:30 with an interval of 5.5 hours [51] and adjusted each week according to the results of weekly population samples. After four weeks of treatment, fry in all treated batches were fed 10% of their biomass with commercial granular feed (Skretting at 30% crude protein).
Figure 2: Experimental set-up.
Every morning and evening at 8am and 4pm respectively, the physico-chemical parameters of the water (temperature, pH, and dissolved oxygen) were taken. These parameters, which provide information on water quality, were monitored regularly to ensure optimum rearing conditions for O. niloticus juveniles. The survival and growth of the fishes were monitored from the second week of experimentation, respectively by counting the dead individuals counted and by weighing a sample of 30 individuals taken at random from each of the treatments, at the end of the treatments and then every fortnight until the end of the experiments. The growth performance of O. niloticus juveniles at the end of this experiment (in terms of Average Weight Gain (AWG), Daily Weight Gain (DWG), Specific Growth Rate (SGR), Total Fish Length (TL), Condition Factor (CF) and Survival Rate (SR)) were determined using the following formulae borrowed from various authors [52-57]. These various parameters were calculated at the end of the experiment. These formulae are as follows:
Average Weight Gain: AWG (g) = (Average Final Weight − Average Initial Weight) (g)
Specific Growth Rate (SGR in %.day): SGR = 100 × (ln FAW − ln IAW) ÷ t where IAW: Initial Average Weight (g); FAW: Final Average Weight (g)
Food Conversion Ratio (FCR): FCR = Rd ÷ (Bf − Bi) where Bi: Initial Biomass (g), Bf: Final Biomass (g), Rd: Ration or quantity of feed consumed or distributed (g)
Condition Factor (CF): CF = (W × 100) ÷ LT3 where W: weight (g), LT: Total length (cm)
Survival Rate (%): SR = 100 × (final number of individuals ÷ initial number of individuals)
Sex identification
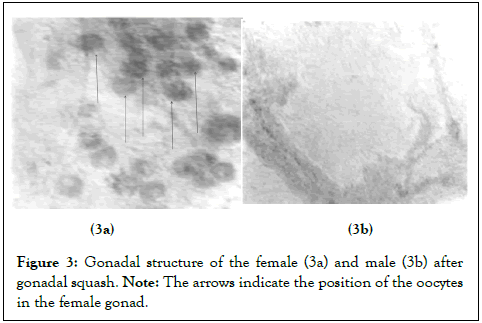
Thirty (30) fishes from each replicate were randomly selected and prepared for sex identification following the gonad squash technique by Guerrero and Shelton. The experimental fish were dissected, and the gonads were placed on a glass slide. A few drops of acetocarmine were added, and the gonads were gently squashed with a cover slip and then observed under an optical microscope in order to determine the sex based on the gonadal structure (Figure 3).
Figure 3: Gonadal structure of the female (3a) and male (3b) after gonadal squash.
Note: The arrows indicate the position of the oocytes in the female gonad.
% male = (number of identified males ÷ total number of fish sample) × 100.
Statistical analysis
Results are expressed as mean ± standard deviation. The homoscedacity and normality of the datasets were checked beforehand using Hartley’s test. Once the conditions of normality and homoscedacity had been met, a one-way analysis of variance (one-factor ANOVA) was used to analyse the differences between the treatments. The 2-to-2 comparisons were made using the post-test for multiple comparisons of means (Turkey test). Differences were considered significant at p<0.05. Statistical tests were performed using STATGRAPHICS Centurion version 19.6 software.
Results
Phytochemical characterisation of M. pruriens seeds ethanolic extract
Qualitative phytochemical screening revealed the presence of phenolic compounds, flavonoids, alkaloids and tannins, saponins, and steroids in M. pruriens seeds ethanolic extract. Quantitative evaluation of M. pruriens seeds ethanolic extracts reveals that Tannins with an average 234.20 ± 1.63 mg/g is the most important compound in the extract of M. pruriens seeds. The lowest compound is Saponins with an average of 0.35 ± 0.02 mg/g (Table 1).
| Type of Extract | Phytochemicals | Qualitative test | Quantitative test (mg/g of extract) |
|---|---|---|---|
| Mucuna pruriens seeds ethanolic extract | Alcaloids | ++ | 131.02± 1.58 |
| Flavonoids | + | 6.34± 0.47 | |
| Polyphenols | + | 2.85± 0.33 | |
| Saponins | + | 0.35±0.02 | |
| Steroids | + | 0.64±0.05 | |
| Tannins | ++++ | 234.20 ±1.63 |
Note: [+]: Presence of constituent; [++]: Moderate concentration of constituent; [+++]: High concentration of constituents; [-]: Shows absence of constituents.
Table 1: Qualitative and quantitative phytochemical composition of M. pruriens seeds ethanolic extract.
From 7.71 ± 0.1 to 8.28 ± 0.44. The average dissolved oxygen values varied from 5.27 ± 0.5 to 5.63 ± 0.81 mg/l. These main values of temperature and dissolved oxygen presented are within the acceptable standards for the breeding of O. niloticus.
Survival and growth characteristics of O. niloticus fry treated with different doses of M. pruriens seeds ethanolic extracts
A comparative analysis at the end of the experimental phase of the different survival rates of O. niloticus fry in control and treated group with different doses of M. pruriens seeds ethanolic extracts (0.15; 0.20 and 0.30 g/kg) showed a significant difference (p<0.05) between treatments (Table 2). Fishes from group treated at 0.20 g/kg of M. pruriens seeds ethanolic extracts obtained the highest survival rates (96.47 ± 2.41%), while the lowest was obtained from fishes from control group treated with 0 g/kg with an average value of 82 ± 1.12%. These results showed that the dose of M. pruriens seeds ethanolic extract did not significantly affect subjects mortality.
| Dietary Mucuna pruriens seeds ethanolic extract g.kg-1 of diet | TMT(control) | T0(control) | MPT 0.15 | MPT 0.20 | MPT 0.30 | P-value |
|---|---|---|---|---|---|---|
| IBW (g) | 8,53 ± 0,01 | 8.39 ± 0.27 | 8.31 ± 0.31 | 8.60 ± 0.69 | 8.45 ± 0,73 | - |
| FBW(g) | 23,25 ± 0,08a | 18,55 ± 0,36b | 16,87 ± 0,4c | 21,5 ± 0,66a | 19,78 ± 0,22b | 0,029 |
| WG (g) | 21,65 ± 6,24a | 16,84 ± 5,21bc | 15,5 ± 4,09c | 20,01 ± 6,33a | 18,07 ± 5,51b | 0,0004 |
| ADG (g.day-1) | 0,36 ± 0,02a | 0,28 ± 0,07ab | 0,26 ± 0,05b | 0,35 ± 0,09a | 0,30 ± 0,54ab | 0.037 |
| SGR (g.day-1) | 6,93 ± 0,35a | 5,96 ± 0,70c | 6,22 ± 0,83b | 6,98 ± 0,06a | 6,07 ± 0,42c | 0,0001 |
| FCR | 1,43 ± 0,05b | 0,89 ± 0,01c | 1,66 ± 0,04a | 1,46 ± 0,02b | 1,68 ± 0,08a | 0,049 |
| FL(cm) | 10,9 ± 1,03a | 9,7 ± 0,17ab | 9,04 ± 0,45b | 10,22 ± 1,14a | 9,93 ± 0,81ab | 0,024 |
| CF(%g/cm3) | 1,82 ± 0,12c | 2,09 ±0,33b | 2,31 ± 0,75a | 2,09 ± 0,18b | 2,02 ± 0,14b | 0.041 |
| SR (%) | 98.5 ± 1.04a | 82 ± 1.12c | 93,89 ± 1;7ab | 96,47 ± 2,41a | 88,67 ± 2.02b | 0.041 |
Note: Data are expressed as means ± standard deviations. Values with the same superscripts of the same row are not significantly different (pË?0.05). Where, IBW= Initial Body Weight, FBW=Final Body Weight, WG=Weight Gain, ADG=Average Daily Gain, SGR=Specific Growth Rate, FCR=Food Conversion Ratio, FL=Fish Length, CF=Condition Factor, SR=Survival Rate T0=Control Treatment 1; TMT=Control treatment 2, Methyltestosterone (60 mg/kg of feed); MPT 0.15=M. pruriens seeds ethanolic extract treatment at 0.15 g/kg of feed; MPT 0.20 g/kg=M. pruriens seeds ethanolic extract treatment at 0.20 g/kg of feed; MPT 0.30/kg=M. pruriens seeds ethanolic extract treatment at 0.30/kg of feed.
Table 2: Survival and growth parameters of O. niloticus fry treated with different doses of M. pruriens seeds ethanolic extract, compared with untreated group and group treated with Methyltestosterone at 60 mg/ Kg of feed.
Growth characteristics varied significantly according to the treatment applied. A comparative analysis of the control group and group treated with different doses of M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) of the different progeny shows a significant difference (p<0.05) between the treatments. The results show that the offspring treated at 0.20 g/kg and those treated with Methyltestosterone at 60 mg/kg of feed had a significantly greater effect (p<0.05) than the other treatments applied in terms of Average Weight Gain (20.01 ± 6.33 g (MP 0.20) and 21.65 ± 6.24 g (MT)), Average Daily Gain (0.35 ± 0.09 g/d (MP 0.20) and 0.36 ± 0.02 g/d (MT)), Specific Growth Rate (6.98 ± 0.06 %/d (MP 0.20) and 6.93 ± 0.35 %/d (MT)) and Average Fish Length (10.22 ± 1.14 cm (MP 0.20) and 10.9 ± 1.03 cm (MT)).
The poorest growth performance was recorded with the treatment containing 0.15 g/kg of M. pruriens seeds ethanolic extract in view of the values obtained for Average Weight Gain (15.5 ± 4.09 g) and Average Daily Gain (0.26 ± 0.05 g/d). However, the offsprings of the group treated at 0.15 g/kg of M. pruriens seeds ethanolic extract had obtained a high Condition Factor (with an average of 2.31 ± 0.75) compared with the other treated group. These results show that the treatment at 0.20 g/kg of M. pruriens seeds ethanolic extract gave the best growth performance of the offspring compared with the other treated group (0.15 and 0.30 g/kg of M. pruriens seeds ethanolic extract).
A comparative analysis of the Consumption Index of the different group fed with feed based on M. pruriens seeds ethanolic extract compared with the control group showed a significant difference (p<0.05) between the treatments. The offsprings of the untreated group had a low Consumption Index (with an average of 0.89 ± 0.01) compared with the other treated group. The highest value was obtained with the group treated at 0.30 g/kg and 0.15 g/kg of M. pruriens seeds ethanolic extract with respective average value of 1.68 ± 0.08 and 1.66 ± 0.04.
Sex reversal
Sexing of fish was done by the standard acetocarmine squash technique of gonads after 60 days of treatment. Microscopic observation after gonadal squash showed that all individuals had normal gonadal development and no intersex individuals were identified.
An analysis of the sex ratio obtained following sexing in the different treated group with different doses of M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) compared with the control group (Methyltestosterone-treated group and untreated group) showed a significant effect (p<0.05) of treatment on the sex ratio. The highest masculinization rate was obtained in group treated with Methyltestosterone at 60 mg/kg (TMT), with an average rate of 92.22 ± 2.08%. A comparative analysis of the different group treated with M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) reveals a significant effect of the treatment at the dose of 0.20 g/kg with an estimated masculinization rate of 88.81 ± 1.53% (Table 3). However, the lowest rate of masculinization was obtained in the untreated batches with an average value of 51.11 ± 0.58%.
| Treatments | Total individuals | Male | Female | Masculinization rate (%) |
|---|---|---|---|---|
| TMT(control) | 90 | 83 | 7 | 92.22 ± 2.08a |
| T0 (control) | 90 | 46 | 44 | 51.11 ± 0.58d |
| MPT 0.15 g/kg | 90 | 66 | 24 | 73.33 ± 3.60cd |
| MPT 0.20 g/kg | 86 | 76 | 10 | 88.37 ± 1.53b |
| MPT 0.30/kg | 88 | 72 | 16 | 81.81 ± 2.64c |
| P-value | - | - | - | 0.031 |
Note: Data are expressed as means ± standard deviations. Values with the same superscripts of the same column are not significantly different (pË?0.05). T0=Control Treatment 1; TMT=Control Treatment 2, Methyltestosterone (60 mg/kg of feed); MPT 0.15=M. pruriens seeds ethanolic extract treatment at 0.15 g/kg of feed; MPT 0.20 g/kg=M. pruriens seeds ethanolic extract treatment at 0.20 g/kg of feed; MPT 0.30/kg=M. pruriens seeds ethanolic extract treatment at 0.30/kg of feed.
Table 3: Sex ratio at 60 days post-treatment of O. niloticus offspring treated with different doses of M. pruriens seeds ethanolic extract ((0.15; 0.20 and 0.30 g/kg) compared with the control group (Methyltestosterone-treated group and untreated group).
Discussion
The main physical and chemical parameters measured during this experiment
were temperature, pH and dissolved oxygen. The average values for temperature, pH and dissolved oxygen remained relatively stable during the experimental period. In fact, the temperature values oscillated in the average range of 27.39 ± 0.74 °C and 28.74 ± 0.91°C, while the pH values ranged from 7.71 ± 0.1 to 8.28 ± 0.44. The average dissolved oxygen values varied from 5.27 ± 0.5 to 5.63 ± 0.81 mg/l. These main temperature and dissolved oxygen values presented are within the acceptable norms for rearing O. niloticus as reported by Omitoyin [59], since the optimum temperature for growth of O. niloticus is between 24 and 28°C, while the pH is between 7-8. The optimum dissolved oxygen concentration is 5 mg/l [60].
Phytochemical screening
Phytochemical screening revealed the presence of phenolic compounds, flavonoids, alkaloids, tannins, saponins and steroids in M. pruriens seeds ethanolic extract. Quantitative evaluation of M. pruriens seeds ethanolic extracts reveals that tannins with an average of 234.20 ± 1.63 mg/g is the most important compound in the extract of M. pruriens seeds. The lowest compound is Saponins with an average of 0.35 ± 0.02 mg/g. These results are in line with those obtained by Mukherjee et al. [39], who detected the presence of flavonoids, saponins, tannins, and flavonoids in M. pruriens seeds ethanolic extracts. These phytoconstituents might render the androgenic activity of the extracts. Previous phytochemical investigation revealed an array of alkaloids and flavonoids in the seed such as the alkaloids bufotenin, dimethyltryptamine, tetrahydroquinolone alkaloids, stizolamine, mucunine, mucunadine, prurienidine, nicotine and the flavonoids namely genistein, medicarpin, kievitone, cajanol etc [61, 62].
Survival rate
Total survival rates in all treatments and controls were ranging from 96.47 ± 2.41 to 82 ± 1.12% (p<0.05). Indeed, fishes from group treated at 0.20 g/kg of M. pruriens seeds ethanolic extracts obtained the highest survival rates (96.47 ± 2.41%), while the lowest was obtained from fishes from control group treated with 0 g/kg with an average value of 82 ± 1.12%. These high values of the survival rates in the entire group treated with different doses of M. pruriens seeds ethanolic extract as well as the control group show that these treatments could not have a deleterious effect on the survival of the various offspring. In another study as well, toxic changes, stress and changes in behavior were not observed in rats treated with differential doses of ethanolic extracts of M. pruriens in a feeding trial with Nile tilapia, fish fed diets containing differentially processed Mucuna seeds for 56 days showed no mortality for the entire period of experiment [63]. However, the survival rate obtained in this study are lower than those obtained by Mukherjee et al. [37] in Nile tilapia juvenile subjected to dietary treatment with powdered M. pruriens seeds (0.0, 2.0, 3.5 and 5.0 g/kg feed) and immersion treatment with aqueous extract of the plant seeds (0.02, 0.035, 0.05 g/l). This difference could be associated with the types of treatment applied, which would have a differential effect on survival rate of the offspring.
Growth characteristics
Growth characteristics varied significantly according to the treatment applied. The results show that offsprings treated at 0.20 g/kg of M. pruriens seeds ethanolic extract and those treated with Methyltestosterone at 60 mg/kg feed had a significantly greater effect (p<0.05) than the other treatments applied in terms of Average Weight Gain (20.01 ± 6.33 g (MP 0.20) and 21.65 ± 6.24 g (MT)), Average Daily Gain (0.35 ± 0.09 g/d (MP 0.20) and 0.36 ± 0.02 g/d (MT)), Specific Growth Rate (6.98 ± 0.06 %/d (MP 0.20) and 6.93 ± 0.35 %/d (MT)) and Average Fish Length (10.22 ± 1.14 cm (MP 0.20) and 10.9 ± 1.03 cm (MT)). These result is higher than those obtained by Saiyad et al. [64] in O. mozambicus juveniles fed with M. pruriens seed meal enriched diet at 2, 4, 6 and 8 g/kg feed. This difference could be associated with the types of treatment applied, the species, the age of the subjects used which would have a differential effect on the growth performance of the offspring.
Recently Ojha et al. [65] has reported that the M. pruriens seed meal significantly enhances growth performance, metabolic activity, and immune response in Labeo rohita. At the same time Siddhuraju et al. [66] stated that the higher inclusion rate of M. pruriens seed meal significantly reduced the growth parameters in Cyprinus carpio because most of the plant-based feedstuffs have a wide variety of anti-nutritional factors such as phytin, Non-Starch Polysaccharides (NSP) and protease inhibitors, which may impair nutrient utilization, as well as impair fish growth performance and health [67].
Sex ratio and masculinization
An analysis of the sex ratio obtained following sexing in the different treated group with different doses of M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) compared with the control group (Methyltestosterone-treated group and untreated group) showed a significant effect (p<0.05) of treatment on the sex ratio. A comparative analysis of the different group treated with M. pruriens seeds ethanolic extract (0.15; 0.20 and 0.30 g/kg) reveals a significant effect of the treatment at the dose of 0.20 g/kg with an estimated masculinization rate of 88.37 ± 1.53%. It should also be noted that the average rate of 51.11 ± 0.58% obtained in the untreated group confirms the ratio of 1:1 ratio (i.e. 50% male and 50% female) expected from such a cross. The results show highest percentage of males in all treated groups except the control (untreated group), this infers that M. pruriens seeds possess androgenic property which has been found to be effective in O. niloticus.
This finding is in agreement with the study done by Mukherjee et al. [37], who found higher percentage of male Nile tilapia when treated with M. pruriens seeds ethanolic extract. The masculine effect might be due to the presence of flavonoids, saponins and steroids in M. pruriens seeds ethanolic extract, which are natural compounds characterized by androgenic activity. Indeed the ethanolic extract of M. pruriens seeds was found to significantly increase testosterone, LH, FSH and prolactin hormone levels, levator ani muscle weight, sperm count and motility in infertile obese mutant rat models [68]. Suresh et al. [27] revealed that ethanolic extracts of M. pruriens seed produced a significant and sustained increase in the sexual activity of normal male rats and improved mount, intromission and ejaculation and decreased latencies at a particular dose.
These results obtained at 0.20 g/kg of M. pruriens seeds ethanolic extract are higher than those found by Mukherjee et al. [37] who obtained an average masculinization rate of 85.34 ± 0.93%. This difference could be due to the genetic variability inherent in each individual, which would have an impact on the response to the different treatments applied. Authors such as Gennotte et al. [69] also attribute this variability to differential expression of sex determinants or endogenous steroid balance during ontogeny. However, the highest percentage of males produced by M. pruriens seeds ethanolic extract was found to be below the ideal requirement of 100% male population. Thus, further studies would be required to establish an ideal treatment regime for production of all-male tilapia population using the M. pruriens seeds and to provide conclusive evidence regarding their efficacy to be used as a sex-reversal agent in tilapia culture [69].
Conclusion
The aim of the present study was to evaluate the effect of M. pruriens seeds ethanolic extract on the survival, growth performance and masculinization of O. niloticus juvenile. Phytochemical screening revealed the presence of phenolic compounds, flavonoids, alkaloids, tannins, saponins and steroids in M. pruriens seeds ethanolic extract. These phytoconstituents might render the androgenic activity of the extract. The result showed that the dose of extract did not significantly affect juvenile mortality. Analysis of the growth parameters revealed that offsprings treated at 0.20 g/kg of M. pruriens seeds ethanolic extract and those treated with Methyltestosterone at 60 mg/Kg feed had a significantly greater effect (p<0.05) than the other treatments applied. A comparative analysis of the sex ratio of treated group with M. pruriens seeds ethanolic extract revealed a significant effect of the treatment at 0.20 g/kg with an estimated masculinization rate of 88.37 ± 1.53%. The highest percentage of males in all treated groups except the control (untreated group) shows that M. pruriens seeds ethanolic extract enhances the masculinization of O. niloticus juvenile. Based on our study we conclude that M. pruriens seeds ethanolic extract can be used as an alternative method to produce all-male tilapia population in an environment friendly manner using a natural product. However, the highest percentage of males produced by plant extracts was discovered to be significantly lower than the optimal criterion of a 100% male population. Thus, more research is needed to develop an appropriate treatment regimen for producing all-male tilapia population using plant extracts and to offer solid evidence of their efficiency as a sex-reversal agent in tilapia culture.
Acknowledgment
The authors would like to thank Mr NGUESSIE Joseph, promoter of the NGOMSI FISH INDUSTRY OF CAMEROUN farm, for making the facilities of his farm available to us for this research work. We would also like to thank Mr NGUESSIE DIFFO David Yvan, Aquaculture Engineer, for his contribution to this activity.
Author Contributions
• Conceptualization: M.M. and ZP
• Methodology: M.M, ZP and N.D.D.Y
• Software: M.M
• Validation: M.M, ZP and N.D.D.Y
• Formal analysis: M.M
• Investigation: N.D.D.Y
• Resources: M.M, ZP and N.D.D.Y
• Data curation: N.D.D.Y
• Writing-original draft preparation: M.M
• Writing-review and Editing: M.M and ZP
References
- Meyer C. Dictionnaire des sciences animals.
- El-Sayed AF. Tilapia culture. 2006.
- Burel C, Médale F. Quid de l’utilisation des protéines d’origine végétale en aquaculture?. OCL. 2014;21(4):D406.
- Trosvik KA, Rawles SD, Thompson KR, Metts LA, Gannam A, Twibell R, et al. Growth and body composition of Nile tilapia, Oreochromis niloticus, fry fed organic diets containing yeast extract and soybean meal as replacements for fish meal, with and without supplemental lysine and methionine. J World Aquac Soc. 2012;43(5):635-647.
- FAO. La situation mondiale des pêches et de l’aquaculture. Rome: FAO; 2012.
- El-Sayed AFM. Alternative dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture. 1999;179(1–4):149-168.
- Lovell T. Opportunities in aquaculture nutrition: Practical considerations in making tilapia feeds. Feed Management. 1995;46:13–22.
- Lim C. Practical feeding-tilapias. Nutr Feed Fish. 1989:163-183.
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208(3-4):191-364.
- Nakamura M, Bhandari RK, Higa M. The role estrogens play in sex differentiation and sex changes of fish. Fish Physiol Biochem. 2003;28:113-117.
- Baroiller JF, Jalabert B. Contribution of research in reproductive physiology to the culture of tilapias. Aquat Living Resour. 1989;2(2):105-116.
- Mair GC, Abucay JS, Beardmore JA, Skibinski DO. Growth performance trials of Genetically Male Tilapia (GMT) derived from YY-males in Oreochromis niloticus L: On station comparisons with mixed sex and sex reversed male populations. Aquaculture. 1995;137(1-4):313-323.
- Mair GC, Little DC. Population control in farmed tilapias.
- Pandian TJ, Sheela SG. Hormonal induction of sex reversal in fish. Aquaculture. 1995;138(1-4):1-22.
- Phelps RP, Popma TJ. Sex reversal of tilapia. 2000;2:34-59.
- Baroiller JF, d'Cotta H. Environment and sex determination in farmed fish. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130(4):399-409.
- Turan F, Akyurt I. Effects of androstenedione, a phytoandrogen, on growth and body composition in the African catfish Clarias gariepinus. Isr J Aquac.–Bamidgeh. 2005;57(1):62-66.
- Bradbury RB, White DE. Estrogens and related substances in plants. InVitamins & hormones.1954;12:207-233.
- Rempel MA, Schlenk D. Effects of environmental estrogens and antiandrogens on endocrine function, gene regulation, and health in fish. Int Rev Cell Mol Biol. 2008;267:207-252.
- Citarasu T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac Int. 2010;18(3):403-414.
- Chakraborty SB, Hancz C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev Aquac. 2011;3(3):103-119.
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture. 2014;433:50-61.
- Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture. 2015;446:88-96.
- Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The magic velvet bean of Mucuna pruriens. J Tradit Complement Med. 2012;2(4):331-339.
- Sathiyanarayanan L, Arulmozhi S. Mucuna pruriens Linn.-a comprehensive review.
- Yusuf M, Begum J, Hoque MN, Chowdhury JU. Medicinal plants of Bangladesh. 2009.
- Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33(1):22-32.
- Ignacimuthu S, Ayyanar M, Sankarasivaraman K. Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia. 2008;79(7-8):562-568.
- Pugalenthi M, Vadivel V, Siddhuraju P. Alternative food/feed perspectives of an underutilized legume Mucuna pruriens var. utilis-a review. Plant Foods Hum Nutr. 2005;60:201-218.
- Sridhar KR, Bhat R. Agrobotanical, nutritional and bioactive potential of unconventional legume-Mucuna. Livest Res Rural Dev. 2007;19(9):126-30.
- Giuliano F, Allard J. Dopamine and sexual function. Int J Impot Res. 2001;13(3):S18-S28.
- Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7(1):137-144.
- Subramanian Arulkumar SA, Muthukumaran Sabesan MS. Rapid preparation process of antiparkinsonian drug Mucuna pruriens silver nanoparticle by bioreduction and their characterization.
- Chandrashekharaiah KS. Physico-chemical and antifungal properties of trypsin inhibitor from the seeds of Mucuna pruriens. Orient J Chem. 2013;29(3):1061.
- Suresh S, Prithiviraj E, Prakash S. Dose-and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. Seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122(3):497-501.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90(3):627-635.
- Mukherjee D, Ghosal I, Chakraborty SB. Production of monosex Nile Tilapia, Oreochromis niloticus using seed of Mucuna pruriens. IOSR j pharm biol sci. 2015;10:55-59.
- Mukherjee D, Ghosal I, Hancz C, Chakraborty SB. Dietary administration of plant extracts for production of monosex tilapia: Searching a suitable alternative to synthetic steroids in tilapia culture. Turk J Fish Aquat Sci. 2018;18(2):267-275.
- Ahmad N, Rahman ZU, Akhtar N, Ali S. Testosterone like activity of ethanolic and aqueous extracts of Mucuna pruriens seeds and its effects on serum biochemical metabolites in immature male rats. Pak Vet J. 2012;32(1):60-64.
- Muthu K, Krishnamoorthy P. Evaluation of androgenic activity of Mucuna pruriens in male rats. Afr J Biotechnol. 2011;10(66):15017-15019.
- Diarra MN. Etude phytochimique d’une plante antipaludique utilisée au Mali: Spilanthes oleraceae Jacq (Asteraceae) (Doctoral dissertation, Thèse Pharmacie, FMPOS, Université Bamako, Bamako).
- Mousa HA, Mousa MA. Immunocytochemical and histological studies on the hypophysealâ?gonadal system in the freshwater Nile tilapia, Oreochromis niloticus (L.), during sexual maturation and spawning in different habitats. J Exp Zool. 1999;284(3):343-354.
- Akrout A, Ayadi A, Bonnet P. Effect of dietary supplementation with olive oil by-products on growth performance, flesh quality and antioxidant status in Oreochromis niloticus (Nile tilapia). Aquac Res. 2011;42(12): 1816-1826.
- Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J AOAC Int. 2003;86(6):1124-1127.
- Kouadio PA, Attioua BK, Kabran FA, Kassi BA, Yao RA, Yeboue HK, et al. Comparative physico-chemical study of fermented and unfermented cocoa beans from Côte d'Ivoire. Int J Biosci. 2020;16(1):355-362.
- Ba K, Tine E, Destain J, Cissé N, Thonart P. Étude comparative des composés phénoliques, du pouvoir antioxydant de différentes variétés de sorgho sénégalais et des enzymes amylolytiques de leur malt. Biotechnologie, Agronomie, Société et Environnement. 2010;14(1).
- Madhu M, Sailaja V, Satyadev TN, Satyanarayana MV. Quantitative phytochemical analysis of selected medicinal plant species by using various organic solvents. J Pharmacogn Phytochem. 2016;5(2):25-29.
- Jauncey K. Nutritional requirements–In: Tilapias: Biology and exploitation (Eds) MCM Beveridge, BJ McAndrew.
- Rothbard S, Solnik E, Shabbath S, Amado R, GrabieI. The technology of mass production of hormonally sex-inversed all male Tilapias.
- Varadaraj K, Kumari SS, Pandian TJ. Comparison of conditions for hormonal sex reversal of Mozambique tilapias. Prog Fish-Cult. 1994;56(2):81-90.
- Eduardo Ferrari Sanches L, Hayashi C. Effect of feeding frequency on Nile tilapia, Oreochromis niloticus (L.) fries performance during sex reversal in hapas. Acta sci Anim sci. 2001:871-876. [CrossRef][Google Scholar][PubMed]
- Jegede T. Control of reproduction in Oreochromis niloticus (Linnaeus 1758) using Hibiscus rosa-sinensis (Linn.) leaf meal as reproduction inhibitor. J Agric Sci. 2010;2(4):149.
- Akinwande A, Dada A, Moody F. Effect of dietary administration of the phytochemical “Genistein”(3, 5, 7, 3, 4 Pentahydroxyflavone) on masculine tilapia. Oreochromis niloticus. 2011:2231-2233.
- Khalil WK, Hasheesh WS, Marie MA, Abbas HH, Zahran EA. Assessment the impact of 17α-methyltestosterone hormone on growth, hormone concentration, molecular and histopathological changes in muscles and testis of Nile tilapia; Oreochromis niloticus. Life Sci J. 2011;8(3):329-343.
- Kefi AS, Kang’ombe J, Kassam D, Katongo C. Growth, reproduction and sex ratios in Oreochromis andersonii (Castelnau, 1861) fed with varying Levels of 17α-methyl testosterone. J Aquac Res Dev. 2012;3:130-137.
- Ogunji JO, Wirth M. Partial Replacement of Fish Meal with Some Alternative Protein Sources in the Diet of Tilapia Oreochromis niloticus (Linn). Isr J Aquac-Bamidgeh. 2001;53(1):34-43.
- Ahmad M, Abdel-Tawwab M, Shalaby A, Khattab Y. Effects of 17 a-Methyltestosterone on growth performance and some physiological changes of nile tilapia, Oreochromis niloticus L.) Fingerllngs. Egypt J Aquat Biol Fish. 2002;6(2):1-23.
- Guerrero III RD, Shelton WL. An aceto-carmine squash method for sexing juvenile fishes. Prog Fish-Cult. 1974;36(1):56.
- Omitoyin BO. Introduction to fish farming in Nigeria. 2007.
- Malcolm C, Beveridje H, Mc Andrew BJ. Tilapias: Biologie and exploitation. Institute of aquaculture. University of stirling, Scotland.
- Janardhanan VV. Nutritional and anti-nutritional composition of velvet bean: An under-utilized food legume in South India. Int J Food Sci Nutr. 2000;51(4):279-287.
- Prakash D, Niranjan A, and Tewari SK. Some nutritional properties of the seeds of three Mucuna species. Int J Food Sci Nutr.2001; 52:79-82.
- Siddhuraju P, Becker K. Comparative nutritional evaluation of differentially processed mucuna seeds [Mucuna pruriens (L.) DC. var. utilis (Wall ex Wight) Baker ex Burck] on growth performance, feed utilization and body composition in Nile tilapia (Oreochromis niloticus L.). Aquac Res. 2003;34(6):487-500.
- Musthafa MS, Asgari SM, Kurian A, Elumalai P, Ali AR, Paray BA, et al. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish & shellfish immunology. 2018;75:374-380.
- Ojha ML, Chadha NK, Saini VP, Damroy S, Chandraprakash SP, Sawant PB, et al. Effect of ethanolic extract of Mucuna pruriens on growth, metabolism and immunity of Labeo rohita (Hamilton, 1822) fingerlings. Int J Fauna Biol Stud. 2014;1(5):01-9.
- Siddhuraju P, Becker K. Preliminary nutritional evaluation of Mucuna seed meal (Mucuna pruriens var. utilis) in common carp (Cyprinus carpio L.): An assessment by growth performance and feed utilisation. Aquaculture. 2001;196(1-2):105-123.
- Dictionnaire des sciences animals
- Kumar S, Ali MY, Sailaja P, Mahesh S, Surekha MV, Giridharan NV, et al. Male reproductive enhancing activity of Mucuna pruriens Linn. seed extract in WNIN/GR-OB obese rats–an infertile obese mutany rat model with prediabetes. Int J Curr Res. 2011;33:323-327.
- Gennotte V, Mélard C, d'Cotta H, Baroiller JF, Rougeot C. The sensitive period for maleâ?toâ?female sex reversal begins at the embryonic stage in the Nile tilapia and is associated with the sexual genotype. Mol Reprod Dev. 2014;81(12):1146-1158.
Citation: Melvin M, Paul Z, Diffo David Yvan N, Eyango Minette T. (2025) Efficacy of Velvet Bean (Mucuna pruriens) seeds Ethanolic Extract on Survival, Growth Performance and Sex Reversal of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758). J Aquac Res Dev. 16:967.
Copyright: © 2025 Melvin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.