Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 10, Issue 9
Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Salt Stress Tolerance of Barnyard Millet (Echinochloa frumentacea)
Rakesh Singh1*, Yashwant Singh Tariyal2 and J.S.Chauhan12High Altitude Plant Physiology Research Centre, HNB Garhwal University (A Central University), Uttarakhand, India
Received: 10-Aug-2021 Published: 31-Aug-2021
Abstract
Salinity stress is a crucial environmental stress adversely affecting crop production by plummeting crop growth. The impacts of rhizobacteria to alleviate salinity stress on germination and growth of barnyard millet were assessed using different concentration of NaCl. It is a well acknowledged strategy to improve plant salt tolerance through inoculation with beneficial microorganisms. The results indicated that the PGPR significantly improved germination percent, root and shoot length and fresh and dry weight of seedling and leaf chlorophyll under salt stress in comparison to non-stressed and plants without inoculants. The results of present study showed that PGPR could be helpful to alleviate the salinity stress at the time of seed germination and growth. It can be concluded that PGPRs are the cost effective and economical tool for salinity tolerance and growth promotion in crop plants.
Keywords
Salinity; Barnyard millet; Germination; Vigour; PGPR
Introduction
Salinity stress significantly affects the productivity of soil and possesses enormous impact on the agriculture production and economy of the nation. Major factor for increases salinity of soil may be anthropogenic or natural which causes increases in concentration of soluble salts in soil. Naturally salinity increases due to the weathering of rocks, mobilization of minerals deposition of salt transported through wind currents from ocean and influx of seawater followed by subsequent retreat [1,2]. Cultivation operations such as land clearing, excessive irrigation and fertilizer use are the reasons for anthropogenic induced salinity. Irrigated lands are observed more prone to salinity than dryland. Anthropogenic induced salinity has degenerated vast tracts of irrigated land to the point that they are no longer productive. Salt is also discharged and redistributed by surface runoff or leached down into soil profile by rainfall and then move laterally to watercourses. Higher salt concentrations in soil inhibit the growth of main and lateral roots by suppressing cell activity such as cell division and elongation [3]. It also imitates soil area for root system to gain access to larger pools of water and nutrients. Salinity affects the symbiotic events, colonization and infection of root hairs by bacterial strains [4- 6]. Subsequently, the number of bacteria colonizing roots, root nodulation reduces thus rate of nitrogen fixation abridged, which finally result in poor plant growth in salt-affected soil ) [7]. At later stages of plant growth, soil salinity interferes with root turgor that led to reduction in water absorpion consequently reduces plant water column that progresses through dehydration, wilting and osmotic stress, inhibition of metabolic processes, alteration in the transpiration system, and most importantly interference in traits attributing to ph) [8]. Photosynthesis attributes in dry matter accumulation and as such in plant productivity showing a decrease under saline condition owing to the reduction in leaf turgor and reduced leaf surface area [9,10]. It occur either through stomatal closer and decreased CO2 uptake which is associated with the reduction in stomatal conductance or less-efficient Calvin cycle due to limited chlorophyll content [11,12]. Stunted growth (seedling) with reduced biomass and leaf area are observed effects of salinity in the growth (vegetative stage) of plants [13]. Plants are inherently equipped with tolerance mechanism for salt stress mitigation. Plant response to salinity stress by hormonal stimulation, ion exchange, synthesis of antioxidant enzymes and activation of signaling cascades on their metabolic and genetic frontiers that sooth the stressed condition. Additional to the plant inherent mechanisms, Plant Growth Promoting Rhizobacteria (PGPR) also have specialized mechanism that play key role in salt stress tolerance and plant growth promotion. The effectiveness of PGPRs is variable under different biotic and abiotic conditions. Abiotic factors may negatively affect the beneficial properties and efficiency of the introduced PGPR inoculants. It has been observed that the pre-treatment of seeds with different PGPR promotes seed germination and seedling growth [14-16]. As part of the mechanism, it is assumed that PGPR helps in maintaining the hormonal balance e.g., auxin to cytokinin levels during germination and initial plant development, thereby playing a critical role in dictating the genetic program that controls post-embryonic roots and shoot growth [17,18]. These bacteria also produce growth regulators like siderophore, helps in nitrogen fixation, solubilization of organic and inorganic phosphate. PGPR encouraging plant growth prominently by enhancing nutrient availability and securing mineral assets such as phosphorus, phytohormones production, production of volatile compounds in controlling seed and soil-borne phytopathogen, and synergism with other plant-beneficial microorganisms in enhancing resistance against various stresses [19-23].
Barnyard millet (Echinochloa sp.) is one of the oldest domesticated millets of semi-arid tropics of Asia and Africa. The genus Echinochloa includes about 20 species that are distributed throughout the warmer parts of the world. The two main species E. crus-galli and E. frumentacea are grown as cereals. In addition to these two species genus Echinochloa includes about 30 annual and biennial wild species distributed worldwide [24]. These millet species have distinctive morphological variations. Indian barnyard millet (E. frumentacea) can easily be distinguished from Japanese barnyard millet (E. crus-galli) by its panicle, thinner texture of the glumes and lower lemma [25]. It is the fastest growing crop among all millets and can be harvested in a short period of time in about nine weeks. It is valued for its drought tolerance and superior nutritional value. Its grains contain 6.2 % protein, 9.8 % crude fiber, 65.5 % carbohydrates and are consumed just like rice.
Materials and Methods
The Experiment was conducted in pot culture during kharif season at the Department of Seed Science and Technology, HNB Garhwal University, Srinagar, Uttarakhand. The seeds of Barnyard millet were collected from Local farmers of Chauras village. The PGPR Bacillus amyloliquefaciens (BS-56 and Bs-10) and Pseudomonas fluorescens(S-90) bacterial strains (chalk powder) were procured from Microbiology laboratory, Department of Basic Science, College of Forestry (VCSG UUHF, Bharsar) Ranichauri, Tehri Garhwal.
Seed treatment
Barnyard millet seeds were surface disinfected by immersing in 1% Sodium hypochlorite (NaOCl) for 5 min, and then washed three times in distilled water. Seeds were inoculated (Bacterization) with talk formulation, (@10g kg-1seeds) to make the mixture and kept for 24 h at room temperature. The trial was conducted in triplicate in a completely randomized design each containing 25 seeds of Barnyard millet were prepared for each extract concentration using Pot culture and salinity stress were provided by adding 10 ml of the 5% of NaCl solution. Pots are filled by Sand, FYM and soil, Seeds were evenly distributed on the Pot and 10 ml of NaCl solution was added to each pot. The seeds used as controls were treated with only distilled water of same amount. Moisture in the pot was maintained by adding 5 ml of NaCl solution or distilled water every 2 days.
Biochemical analysis of plants
1. Chlorophyll content: Chlorophyll was estimated by method given by Khan et al. 100 mg of fresh leaves were homogenized in 5 ml of 80% acetone and incubated for 5 min at 90ºC in the water bath. The homogenate was centrifuged at 3000 rpm for 10 min. The absorbance of supernatant was recorded for chlorophyll content at 663 and 645 nm against 80% acetone as blank [27].
Chlorophyll a=(12.7 × A_663 )-(2.69 × A_645)
Chlorophyll b=(22.9 × A_645 )-(4.68 × A_663)
Total Chlorophyll=(12.7 × A_663 )+(22.9 × A_645)
Protein content: The protein content was determined by using the method of Khan et al. 100 mg of fresh leaves were homogenized in 1 ml of phosphate buffer and centrifuged for 10 min at 3000 rpm. The supernatant (0.1 ml) was poured in a test tube and total volume made upto 1 ml by distilled water. Reagent C (1 ml) was added followed by shaking for 10 minutes following addition of 0.1 ml of reagent D. The absorbance of each sample was recorded at 650 nm after 30 minutes of incubation. The concentration of protein was determined by the following formula:
Protein (mg/g)=K Value × Dillution Factor×Absorbance/(Weight of Sample)
Antioxidant enzymatic activities
Enzymatic activities were estimated by taking about 500 mg of frozen shoots and homogenize in extraction buffer (1:6 w/v) containing 50 mM potassium phosphate buffer (pH 7.5), 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 mM polyethylene glycol (PEG), 5% (w/v) Polyvinylpolypyrrolidone (PVP) and 0.1% (v/v) Triton X-100. The homogenate was then centrifuged at 10000 rpm at 4º C for 30 min, and the supernatant recovered and stored at -8ºC.
Catalase (CAT; EC 1.11.1.6) activity: Catalase (CAT; EC 1.11.1.6) activity was estimated by measuring the rate of decomposition of H2O2 at 240 nm for 3 min as described by Aebi [28]. The reaction mixture containing 200 μl of extract and 2 mL of 10 mM H2O2 prepared in potassium phosphate buffer (100 mM, pH 7). The reaction was initiated by the addition of the extract. CAT activity was expressed in mmol H2O2 min-1 g-1 FW using the molar absorption coefficient of 39.6 mM-1 cm-1.
Polyphenoloxidase (PPO) (EC 1.14.18.1) activity: The Polyphenoloxidase (PPO) (EC 1.14.18.1) activity was determined by the method described by Hori et al. The reaction mixture containing 500 μl of 1.6% (v/v) catechol prepared in potassium phosphate buffer (100 mM, pH 6) and incubated for 3 min at room temperature after the addition of 100 μl of extract. The optical density was measured at 410 nm. PPO activity was expressed as the amount of enzyme used to increase one unit of absorbance (475 nm) min-1 g-1 FW.
Peroxidase (POD; EC 1.11.1.7) activity: The Peroxidase (POD; EC 1.11.1.7) activity was measured using guaiacol as a hydrogen donor [29]. In a cuvette take 100 μl of the enzyme extract 300 μl of 20 mM guaiacol and 2 mL of potassium phosphate buffer (100 mM, pH 6). To initiate reaction add 200 μl of 0.3% (v/v) hydrogen peroxide. The formation of tetra-guaiacol was determined spectrophotometrically at 470 nm. POD activity was calculated using the extinction coefficient of 26.6 mM-1 cm-1 and expressed as mmol tetra-guaiacol min-1 g-1 FW.
Statistical analysis
The data recorded from was subjected to statistical analysis through method suggested by Cochran and Cox et al. The data was analyzed through OPSTAT online analysis software developed by CCS Hisar Agriculture University, Haryana. Principal Component Analysis (PCA) was carried out to determine the statistical correlation among different treatments for trait under study using XLSTAT 2020 version 22.5.1040.0 add-in software in Microsoft Excel 2007.
Results
Seed germination and seedlings growth
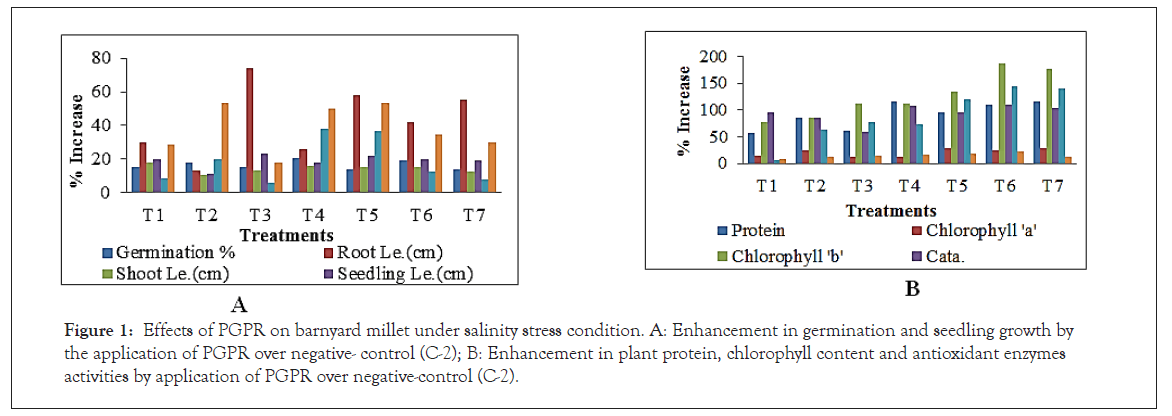
From the observations it is evident that NaCl (5%) induced salinity had an adverse effect on seed germination and seedling growth in Barnyard millet. The bacterial isolates further enhance the seed germination and growth attributing traits (Table 1). The effect was seen in negative control(C-2) with 5% inhibition of germination than in control (C-1). Results showed that the seed treatment with pseudomonas and Bacillus strains reduced salinity stress (Nacl 5%) and significantly enhanced the different growth parameters (Table 2). The germination % was significantly increased 20.89% in T-4, root length 73.77% increase in T-3, shoot length 18.17% increase in T-1, seedling length 23.03 % increase in T-3, seedling fresh weight increased 38.09% in T-4, seedling dry weight increased 53.43% in T-5, vigour –I increased 42.80% in T-6 and vigour-II increased 81.23% in T-2 as compared to negative control negative control (Figure 1a and b).
Figure 1: Effects of PGPR on barnyard millet under salinity stress condition. A: Enhancement in germination and seedling growth by the application of PGPR over negative- control (C-2); B: Enhancement in plant protein, chlorophyll content and antioxidant enzymes activities by application of PGPR over negative-control (C-2).
| Control (C1) | Soil and seeds without any treatment |
|---|---|
| Negative control(C2) | Soil +Nacl 5% and seeds received no treatment |
| T1 | Soil+ Nacl 5% and seed treated with BS-56@10g/kg Seeds. |
| T2 | Soil+ Nacl 5% and seed treated with BS-10@10g/kg Seeds. |
| T3 | Soil+Nacl 5% and seed treated with S-90@10g/kg Seeds. |
| T4 | Soil+ Nacl 5% and seed treated with BS-56+BS-10@10g/kg Seeds(5 g each) |
| T5 | Soil+Nacl 5% and seed treated with BS-56+S-90@10g/kg Seeds(5 g each) |
| T6 | Soil+ Nacl 5% and seed treated with BS-10+S-90@10g/kg Seeds(5 g each) |
| T7 | Soil+Nacl 5% and seed treated with BS-56 +BS-10+S-90@10g/kg Seeds(3.33 g each) |
Table1: Detail of treatments used in pot experiment.
| Treatment | Germination % | Root length (cm) | Shoot length (cm) | Seedling length (cm) | Seedling fresh weight (g) | Seedling dry weight.(g) | Vigour- I | Vigour-II |
|---|---|---|---|---|---|---|---|---|
| C-1 | 85 | 11.28 | 39.02 | 50.3 | 18.24 | 3.56 | 4276.18 | 302.87 |
| C-2 | 71.33 | 6.66 | 34.73 | 41.39 | 14.25 | 2.39 | 2952.38 | 170.48 |
| T1 | 82 | 8.65 | 41.04 | 49.69 | 15.52 | 3.08 | 4074.25 | 252.57 |
| T2 | 84.34 | 7.56 | 38.38 | 45.93 | 17.05 | 3.66 | 3874.33 | 308.96 |
| T3 | 82 | 11.57 | 39.36 | 50.93 | 15.07 | 2.82 | 4176.6 | 231.23 |
| T4 | 86.23 | 8.39 | 40.36 | 48.76 | 19.67 | 3.58 | 4204.29 | 308.7 |
| T5 | 81.34 | 10.52 | 40.06 | 50.58 | 19.51 | 3.67 | 4114.52 | 298.24 |
| T6 | 85.13 | 9.46 | 40.06 | 49.52 | 16.07 | 3.22 | 4215.99 | 274.11 |
| T7 | 81.23 | 10.36 | 39.13 | 49.5 | 15.34 | 3.11 | 4020.94 | 252.9 |
| C.D. (at 5%) | 1.659 | 0.889 | 0.052 | 0.894 | 0.027 | 0.025 | 136.841 | 4.865 |
| SE(m) | 0.549 | 0.294 | 0.017 | 0.296 | 0.009 | 0.008 | 45.254 | 1.609 |
| SE(d) | 0.776 | 0.416 | 0.024 | 0.418 | 0.013 | 0.012 | 63.999 | 2.275 |
| C.V. | 1.158 | 5.425 | 0.075 | 1.056 | 0.094 | 0.447 | 1.965 | 1.045 |
Table 2: Effect of PGPR on Germination and seedling growth of barnyard millet at salinity stress (NaCl) compared to un-inoculated (absolutely control).
Biochemical parameters
Protein content:Analysis of protein content showed that NaCl salt's presence causes a reduction in the protein contents in Barnyard millet. While on bacterial inoculation, they were considerably higher compared to stress control, which led to the improvement of plant growth and biomass production. The maximum (7 mg/g FW) protein content was observed in T-4 and minimum (3.25 mg/g FW) in C-2 (Table 3).
Photosynthetic pigments (chlorophyll ‘a’ and ‘b’):When the plants are grown with PGP bacteria under saline conditions, the plants have the highest chlorophylls, carotenoid contents, and healthy leaves compared to NaCl induced plants. The maximum (8.46 mg g-1FW) chlorophyll ‘a’ was observed in T-5 and chlorophyll ‘b’ (2.87 mg g-1FW) in T-6 over C-2 (Table 3).
Antioxidants enzymes: To overcome the deleterious effect of ROS, the plant cells have developed the antioxidants machinery. The antioxidants enzymatic activities were evaluated in un- inoculated and inoculated plants under salinity stress treatment. The antioxidants enzymatic activities of CAT, POX, and PPO in barnyard millet plants were increased as compared to salt inoculated plants than PGPR inoculated. Application of NaCl (salt) stress leads to induction of antioxidant enzymes irrespective of PGP bacterial inoculation. However, antioxidant activity was higher when treated with PGPR isolates than in un-inoculated plants and salinity stressed plants. The highest Catalase (4.14 min-1g-1 fresh weight), Peroxidase (433.67 min-1g-1 fresh weight) and Polyphenoloxidase (22.58 min-1g-1 fresh weight) activities were observed in T-6 over C-2 (Table 3).
| Treatment | Protein (mg/g FW) |
Chlorophyll 'a' (mg/g FW) |
Chlorophyll 'b' (mg/g FW) |
Catalase (min-1 g-1 FW) |
Peroxidase (min-1 g-1 FW) |
PPO (min-1 g-1 FW) |
|---|---|---|---|---|---|---|
| C-1 | 4.21 | 8.03 | 1.13 | 3.22 | 198.11 | 19.13 |
| C-2 | 3.24 | 6.56 | 1 | 1.98 | 177.13 | 18.28 |
| T1 | 5.11 | 7.56 | 1.77 | 3.87 | 189.6 | 19.67 |
| T2 | 6.01 | 8.25 | 1.85 | 3.67 | 288.35 | 20.66 |
| T3 | 5.23 | 7.44 | 2.12 | 3.15 | 313.24 | 21.01 |
| T4 | 7 | 7.45 | 2.12 | 4.11 | 308.07 | 21.49 |
| T5 | 6.35 | 8.46 | 2.35 | 3.88 | 388.46 | 21.82 |
| T6 | 6.78 | 8.14 | 2.87 | 4.14 | 433.67 | 22.58 |
| T7 | 6.97 | 8.43 | 2.77 | 4.03 | 424.32 | 20.69 |
| C.D (at 5%) | 0.019 | 0.024 | 0.033 | 0.012 | 1.813 | 0.977 |
| SE(m) | 0.006 | 0.008 | 0.011 | 0.004 | 0.599 | 0.323 |
| SE(d) | 0.009 | 0.011 | 0.015 | 0.006 | 0.848 | 0.457 |
| C.V. | 0.191 | 0.177 | 0.944 | 0.198 | 0.343 | 2.719 |
Table 3: Effect of PGPRs on biochemical parameters and antioxidant activity of barnyard millet under salinity stress (NaCl). Values are the mean of three replicates, significant difference (P<0.05).
Discussion
Crops growth and productivity is highly influenced by the salinization of water and soil. Soil salinity is a critical worldwide hurdle in crop production, so it is imperative to found solutions for plants to have the ability to grow in these salinized areas [30]. In present investigation, the inoculation of Bacillus and Pseudomonas was found to increase the seedling morphological and biochemical parameters under NaCl induced stress conditions. These bacterial isolates promote plant growth which might be due to their ability to produce IAA, P-solubilization, ammonia, and siderophores. Similar plant growth promoting activities were also reported by [31]. Salinity has an inhibitory effect on seed germination due to osmotic/ionic effects [32]. resulting in the disturbed water uptake. The unfavorable effect of NaCl on seed germination and its growth parameters has also been committed by many other scientists [33,34]. In present study, salinity has a considerable effect on germination percent, seedling vigor indexes, fresh and dry biomass, root and shoot lengths etc. similar results were observed. This work may have actual consequences in the agriculture sector as these factors are responsible for determining plant productivity [35]. It has also been reported by that salinity has more adverse effects on shoot compared to roots. Many research also validated that PGPR inoculation on pepper seeds exhibited higher morphological parameters including plant height, greater root length, larger leaf size, and an increase in dry matter in saline soils [36]. PGPR have the enormous capability to reduce salt stress and improve plant development, playing a significant role in ensuring food security by boosting crop productivity. Use of PGPR under salinity stress enhances plant growth by increasing ACC deaminase activity, synthesis of plant hormones (IAA, GA, ABA, cytokinin, and exopolysaccharides) or by lowering plant ethylene levels enzymatically [37]. Synthesis of phyto-hormones stimulates plant growth by enhancing nutrient uptake [38]. It has been suggested that auxins synthesis by root associated microbes is one of the most important mechanisms through which microbes regulate plant growth [39]. Soil salinity adversely affects the microbial process, diminishing bacterial diversity and controlling microbial wealth, composition, and functions [40]. Microbial inoculation with PGPR Bacillus and Pseudomonas and co-inoculation can mitigate these negative effects of salinity. Plant growth-promoting rhizobacteria enhances plant tolerance toward various abiotic stresses including salinity. Our study demonstrated enhancements in plant growth traits under salinity-stressed in response to inoculation with Bacillus and pseudomonas. Similar improvements in plant growth due to inoculation with halotolerant plant growth promoting bacterium Alcaligenes faecalis were evidenced in the study of stated that vegetative growth of salinity-stressed rice and wheat plants was increased significantly linked the augmentation of plant growth with the ability of PGPR to produce plant growth regulators, phosphate solubilization, and nitrogen fixation [41-46]. These features are found in the selected isolates and increase nutrients uptake efficiency and improve its growth, especially under salinity- stress conditions. Photosynthetic pigments are a fundamental physiological trait directly associated with photosynthesis efficiency under abiotic stresses. In present investigation chlorophyll content was observed to increase significantly under salinity stress. These increases were due to the soil supplementation with the tested PGPR. An enhancement in photosynthetic pigments in PGPR-inoculatedz plants under different saline conditions was also observed in previous studies [47-49]. The augmentation in photosynthetic pigments in PGPR-inoculated plants suggests the potency of bacterial inoculation to nullify the harmful impacts of salinity stress by improving the activities of electron transporters associated with photosynthesis as well as the biosynthesis of proteins and enzymes that related to pigment stabilization [50,51]. The current study clarified that protein content in salinity-stressed Barnyard millet plants was increased due to the inoculation with PGPR. Various studies on crop plants have well documented the positive impacts of rhizobacterial inoculation on increasing the protein content [52]. A possible strategy behind this increase might be that bacterial inoculation inhibits the activity of protein-hydrolyzing enzymes in addition to the ability of bacteria in promoting the efficiency of proline in protecting soluble proteins and thus increasing their amounts under the salt-stress conditions [53-54]. To mitigate the oxidative stress induced by salinity stress, the plants developed a group of physiological and biochemical strategies made of various enzymes that can scavenge the ROS species. Antioxidant enzymes act in a network to achieve the detoxification of ROS species [55-57]. In our study, we noticed different increases in Catalase, Polyphenoloxidase (PPO), and Peroxidase (POD) activities in the inoculated barnyard millet plants with the mentioned PGPR under saline conditions. Our findings comply with the reports of [58] on mung bean. This positive role may be attributed to the finding that PGPR facilitate the entity of essential elements in the soil to be easily absorbed by the plant or due to roots’ exudates initiated by PGPR, increasing the availability of some micronutrients [59,60].
Principle component analysis
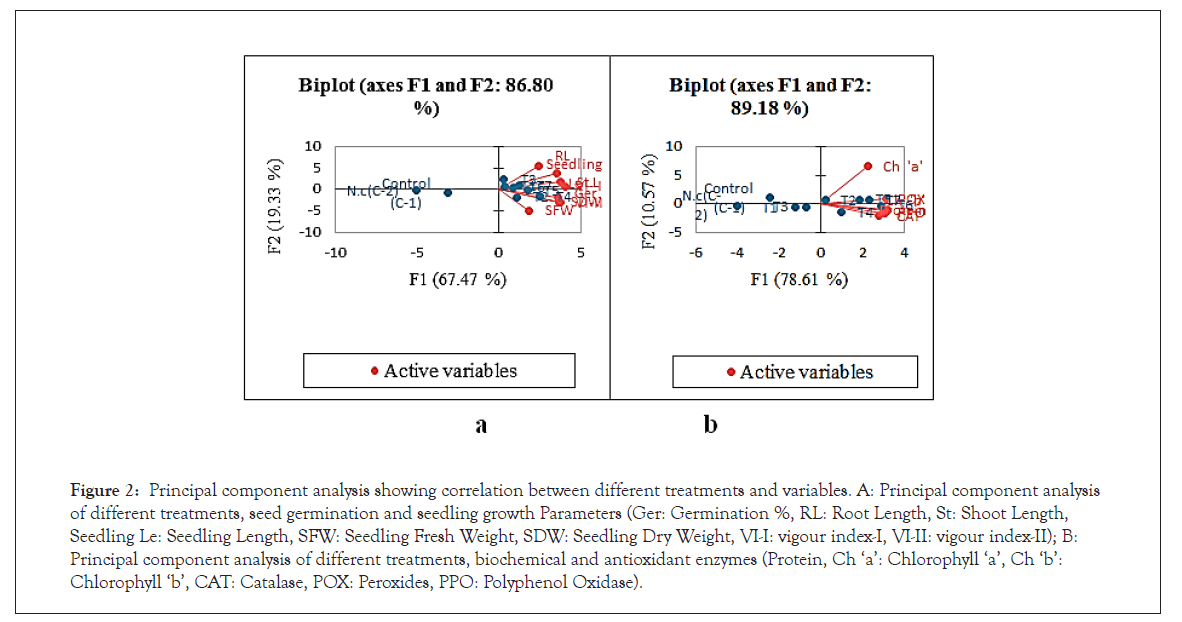
The principle component analysis was carried out to evaluate the statistical correlation between different treatments and their effect on germination, growth parameters and biochemical, and antioxidant enzymes (Figure 2a). The principle component analysis for different treatments, seed germination and growth (Figure 2b) is explained with component 1(F1: 67.47 %) and component 2 (F2: 19.33 %). The statistical correlation between the treatments, biochemical and antioxidant enzymes in plant leaves was also evaluated (Figure 2b) and is explained with component 1 (F1: 78.61 %) and component 2 (F2: 10.57 %).
Figure 2: Principal component analysis showing correlation between different treatments and variables. A: Principal component analysis of different treatments, seed germination and seedling growth Parameters (Ger: Germination %, RL: Root Length, St: Shoot Length, Seedling Le: Seedling Length, SFW: Seedling Fresh Weight, SDW: Seedling Dry Weight, VI-I: vigour index-I, VI-II: vigour index-II); B: Principal component analysis of different treatments, biochemical and antioxidant enzymes (Protein, Ch â??aâ??: Chlorophyll â??aâ??, Ch â??bâ??: Chlorophyll â??bâ??, CAT: Catalase, POX: Peroxides, PPO: Polyphenol Oxidase).
Results suggest that application of biofertilizers significantly affected the seed germination seedling growth, biochemical and antioxidant enzymes in plants under salinity stress. The results also revealed that application of PGPR alone was less effective than combined effect of two PGPR. Among the different treatments T3 to T4, T5 and T6 showed positive effect on seed germination and growth parameters. Among these, T4 and T3 were most effective to enhance the germination and growth parameters (Figure 2a), however, T6 was most effective to increase biochemical and antioxidant enzymes activity (Figure 2b). The PCA analysis also revealed that seedling fresh weight was independent and negatively correlated with root length. It also suggests that increase in seedling fresh weight not dependent on root length (Figure 2a). However, seedling dry weight and vigour index-II were closely related and dependent to each other. It also suggests that increase in seedling vigour index-II dependent on dry weight of seedling (Figure 2a). Similarly, among the biochemical parameters, ‘chlorophyll a’ was independent and did not affect the other parameters. In addition to the above, the antioxidant enzymes were positively correlated to chlorophyll and protein content. Among these, CAT and PPO were found most closely associated to chlorophyll ‘b’ and protein content followed by POX. This clearly indicates that higher activity of antioxidant enzymes can directly influence the chlorophyll and protein content in barnyard millet under salinity stress condition [61].
Conclusion
From the outcome of the obtained results, it seems likely to conclude that using of Bacillus and Bacillus amyloliquefaciens and Pseudomonas fluorescens brought about enhancements in different growth indices of barnyard millet plants grown in saline soil. The co-inoculation with both bacterial isolates brought about significant improvements in germination and most seedling growth parameters, photosynthetic pigments and protein contents. Additionally, Catalase, Peroxidase and Polyphenoloxidase activities were promoted as a reason for the single inoculation and co-inoculation with the mentioned isolates, thus boosting the tolerance of plants to cope with salinity stress. We suggest using the co-inoculation with Bacillus amyloliquefaciens and Pseudomonas fluorescens as an effective and important approach for ameliorating salinity stress. PGPR can be used as a cost effective and economical tool for salinity tolerance and growth promotion in plants. Under salt stress bacterial strains has reported to significantly increase the root and shoot length and total fresh weight of the plants. Soil salinity is considered as one of the most serious environmental problems in arid and semi-arid regions that cause economic losses in agriculture.
REFERENCES
- Pitman MG, Läuchli A. Global impact of salinity and agricultural ecosystems. InSalinity: environment-plants-molecules. Springer, Dordrecht.2002; 3-20.
- Rengasamy P. Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Aust J Exp Agric. 2002;42(3):351-361.
- Zolla G, Heimer YM, Barak S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot. 2010;61(1):211-224.
- Räsänen LA, Saijets S, Jokinen K, Lindström K. Evaluation of the roles of two compatible solutes, glycine betaine and trehalose, for the Acacia senegal–Sinorhizobium symbiosis exposed to drought stress. Plant Soil. 2004;260(1):237-251.
- Egamberdieva D, Li LI, Lindström K, Räsänen LA. A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl Microbiol Biotechnol.. 2016;100(6):2829-2841.
- Karmakar K, Rana A, Rajwar A, Sahgal M, Johri BN. Legume-rhizobia symbiosis under stress. InPlant microbes symbiosis: applied facets. Springer, New Delhi. 2015;241-258.
- Latrach L, Farissi M, Mouradi M, Makoudi B, Bouizgaren A, Ghoulam C. Growth and nodulation of alfalfa-rhizobia symbiosis under salinity: electrolyte leakage, stomatal conductance, and chlorophyll fluorescence. Turk J Agric. 2014;38(3):320-326.
- Kaushal M, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Annals of Microbiology. 2016;66(1):35-42.
- Qin J, Dong WY, He KN, Yu Y, Tan GD, Han L, et al. NaCl salinity-induced changes in water status, ion contents and photosynthetic properties of Shepherdia argentea (Pursh) Nutt. seedlings. Plant Soil Environ. 2010;56(7):325-332.
- Tanveer M, Shah AN. An insight into salt stress tolerance mechanisms of Chenopodium album. Environ Sci Pollut Res. 2017;24(19):16531-16535.
- Lycoskoufis IH, Savvas D, Mavrogianopoulos G. Growth, gas exchange, and nutrient status in pepper (Capsicum annuum L.) grown in recirculating nutrient solution as affected by salinity imposed to half of the root system. Sci. Hortic. 2005;106(2):147-161.
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103(4):551-560.
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1-4.
- Poupin MJ, Timmermann T, Vega A, Zuñiga A, González B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS One. 2013;8(7):e69435.
- Rahmoune B, Morsli A, Khelifi-Slaoui M, Khelifi L, Strueh A, Erban A, et al. Isolation and characterization of three new PGPR and their effects on the growth of Arabidopsis and Datura plants. J. Plant Interact.. 2017;12(1):1-6.
- Bakhshandeh E, Gholamhosseini M, Yaghoubian Y, Pirdashti H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 2020;90(1):123-136.
- Chu TN, Tran BT, Hoang MT. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes. 2019;12(1):1-7.
- Qessaoui R, Bouharroud R, Furze JN, El Aalaoui M, Akroud H, Amarraque A, et al. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 2019;9(1):1-0.
- Bhattacharyya D, Yu SM, Lee YH. Volatile compounds from Alcaligenes faecalis JBCS1294 confer salt tolerance in Arabidopsis thaliana through the auxin and gibberellin pathways and differential modulation of gene expression in root and shoot tissues. Plant Growth Regul. 2015;75(1):297-306.
- Bell CW, Asao S, Calderon F, Wolk B, Wallenstein MD. Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol. Biochem. 2015;85:170-182.
- Kurepin LV, Park JM, Lazarovits G, Bernards MA. Burkholderia phytofirmans-induced shoot and root growth promotion is associated with endogenous changes in plant growth hormone levels. Plant Growth Regul. 2015;75(1):199-207.
- Bach E, dos Santos Seger GD, de Carvalho Fernandes G, Lisboa BB, Passaglia LM. Evaluation of biological control and rhizosphere competence of plant growth promoting bacteria. Appl Soil Ecol. 2016 Mar 1;99:141-149.
- Yuan S, Li M, Fang Z, Liu Y, Shi W, Pan B, et al. Biological control of tobacco bacterial wilt using Trichoderma harzianum amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol Control. 2016;92:164-171.
- Clayton WD, Renvoize SA. Genera graminum. Grasses of the world. Genera graminum. Grasses of the World.. 1986;13.
- Yabuno T. A note on barnyard millet. SABRAO Newsletter. 1971;3(1):43-5.
- Ruiz-Santaella JP, Bastida F, Franco AR, De Prado R. Morphological and molecular characterization of different Echinochloa spp. and Oryza sativa populations. J Agric Food Chem. 2006;54(4):1166-1172.
- Khan N, Zandi P, Ali S, Mehmood A, Adnan Shahid M, Yang J. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front Microbiol. 2018;9:2507.
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121-6.
- Hori K, Wada A, Shibuta T. Changes in phenoloxidase activities of the galls on leaves of Ulmus davidana formed by Tetraneura fuslformis (Homoptera: eriosomatidae). Appl Entomol Zool. 1997;32(2):365-71.
- Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19(6):371-379.
- Qin S, Feng WW, Wang TT, Ding P, Xing K, Jiang JH. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil. 2017;416(1):117-132
- Nasri N, Saïdi I, Kaddour R, Lachaâl M. Effect of salinity on germination, seedling growth and acid phosphatase activity in lettuce. Am J Plant Sci. 2015;6(01):57.
- Bybordi A. The influence of salt stress on seed germination, growth and yield of canola cultivars. Not Bot Horti Agrobot Cluj Napoca. 2010;38(1):128-33.
- Ghorbannejad H, Amooaghaie R. Differential changes of proline content and activities of antioxidant enzymes results in varied salt-tolerance in canola genotypes. Plant Genet. Resour. 2017;3(1):36-46.
- Shahrajabian MH, Khoshkharam M, Wenlis, Cheng Qİ. The effects of pretreatment factors on seed germination and seedling growth of anise (Pimpinella anisum L.). Middle East J Sci Res. 2019;5(1):86-93.
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25(2):239-250.
- Bhise KK, Dandge PB. Mitigation of salinity stress in plants using plant growth promoting bacteria. Symbiosis. 2019;79(3):191-204.
- Kohler J, Caravaca F, Carrasco L, Roldan A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions. Soil Use Manag. 2006;22(3):298-304.
- Foo E, Plett JM, Lopez-Raez JA, Reid D. The Role of plant hormones in plant-microbe symbioses. Front. Plant Sci. 2019;10:1391.
- Omer AM. Inducing plant resistance against salinity using some rhizobacteria. Egypt J Desert Res. 2017;67(1):187-208.
- Fatima T, Mishra I, Verma R, Arora NK. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech. 2020;10(8):1-2002E
- Shukla PS, Agarwal PK, Jha B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012;31(2):195-206.
- Ullah S, Bano A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015;61(4):307-13.
- Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016;47:621-627.
- Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Plant Sci. 2015;6:198.
- Etesami H, Glick BR. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ Exp Bot. 2020;178:104124.
- Yildirim E, Turan M, Ekinci M, Dursun A, Cakmakci R. Plant growth promoting rhizobacteria ameliorate deleterious effect of salt stress on lettuce. Sci Res Essays. 2011;6(20):4389-96.
- Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact.. 2014;9(1):673-82.
- Aslam F, Ali B. Halotolerant bacterial diversity associated with Suaeda fruticosa (L.) forssk. improved growth of maize under salinity stress. Agronomy. 2018;8(8):131.
- Pinnola A, Staleva-Musto H, Capaldi S, Ballottari M, Bassi R, Polívka T. Electron transfer between carotenoid and chlorophyll contributes to quenching i006E the LHCSR1 protein from Physcomitrella patens. Biochim Biophys Acta Bioenerg BBA-BIOENERGETICS. 2016;1857(12):1870-1878.
- Enebe MC, Babalola OO. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl. Microbiol. Biotechnol. 2018;102(18):7821-35.
- Egamberdieva D, Lugtenberg B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. InUse of Microbes for the Alleviation of Soil Stresses. Springer. 2014; 1(4):73-96.
- Hamdia MA, Shaddad MA, Doaa MM. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004;44(2):165-174.
- Beneduzi A, Ambrosini A, Passaglia LM. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol. 2012;35:1044-1051.
- Ahammed GJ, Li Y, Li X, Han WY, Chen S. Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J Plant Growth Regul. 2018;37(4):1349-1356.
- Abbas T, Pervez MA, Ayyub CM, Ahmad R. Assessment of morphological, antioxidant, biochemical and ionic responses of salttolerant and salt-sensitive okra (Abelmoschus esculentus) under saline regime. Pak J Life Soc Sci. 2013;11(2):147-153.
- AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016;7:276.
- Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016;80(1):23-36.
- Etesami H, Mirsyed Hosseini H, Alikhani HA. In planta selection of plant growth promoting endophytic bacteria for rice (Oryza sativa L.). J. Plant Nutr Soil Sci. 2014;14(2):491-503
- Kumar A, Singh S, Gaurav AK, Srivastava S, Verma JP. Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020;11:1216.
- Jaiswal DK, Verma JP, Prakash S, Meena VS, Meena RS. Potassium as an important plant nutrient in sustainable agriculture: a state of the art. Potassium solubilizing microorganisms for sustainable agriculture. Springer. 2016:21-29.
Citation: Singh R, Tariyal YS, Chauhan JS (2021) Effect of Plant Growth Promoting Rhizobacteria (Pgpr) on Salt Stress Tolerance of Barnyard Millet (Echinochloa frumentacea). Biochem Anal Biochem. 10:403.
Copyright: © 2021 Singh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.