Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 11, Issue 2
Effect of Capsicum annuum L. and Capsicum frutescens L. Varieties Extracts on in vitro Growth of Fungal Strains
Koffi Affoué Carole1*, Kouassi Kouassi Clément2, Kossonou Yao Kamelé1, Koffi Ahua René3 and Koffi-nevry Rose42Department of Biochemistry and Microbiology, Laboratory of Agrovalorization, Jean Lorougnon Guede University, Ivory Coast
3Laboratory of Food Biotechnology and Microbiology, Ecology Research Centre, Ivory Coast
4Department of Food Science and Technology, Laboratory of Food Biotechnology and Microbiology, University of Nangui Abrogoua, Abidjan, Ivory Coast
Received: 13-Dec-2018 Published: 15-Apr-2019
Abstract
This study aims to test different extracts (water, 70% ethanolic-water and acetal) of Capsicum sp. on the growth of Alternaria sp., Penicillium sp., Fusarium sp. and Aspergillus flavus. The nature of the antifungal activity, the antifungal parameters (MIC, MFC, IC50) and the percentage of survival of the strains were determined with the dilution method.
On Alternaria sp., Penicillium sp. and Fusarium sp. strains, values of MFC/MIC ratios of water and 70% ethanol extracts of Capsicum annuum antillais were equal to two. Concerning Capsicum frutescens (soudanais, attié, doux and oiseau) varieties, these values varied from 1 to 2 on the fungal strains.
These ratios for most of the strains were lower than 4. These extracts exerted a fungistatic action on chosen strains. The curves obtained had a declining appearance with more or less steep slopes depending on the extracts. These curved were used to obtain the inhibitory concentrations for 50% survival (IC50) of the moulds. The highest activity was achieved at the lowest IC50 values which varied from 0.2 mg/mL to 0.3 mg/mL. Therefore, Capsicum extracts proved to be active on in vitro growth of the fungal strains studied in a dose-dependent manner.
Introduction
Medicinal plants have been used for centuries for various purposes by civilisations [1]. Plants are used as food, energy, clothing, and housing constructions. Sometimes, they are used to cure diseases [2]. According to these some authors, “soft” medicines, particularly herbal medicine, are gaining considerable interest in many parts of Africa, Asia and Europe. Worldwide, there are serious and lifethreatening diseases caused by microorganisms such as bacteria, viruses, fungi and parasites [3]. For a very long time, humans have been relatively disarmed by the diseases caused by fungi. Over the last twenty years, a large increase in this diseases in which affect the wide range of hosts has been observed. These diseases are caused by surprisingly high number of fungal species [4]. They cause serious damage to humans as well as to plant species [5]. Food industries are also facing problems such as appearance changing, organoleptic and chemical qualities spoilage, non-stable altered substances produced, that food can appear during conservation [6]. The need for new prevention strategies against fungal infections and diseases is a major interest, not only for food safety, but also for consumer health, and moreover for the protection of countries’ economy. Thus, at the therapeutic level, there are several antifungals. However, some of these molecules sold on the market and therapeutically used have lost their effectiveness due to antifungal resistance and are, out of reach for African populations with low incomes due to their high cost [7]. Therefore, these populations are increasingly using medicinal plants their health issues. According to the annual report of the World Health Organization (WHO), about 80% of the African population use plants for treatment of ailments [8,9]. However, the overuse of these plants exposes them to various accidents because; traditional medicines may contain other molecules having toxic effects. In Africa, pepper is always cited among the therapeutic arsenal [10]. Especially, Capsicum fruits are used in traditional medicine for their antimicrobial and antifungal properties as condiment [11,12]. As a result, the use of capsicum extracts in the fight against mould from food causes the choice of our subject.
Materials and Methods
Plant materials
Plant materials consisted of fruits of Capsicum annuum variety antillais, Capsicum frutescens variety doux, Capsicum frutescens variety attié, Capsicum frutescens variety soudanais, Capsicum frutescens variety oiseau and Capsicum frutescens variety pendulum. Six varieties from fruits coming from Capsicum annuum, Capsicum frutescens and Capsicum chinense were chosen. These varieties are fresh, mature and firm fruits of Capsicum annuum var. antillais and Capsicum annuum var. pendulum were used. For the other varieties (Capsicum frutescens var. doux, Capsicum frutescens var. attié, Capsicum frutescens var. soudanais, Capsicum frutescens var. oiseau and Capsicum chinense var. pendulum) based on the way they are traditionally used by local people, air dried materials were used from food were used for the tests. These different strains were sub cultured and stored in nutrient agar at 4°C. The varieties of peppers are obtained directly from five main district of Abidjan markets: The large market of Abobo, the large market of Yopougon, the large market of Adjamé, the large market of Treichville and the large market Port Bouët. Fruit varieties of Capsicum annuum variety antillais and Capsicum chinense variety pendulum are obtained fresh while those varieties of Capsicum frutescens (soudanais, attié doux and oiseau) are desiccated (dried form). These varieties have identified all summers University Floristic National Centre Félix Houphouët-Boigny (Côte d’Ivoire).
One hundred and twenty samples of peppers were collected and kept in sterile sampling bags. The fruits of Capsicum were sorted then washed under tap water and dried in an oven at 55°C for eight to ten days for fresh fruits and five days dried fruits. The dried fruits were reduced to fine powder using an electronic mixer at 3000 rpm then sieved (1 mm) to obtain the final powder (Figure 1).

Figure 1. Chilli powder packaged in Stomacher sachets.
Microorganism
Five fungal strains, namely Aspergillus niger, Fusarium sp., Alternaria sp., Aspergillus flavus and Penicillium sp. They are foodborne pathogens and they also cause epidermal diseases, respiratory allergies, vomiting, and sinusitis [13]. In fact, all these strains were provided by the Institute Pasteur in Côte d’Ivoire (IPCI).
Extract preparation
The extracts (Water, 70% water-ethanol and acetate) were prepared according to successive extraction methods developed and described by Guedé Guina et al. [14] from the chili pepper powder previously obtained.
Thus, 50 grams were extracted by homogenization in one litre (1 L) of distilled water. The mixture was filtered through Whatman paper No.2 (Whatman International, Maidstone England) and then oven evaporated at 60°C until completely dry. The powders obtained constitute the crude aqueous extract coded Etaq.
25 grams of the crude aqueous extract were dissolved in 500 mL of ethanol (70%). Solution per homogenization during 24 hours with room temperature (25-30°C). The mixture was filtered through Whatman paper No. 2 and the filtrate was concentrated in a rotavapor at 50°C. The powders obtained constitute the ethanol 70% coded Eeth 70%.
10 grams of ethanolic 70% extract are dissolved in 500 mL of a solution composed of a mixture of ethyl acetate and distilled water (v/v). The mixture was mixed for 24 hours using a magnetic stirrer. The homogenate was filtered through Whatman paper No. 2 and the filtrate was concentrated in a rotavapor at 50°C. The dried extracted product constituted the acetatic 1 coded Eace 1.
Performance calculation
The yield of extract expressed on dry weight basis of pepper powder, was calculated from the following formula: Rd (g/100 g)=(M1 × 100)/M0 where Rd: Yield; M1: weight of the extract residue obtained after solvent removal and M0: weight of the chilli powder taken.
Antifungal tests
Antifungal tests of Capsicum extracts were carried out with subcultures of each fungal species were made on Sabouraud agar. Agar well diffusion was used to determine antimicrobial activity [11,15] and antifungal parameters (MIC, MFC, IC50) were determined by tube dilution method [16].
Minimum inhibitory concentrations determination
Antifungal activities of pepper varieties were determined in sterile haemolysis tubes. The concentration ranges of the Capsicum extracts of each strain initially prepared according to the double dilution method in liquid medium, namely 6.72; 3.36; 1.68; 0.84; 0.42; 0.21 and 0.10 mg/mL were divided into seven sterile haemolysis tubes 1 mL per tube. An inoculum whose turbidity was adjusted to 108 CFU/mL in Sabouraud broth was also prepared for each fungal strain. In each of the seven haemolysis tubes containing 1 mL of each Capsicum extract, 1 mL of the previously prepared fungal inoculum was added. Two control tubes were prepared: A growth control tube containing 1 mL of sterile distilled water and 1 mL of fungal inoculum, followed by a sterility control tube containing 2 mL of Sabouraud broth. The contents of each haemolysis tube thus prepared was homogenized by a vortex mixer and then incubated at 25°C for 72 hours.
The experiments were repeated three times to ensure the reliability of the results. After distribution of the broth inoculated into the seven experimental tubes, the remaining broth was diluted to 10- 4. The dilutions obtained were subsequently seeded onto plates of Petri containing Sabouraud agar, by 5 cm streaks (box A) and incubated at 25°C for 72 hours [17].
Minimum fungicide concentration (MFC)
The MFC was determined in an agar medium after reading the MIC. To do this, the different cultures in each experimental tube were seeded by 5 cm streaks on Sabouraud agar using a calibrated loop. On each seeded agar plate (box B), there is the inoculum of the control tube of growth control, the inoculum where the turbidity was not visible, that of the tube which made it possible to determine the MIC. The different seedlings were incubated at 25°C for 72 hours. The determination of MFC was possible by comparing the number of colonies on the streaks of the dish containing the remainder of the diluted broth up to 10-4 (box A), with that of box B [18].
Number of antimicrobial effect of plant extract
The MFC/MIC ratio was calculated for each extract to determine the nature of antimicrobial effects of plant extracts. When, the MFC/MIC ratio <4, the phytochemical has a fungicidal effect. However, if the MFC/MIC ratio ≥ 4 the plant extract has a fungistatic effect [19].
Percentage survival (Cytotoxic activity)
The fungal flora tested was counted by direct counting using a colony counter. Their growth in the experimental dishes was evaluated as a percentage of survival calculated with respect to 100% survival in the control boxes. The method of calculating the percentage of survival of the seeds in the different experimental boxes is translated by the following formula.
S=(n/N) × 100.
S=survival of fungal germs (%)
n=number of colonies in the experimental petri dishes
N=number of colonies in the controls petri dishes
Results
Yield of different extracts
Table 1 surmises the extraction yield for different solvents and different C annum and C frutescens varieties. The extraction yield is lower for acetate extract of Capsicum frutescens doux (3.2%) and higher for water extract (24.8%) of C. chinense pendulum.
| Capsicum varieties | Extraction solvent (%) | ||
|---|---|---|---|
| Aq | EtOH 70% | Ace | |
| C. annuum antillais | 24.6 | 10.2 | 4.5 |
| C. frutescens soudanais | 23.7 | 9.8 | 3.6 |
| C. frutescens attié | 23.6 | 9.5 | 3.4 |
| C. frutescens doux | 23.1 | 9.1 | 3.2 |
| C. frutescens oiseau | 23.4 | 9.2 | 3.3 |
| C. chinense pendulum | 24.8 | 10.4 | 4.7 |
Table 1: Extraction yield for different extracts and different varieties of Capsicum.
Antifungal parameters
Tables 2 and 3 show antifungal activities of various extract of Capsicum varieties used in Ivorian traditional pharmacopeia. The most interesting activity on Alternaria sp. was obtained with the lowest value of MIC (0.84 mg/mL) was observed with Capsicum chinense pendulum extracts on Alternaria sp.
| Capsicum varieties | Extracts | Alternaria sp. | Penicillium sp. | Fusarium sp. | Aspergillus flavus |
|---|---|---|---|---|---|
| C. annuum var. antillais | water Ethanol 70% Acetate |
2 2 4 |
2 2 - |
2 2 - |
- - - |
| C. frutescens var. soudanais | water Ethanol 70% Acetate |
2 2 - |
- - - |
- 2 - |
- - - |
| C. frutescens var. attié | water Ethanol 70% Acetate |
1 - - |
- - - |
- - - |
- - - |
| C. frutescens var. doux | water Ethanol 70% Acetate |
2 - - |
- 2 - |
- - - |
- - - |
| C. frutescens var. oiseau | water Ethanol 70% Acetate |
2 2 - |
- - - |
- 1 - |
- - 1 |
| C. chinense var. pendulum | water Ethanol 70% Acetate |
1 1 2 |
4 2 - |
- 2 2 |
- - 2 |
Table 2: In vitro antifungal effect of Capsicum sp. extract on the growth of different strains.
| Capsicum varieties |
Extracts | Alternaria sp. | Penicillium sp. | Fusarium sp. | Aspergillus flavus |
|---|---|---|---|---|---|
| C. annuum var. antillais | water Ethanol 70% Acetate |
1.68 1.68 1.68 |
3.36 1.68 - |
3.36 3.36 - |
- - - |
| C. frutescens var. soudanais | water Ethanol 70% Acetate |
3.36 1.68 - |
- - - |
- 3.36 - |
- - - |
| C. frutescens var. attié | water Ethanol 70% Acetate |
6.72 - - |
- - - |
- - - |
- - - |
| C. frutescens var. doux | water Ethanol 70% Acetate |
3.36 - - |
- 3.36 - |
- - - |
- - |
| C. frutescens var. oiseau | water Ethanol 70% Acetate |
3.36 3.36 - |
- - - |
- 6.72 - |
- - 6.72 |
| C. chinense var. pendulum | water Ethanol 70% Acetate |
1.68 0.84 1.68 |
3.36 1.68 - |
- 3.36 3.36 |
- - 3.36 |
Table 3: Minimal Inhibitory Concentrations (MICs) of Capsicum extracts on four fungal strains.
The lowest concentration was observed with the acetal extract of Capsicum chinense var. pendulum (3.36 mg/mL) and Capsicum frutescens (6.72 mg/mL) on Aspergillus flavus.
For the other varieties and extracts, the MIC could not be determined because there was growth in all experimental tubes. The 70% ethanol extract was the most effective extract.
The lowest minimum fungicide concentration was obtained with the 70% ethanol extract of the fruits of Capsicum chinense var. pendulum (0.84 mg/mL) followed by the acetate and the aqueous then ethanol extracts of Capsicum annuum var. antillais with 3.36 mg/mL each.
The MFC/MIC ratios of the aqueous and 70% ethanol extracts of Capsicum annuum var. antillais were equal to 2 on Alternaria sp., Penicillium sp. and Fusarium sp. strains. This ratio is 4 for the acetate extract on Alternaria sp.
Capsicum frutescens species (sudanese, attié, doux and oiseau) provided MFC/MIC ratios, varying from 1 to 2 on the fungal strains.
On Capsicum chinense var. pendulum, the values of these ratios varied from 1 to 4 with the extracts and the fungal strains (Table 2).
The smallest minimal inhibitory concentration of Capsicum annuum var. antillais (MIC) extract was 1.68 mg/mL on Alternaria sp. The smallest MIC was observed for Capsicum chinense var. pendulum extracts on Alternaria sp. (0.84 mg/mL).
On Aspergillus flavus, the lowest MIC was observed with the ethyl acetate extracts of Capsicum chinense var. pendulum (3.36 mg/mL) and Capsicum frutescens (6.72 mg/mL). The MIC for the other varieties and extracts could not be determined, as there was growth in all experimental tubes (Table 3).
The results of the minimum fungicidal concentrations (MFCs) range from 0.84 to 6.72 mg/mL revealed that the 70 % ethanol extract Capsicum chinense var. pendulum was the lowest concentration (0.84 mg/mL), followed by the acetate, water and ethanolic extracts of Capsicum annuum var. antillais (3.36 mg/mL each). On Aspergillus flavus strain, the fungicidal minimal concentration was obtained only in the presence of Capsicum frutescens var. oiseau and Capsicum chinense var. pendulum acetate extracts (6.72 mg/mL each) (Table 4).
| Capsicum varieties |
Extracts | Alternaria sp. | Penicillium sp. | Fusarium sp. | Aspergillus flavus |
|---|---|---|---|---|---|
| C. annuum var. antillais | water Ethanol 70% Acetate |
3,36 3,36 6,72 |
6,72 3,36 - |
6,72 6,72 - |
- - - |
| C. frutescens var. soudanais | water Ethanol 70% Acetate |
6,72 3,36 - |
- - - |
- 6,72 - |
- - - |
| C. frutescens var. attié | water Ethanol 70% Acetate |
6,72 - - |
- - - |
- - - |
- - - |
| C. frutescens var. doux | water Ethanol 70% Acetate |
6,72 - - |
- 6,72 - |
- - - |
- - |
| C. frutescens var. oiseau | water Ethanol 70% Acetate |
6,72 6,72 - |
- - - |
- 6,72 - |
- - 6,72 |
| C. chinense var. pendulum | water Ethanol 70% Acetate |
1,68 0,84 3,36 |
6,72 3,36 - |
- 6,72 6,72 |
- - 6,72 |
Table 4: Minimum Fungicidal Concentrations (MFCs) of Capsicum extracts on fungal strains.
Survival efficiency
Representative survival curves of Alternaria sp., Penicillium sp. and Fusarium sp. in presence of Capsicum annuum var. antillais and C. chinense var. pendulum are presented in Figures 2 and 3.
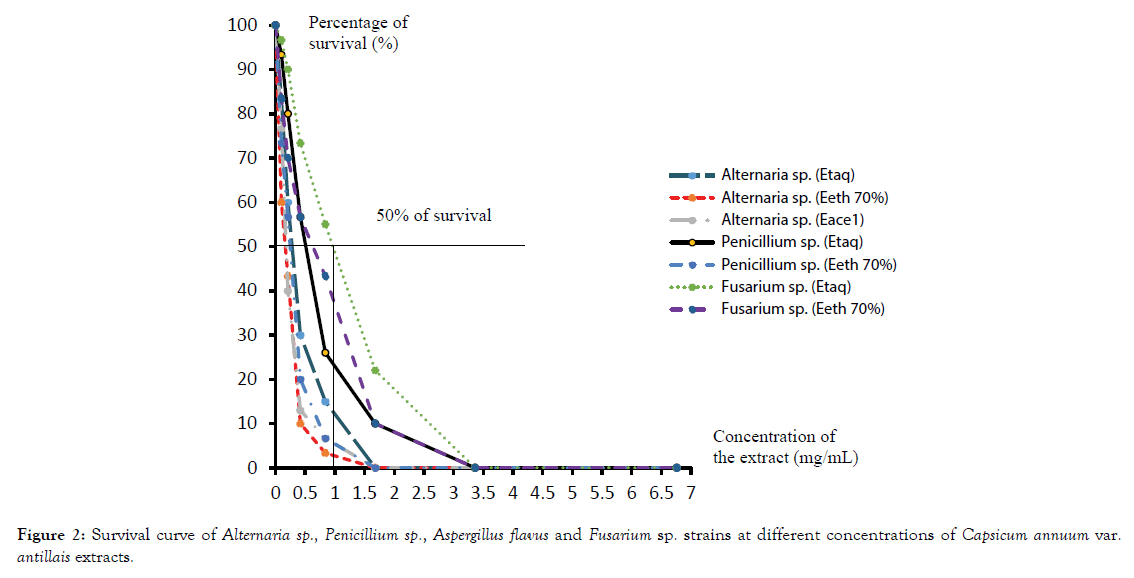
Figure 2. Survival curve of Alternaria sp., Penicillium sp., Aspergillus flavus and Fusarium sp. strains at different concentrations of Capsicum annuum var. antillais extracts.
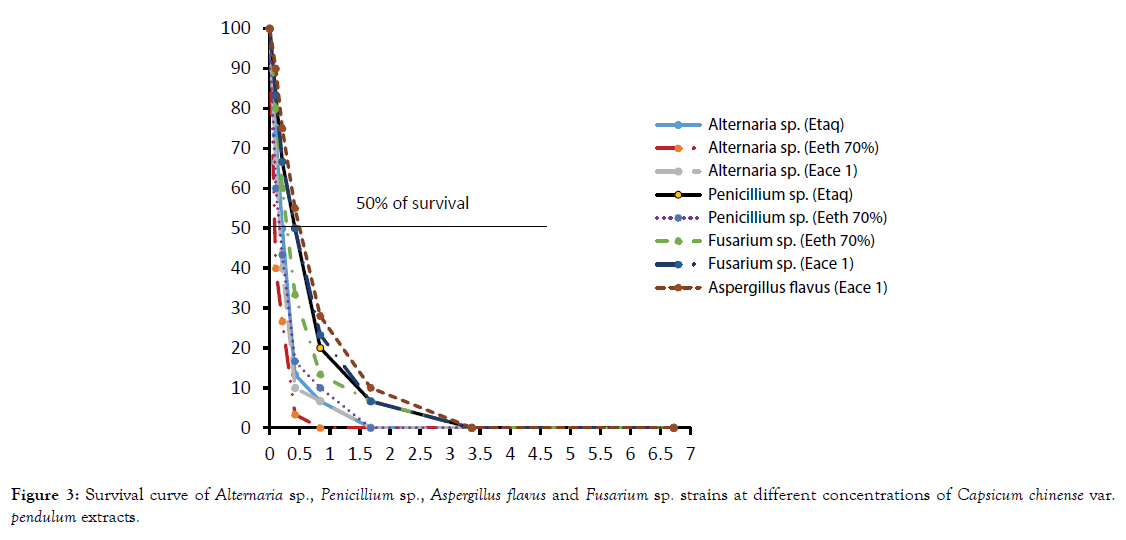
Figure 3. Survival curve of Alternaria sp., Penicillium sp., Aspergillus flavus and Fusarium sp. strains at different concentrations of Capsicum chinense var. pendulum extracts.
With Capsicum annuum var. antillais extracts, the lowest IC50 value was obtained for 70% ethanolic extract on Alternaria sp. and 0.3 mg/mL on the Penicillium sp. The lowest activity of the water extract was obtained at the highest IC50 values. These values were 0.3 mg/ mL on Alternaria sp., 0.5 mg/mL on Penicillium sp. and 1 mg/mL on Fusarium sp. On Alternaria sp. acetone extract and 70% ethanolic extract showed the same IC50 (0.2 mg/mL). Thus, Alternaria sp. is the most sensitive strain.
Indeed, the curves have steeper slopes in the presence of different extracts of Capsicum. The least sensitive strain is Penicillium sp. (Figure 2).
On Capsicum chinense var. pendulum extracts, the lowest IC50 (0.1 mg/mL) was obtained with the ethanol extract while the highest IC50 was obtained with on Alternaria sp.
Water and ethanol extracts showed the same IC50 (0.2 mg/mL). The IC50 of the 70% ethanol extract on Penicilium sp. and Aspergillus flavus were respectively 0.2 and 0.3 mg/mL.
While the IC50 of the acetate extract was the lowest on Fusarium sp. and Aspergillus flavus, 0.4 mg/mL and 0.5 mg/mL respectively. Whatever the extract tested, Alternaria sp. was the most sensitive. While Aspergillus flavus and Aspergillus niger were the most resistant.
Discussion
In order to study the effect of Capsicum extracts on the growth of fungal strains, extractions using different solvents were performed with the fruits of six Capsicum varieties. We found that water extract lead to the best extraction ratio. Thus water may be the best solvent to extract the maximum of component from Capsicum, compared to ethanol and ethyl acetate solvents. However, according to Bouharb et al. [20-22], the yield of extraction should not always be considered as effectiveness criteria. Indeed, the best extraction yields were obtained with the water extracts, while 70% ethanol extracts exhibited the best antifungal activity.
However, it could be difficult to compare the yield extraction results of this study with those of other authors, because it was not only related on the extraction methods and its conditions. The plants and the organ collected may also influence the yield ratio. Indeed, according to Adjou and Soumanou [23], the extraction yield could influence by the harvesting site, the nature of the soil, the stage of development of the plant and the organ used. The different varieties of Capsicum used exhibited antifungal activity on moulds from food. The culture wells method used showed a high diffusion of the extracts in the agar. This study gives consistent results with those obtained by Kouassi and Koffi-Nevry [11] who showed that extracts of Capsicum varieties diffuse better with the well method because the extracts are in direct contact with the agar.
None of the extracts had any effect on the growth of Aspergillus flavus. A. flavus is the less sensitive strain. These results corroborate those of Tournas and Katsoudas [24] and those of Ouattara et al. [25] which showed that Aspergillus sp. are particularly resistant to therapeutic agents. Aspergillus flavus is one of the most resistant microorganisms to antifungals [24]. Koffi-Nevry et al. [26] reported that Aspergillus sp. was the least sensitive strain in presence of Polyhexamethylene-Guanidine Hydrochloride Disinfectant (PHMGH) on Mucor sp., Botrytis sp., Penicillium sp., Geotrichum sp., Aspergillus sp., and Colletotrichum sp. isolated from the papaya Delahousse [27] showed that some plants having good activity also have an inhibitory effect on Penicillium sp., Alternaria sp. and Aspergillus sp. strains.
Capsicum extracts activities vary depending on the nature of the extract and the mould strains. This activity could be due to the phytochemicals present in pepper fruit.
Activities from 70% Ethanolic extracts of caribbean and pendulum varieties exhibited antifungal activity higher than those of water extracts. These results could be explained by the fact that the 70% ethanolic extract could be rich in the specific compounds (phenolic and terpene compounds) capable of destroying fungi. These results may be due to the fact that the 70% ethanolic extract could have more phytocompounds such as phenols and terpenes capable of destroying fungal strains. Moreover, Capsicum annuum var. antillais and Capsicum chinense var. pendulum are rich in these compounds. Ethanol extracts appeared to be more active than aqueous extracts [21,28]. The lowest values of antifungal parameters were obtained with Alternaria sp. strains in the presence of ethanol extracts of Capsicum chinense var. pendulum. The highest values were obtained with the aqueous extracts of Capsicum frutescens attié on Alternaria sp. and with acetate extracts of Capsicum frutescens bird on Aspergillus flavus. According to Daroui-Mokaddem [28], inhibition diameters are related to the desired MIC values, so capsicum extracts with the highest inhibition diameters are those which would possess the lowest MIC. The sensitivity curves to the extracts revealed differences of sensitivity between the fungal strains. In fact, the IC50s of the water, 70% ethanolic and acetate extracts of the pendulum variety on Alternaria sp. were lower than the IC50s of these extracts on Penicillium sp. and Fusarium sp. Our study demonstrates that the ethanol extracts of Capsicum annuum var. antillais and Capsicum chinense var. pendulum are the most active extracts. Alternaria sp. was the most sensitive strain regardless of the extract used.
Conclusions
Both the 70% ethanol and the water extract were the most active extracts on the fungal strains. Alternaria sp., Penicillium sp. and Fusarium sp. were sensitive to the extracts used. However, the most sensitive strain is Alternaria sp. and the lowest one is Aspergillus flavus. In the absence of an extract (0 mg/ml), the germs survival is 100%. Nevertheless, any concentrations of extracts added to the culture medium, lead to progressively decrease the growth of fungal strains. The capsicum extracts are therefore active on the in vitro growth of the fungal strains studied in a dose-dependent manner. This study provides an undeniable scientific argument for the traditional use of capsicum varieties in the treatment of infections due to microorganisms.
REFERENCES
- Tyihák E, Móricz ÁM, Ott PG. Biodetection and determination of biological activity of natural compounds in thin layer chromatography in phytochemistry. CRC Press 2007.
- Yapo YCV, Konkon NG, Coulibaly K, Camara D, Zirihi GN. Botanical study, evaluation of the antifungal activity on the in vitro growth of Candida albicans and the toxicity on HFF cells of leaves of Mallotus oppositifolius (Geiseler) Müller. Arg (Euphorbiaceae). J Anim Plant Sci. 2016;28(1):4330-4339.
- Yao A C. Investigations for a potential development of new antimicrobial drugs from plant extracts, inhibitors of the methylerythritolphosphate (MEP) pathway, one of the isoprenoid biosynthetic pathways. DEA of Botany, UFR Biosciences; Félix Houphouët-Boigny University. 2013;p:58.
- Bitar D, Lortholary O, Dromer F, Coignard B, Che D. Invasive fungal infections and metropolitain France, PMSI, incidence, lethality and trends 2001-2010. Weekly Epidemiological Bulletin. Pp:109-114. 2013.
- Dorrance AE, Berry SA, Bowen P, Lipps PE. Characterization of Pythiumspp. from three Ohio fields for pathogenicity on corn and soybean and metalaxyl sensitivity. Plant Health Prog. 2004.
- Gamal A. Characterization and study of the effect of carbon and nitrogen sources on the production of new secondary metabolites in Aspergillus ochraceus non ochratoxin producing. A PhD thesis. National Polytechnic Institute of Toulouse. 2005;p:175.
- Ahon GM. Evaluation and optimization test of the antifungal activity of extracts of Terminalia superba Engl. And Diels (Combretaceae) on the in vitro growth of Aspergillus fumigatus, Candida albicans and Cryptococcus neoformans. PhD Thesis. Félix Houphouet Boigny University. 2014 ;p:117.
- OMS. Essential medicines and pharmaceutical policies: Provide country support to reduce access to medicines. WHO: Geneva, Switzerland. 2003;p:20.
- Elujoba AA, Odeleye OM, Ogunyemi CM. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr J Tradit Complement Altern Med. 2005;2(1):46-61.
- Dorantes L, Colmenero R, Hermandez H, Mota L, Jaramillo M E, Fernandez E, et al. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annuum extracts. Int J Food Microbiol. 2000;57:125-128.
- Kouassi CK, Koffi-Nevry R. Assessment of the knowledge and use of chilli varieties (Capsicum) grown in Côte d 'Ivoire. Int J Biol Chem Sci. 2012;6(1):175-185.
- Koffi AC, Koffi-Nevry R, Kouassi KC, Loukou YG. Activity extracts of six varieties of pepper (Capsicum) used in Côte d'Ivoire. J Appl Biosci. 2014;82:7379-7388.
- Boxman IL, Tilburg JJ, te Loeke NA, Vennema H, de Boer E, Koopmans M. An efficient and rapid method for recovery of norovirus from food associated with outbreaks of gastroenteritis. J Food Protec. 2007;70(2):504-508.
- Guede-Guina F, Vangah-Manda M, Harouna D, Bahi C. Potencies of MISCA, a plant source concentrate against fungi. J Ethno Pharmacol. 1993;2:45-53.
- CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests methods for antimicrobial susceptibility testing for bacteria isolated from animals-Approved standard- Third edition – CLSI document M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA, USA. 2007.
- SFM. Recommandations of the antibiogram committee of the french microbiology society. 2008 ;p:49.
- Okou OC, Trebissou JND, Bahi C, Guede-Guina F. Effects of tobacoak, a plant extract, on carotid arterial pressure, respiration and duodenal contractions of the rabbit. J Sci Technol. 2008;11:91-102.
- Dosso M, Faye Kette H. Antibiotic Technical Documents. National University of Ivory Coast. 1995 ;p:178.
- Traore Y, Ouattara K, Yeo D, Doumbia I, Coulibaly A. Investigation of antifungal and antibacterial activities of leaves of Annona senegalensis Pers. (Annonaceae). J Applied Biosciences. 2012;58:4234-4242.
- Berche P, Gaillard J L, Simonet M. Bacteria of human infections. Publisher Flammarion: Medicine and Science 1990.
- Bouharb H, Badaoui KE, ZairT, Amri JE, Chakir S, Alaoui T. Selection of some medicinal plants from Zerhoun (Central Morocco) for the antibacterial activity against Pseudomonas aeruginosa. J Appl Biosci. 2014;78:6685-6693.
- Adjou SE, Soumanou MM. Efficacy of plant extracts in the fight against toxinogenic mould isolated from groundnuts in post-harvest Benin. J Appl Biosci. 2013;70:5555-5566.
- Tournas VH, Katsoudas E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int J Food Microbiol. 2005;105:11-17.
- Ouattara S, Kra AKM, Kporou KE, Guede-Guina F. Evaluation of the antifungal activity of extracts of Terminalia ivorensis (tekam 2) on the in vitro growth of Aspergillus fumigatus (evaluation of the antifungal activity of Terminalia ivorensis (tekam 2) extracts in vitro growth of Aspergillus fumigatus). Bull Royal Soci Sci Liège. 2009;78:302-310.
- Koffi-Nevry R, Manizan AL, Tano K, Yue Bi YC, Oule MK, Koussemon M. Assessment of the antifungal activities of polyhexamethylene-guanidine hydrochloride (PHMGH)-based disinfectant against fungi isolated from papaya (Carica papaya L.) fruit. Afr J Microbiol Res. 2011;5:4162-4169.
- Delahousse G. Plants with antifungal properties. PhD Thesis in Pharmacy. University of Nantes (France). 2003 ;p:109.
- Ouattara B, Koffi Mathieu KA, Coulibaly A, Guede-Guina F. Efficacy of the ethanolic extract of Thonningia sanguinea on Cryptococcus neoformans. Fr Stud Res Papers/Health. 2007;17(4):219-222.
- Daroui-Mokaddem H, Kabouche A, Bouacha M, Soumati B, El-Azzouny A, Bruneau C, et al. GC/MS analysis and antimicrobial activity of the essential oil of fresh leaves of Eucalytus globulus, and leaves and stems of Smyrnium olusatrum from Constantine (Algeria). Nat Prod Commun. 2010;5(10):1669-1672.
Citation: Carole KA, Clément KK, Kamelé KY, René KA, Rose KN (2019) Effect of Capsicum annuum L. and Capsicum frutescens L. Varieties Extracts on in vitro Growth of Fungal Strains. J Microb Biochem Technol 11:2. doi: 10.4172/1948-5948.1000412
Copyright: © 2019 Carolel KA et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

