Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2019) Volume 9, Issue 1
Developments on Monovalent Anion-Selective Membranes (MASMs): A Mini-review of Our Recent Contributions
Junbin Liao1, Xing Gao1, Xinyan Yu1, Huimin Ruan1, Jun Li2, Jiangnan Shen1* and Congjie Gao12College of Environment, Zhejiang University of Technology, Hangzhou, 310014, China
Received: 07-Jan-2019 Published: 21-Jan-2019
Abstract
Ion Exchange Membrane (IEM)-based Electrodialysis (ED), as one of the most promising separation techniques, plays a vital role in industrial separation. In particular, for special mono-/multivalent anion separation process in practical industries or academic explorations, highly ion-selective membranes for ED applications is critical but challengeable. To further understand the advances of this specific technique, herein, we have summarized our recent contributions on Monovalent Anion-Selective Membranes (MASMs) fabricated in our research group through:
(1) Surface modification on commercial Anion Exchange Membrane (AEM)
(2) Micro-phase structure regulation of homogeneous AEM. We have also discussed the advantages and disadvantages with respect to some specific cases in detail and the future perspectives of MASMs
Keywords
Monovalent anion selective membranes; Anions; Surface modification; Micro-phase structure regulation; Electrodialysis
Abbrevations
ED: Electrodialysis; MASM: Monovalent Anion- Selective Membrane; AEM: Anion Exchange Membrane; IEM: Ion Exchange Membrane; GO: Graphene Oxide; DC: Dilute Cell; PSS: Poly(sodium 4-styrene sulfonate); HACC: Hydroxypropyltrimethyl Ammonium Chloride Chitosan; L-B-L: Layer-By-Layer; SDA: Sulfonated Dopamine; QPPO: Quaternized Poly(Phenylene Oxide); DAS: 4,4-Diazostilbene-2,2-Disulfonic Acid Disodium Salt
Introduction
Ion Exchange Membranes (IEMs) are typically composed of hydrophobic substrates, immobilized ion-functionalized groups, and movable counter-ions [1,2]. With the growing IEM-based applications in chloride-alkali manufacture, fuel cells, ED, electromembrane reactor batteries, etc., developing IEMs having superior ion perm-selectivity between mono-/multi-valent ions (e.g., Li+/ Mg2+; Cl-/SO42-) or equal valent ions (e.g., NO3 - and Cl-) are highly desirable [1-4]. In recent years, a soaring interest in exploring some green processes to force the migration of anions or cations towards a specific direction has been widely aroused, especially over electrically-driving membrane processes in resource recovery, energy production, seawater desalination, and water treatment/ pre-treatment, etc. [4,5]. These extensive applications of IEMs further promote the investigations in both academic and industrial level. The discrimination of anions by an AEM over ED process remains more challenges, because of the quite similar hydrated radii of some specific anions (e.g., F-, 3.52 Å ; Cl-, 3.32 Å; Br-, 3.30 Å; NO3-, 3.35 Å; CO32-, 3.94 Å; SO42-, 3.79 Å) [6,7]. Thus far, the lack of advanced MASM for some specific systems still limits the development and applications of ED in many fields [4,5]. Thereby, more efforts have been devoted to the preparation of MASMs with perm-selectivity for monovalent anions.
Figure 1 shows the proposed schematic experimental setup for ED fabricated with MASMs. Therein, MASMs allow the smooth transport of monovalent anions (A-), while blocking migration of multivalent anions (Bn-) through the MASM matrix. As a result of the distinguished features, MASMs have been extensively investigated in different applications like ED and reverse ED, etc. [4,8]. Herein, in this mini-review encompasses:
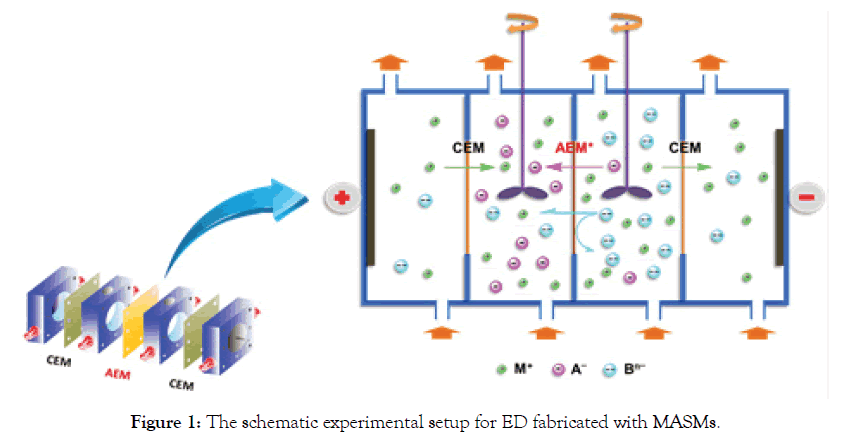
Figure 1. The schematic experimental setup for ED fabricated with MASMs.
(1) Surface modification on AEM with polyelectrolyte layer coating, functional Graphene Oxide (GO) layers and surface covalent crosslinking
(2) Regulation of micro-phase structure of homogeneous AEM to realize the monovalent anion selectivity for ED applications
Mechanism Of The Perm-Selective Separation Of Masms
Perm-selectivity of anions in a mixture solution through MASMs is basically governed by their affinity towards the MASMs (ion exchange equilibrium constant) and the migration speed in the membrane phase (mobility ratio among the cations) [1,5]. In general, the selectivity for monovalent anions can be realized through MASM with the mechanisms of:
(1) Pore-size sieving effect (the different hydrated ionic radii)
(2) Electrostatic repulsion (the difference in electrostatic repulsion between mono-/multi-valent anions)
(3) Hydration energy difference (the different Gibbs hydration energy) etc. [5,9]
Figure 2 illustrates the possible mechanisms of MASMs in ED process (Cl-/SO42- separation system as the example).
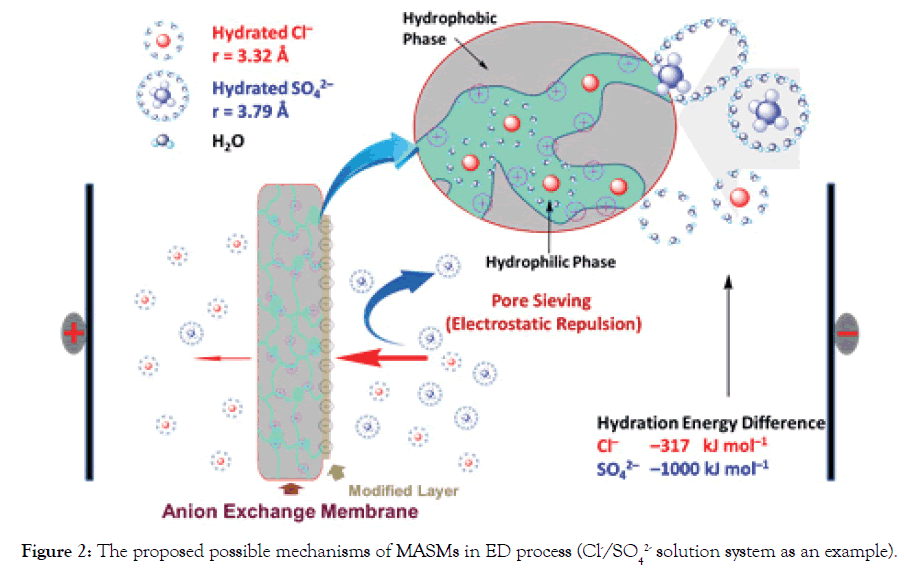
Figure 2. The proposed possible mechanisms of MASMs in ED process (Cl-/SO42- solution system as an example).
To simplify the evaluation system, in our cases, Cl- and SO42- are used as the standard anions in a mixture solution. When one equivalent of SO42- ion permeates through MASMs, the permeated equivalency of a given anion is evaluated. Perm-selectivity between anions Cl- and SO42- is represented as PSO42−Cl− ,which is called as relative transport number ratio of anions Cl- to SO42-.
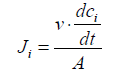 (1)
(1)
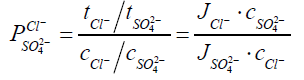 (2)
(2)
Where, Ji=flux (mol.m-2.s-1) of the target anions transferring through MASMs;
tCl − and tSO42− are the transport numbers of Cl- and SO42-, respectively;
c− and cSO42− are the concentrations (M) of Cl- and SO42- in Dilute Cell (DC);
V=volume (L) of the feeding solution in DC;
A=effective area of MASMs.
Technologies For Mapm Fabrication
Surface modification
To fabricate functional layers having perm-selectivity, the surface polymerization, direct coating, electro-deposition of functional layer or multilayers (Layer-By-Layer (L-B-L)) onto the IEM surface and cross-linking of a thin layer have been widely reported [10,11]. In our cases, both surface physicochemical modification and covalently chemical modification have been employed to enhance the monovalent ion perm-selectivity. The modified layer features hydrophilic nature and different charge density from functional groups, resulting in adjusted surface compactness, ion conductivity and perm-selectivity [12,13].
Polyelectrolyte layers: Here, some examples of polyelectrolytes of Poly(Sodium 4-styrene Sulfonate) (PSS) and Hydroxypropyltrimethyl Ammonium Chloride Chitosan (HACC) or modified HACCs deposited on AEMs have been taken to illustrate the physicochemical modifications (L-B-L) [14]. Figure 3a shows a facial avenue to modify the commercial AEM by a coating of (PSS/ HACC)N. Under the external electric force filed, negative PSS and positive HACC molecules were alternately deposited on the surface (HACC and PSS as the bottom and top layers, respectively). As a result, we can find that the coating of (PSS/HACC)N (where N is from 0 to 9) layers boost the perm-selectivity (Cl-/SO42-) of the AEMs obviously from 0.66 to 2.90 and separation efficiency of Cl- and SO42- ions increased from 0.19 to 0.28 with only 9 bilayers within 90 min. Therein, alternative compact charged layers are beneficial to strengthen the pore-size sieving effect and electrostatic repulsion, contributing to the significantly enhanced perm-selectivity.
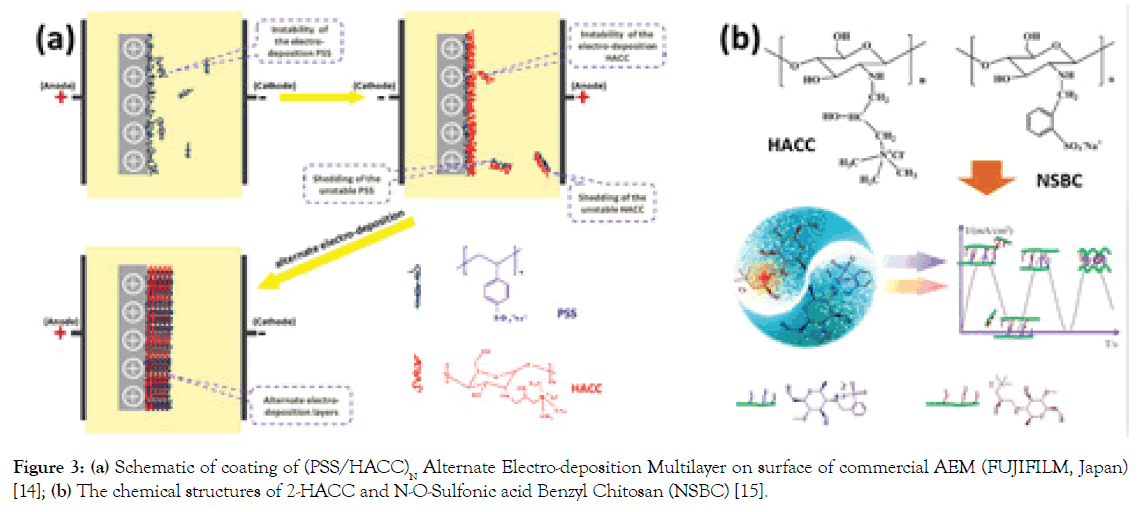
Figure 3. (a) Schematic of coating of (PSS/HACC)N Alternate Electro-deposition Multilayer on surface of commercial AEM (FUJIFILM, Japan) [14]; (b) The chemical structures of 2-HACC and N-O-Sulfonic acid Benzyl Chitosan (NSBC) [15].
Following this strategy, we have also investigated L-B-L layers assembled of AEMs by HACC and N-O-Sulfonic acid Benzyl Chitosan (NSBC) (Figure 3b) via by electric-pulse deposition method [15]. The investigation demonstrates that the constructed NSBC/HACC polyelectrolyte layers with 7.5 bilayers exhibit significantly enhanced monovalent anion (Cl-) selectivity (47.04) with small additional surface area resistance (from 1.31 Ω cm2 to 4.25 Ω cm2). This selectivity stems from an increased electrostatic repulsion effect for divalent anions and hydrophilicity of the modified layers with a separation efficiency of Cl-/SO42 increasing from -8.93% (unmodified AEM) to 94.43% within 20 min. It is noted that the multilayers constructed by electric-pulse deposition technology show much superiority over the traditional electrostatic deposition and the electro-deposition modification methods.
To improve the long-term stability and meanwhile endow this L-B-L type modified AEMs with specific performance, like anti-fouling properties, we have modified a commercial AEM with “sandwich”- like structure, composed of upper/bottom bilayers of PDA and sandwich alternating bilayers of PSS/HACC-nanosilver particles (HACC-Ag Np) (Figure 4a), aiming to enhance the monovalent selectivity and fouling resistance for ED application [16]. Our investigations suggest that the perm-selectivity (PSO42−Cl−)of the modified AEM with 4.5 bilayers of PSS can reach 5.1, significantly outperforming that of commercial standard AEM without modification (0.98) (Figure 4b). Possibly, the strong adherent strength of the top PDA layer from “sandwich”-like structure can well consolidate the sandwich alternating bilayers inside, which contributes greatly to a long-term stability test within 50 hrs.
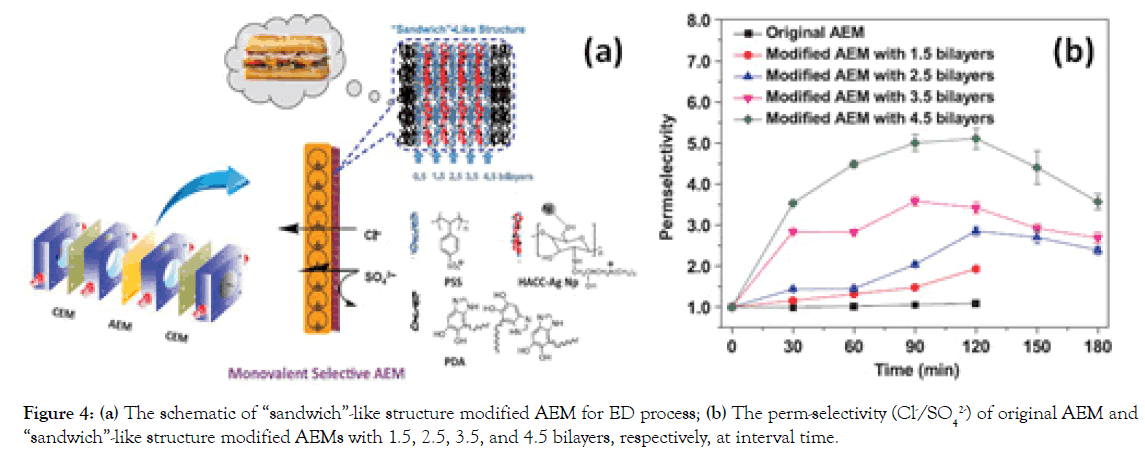
Figure 4. (a) The schematic of “sandwich”-like structure modified AEM for ED process; (b) The perm-selectivity (Cl-/SO42-) of original AEM and “sandwich”-like structure modified AEMs with 1.5, 2.5, 3.5, and 4.5 bilayers, respectively, at interval time.
Encouraged by the superior performance of “sandwich”-like structure gathering anionic and cationic groups in one thin layer, we have been in search of better-performing modification layers for high-performance AEMs. As reported, zwitterionic materials bearing the homogeneously-distributed anionic and cationic groups in one segment have been broadly employed for antifouling porous membrane [17]. Inspired by this, we have further proposed a facile surficial adhesive modification process to fabricate a highly perm-selective AEM for ED application by solely coating polymerlike Sulfonated Dopamine (SDA) adhesive on commercial AEMs under alkaline and aerobic conditions [18], which is shown in Figure 5a. We have found that the SDA coating can significantly enhance the monovalent perm-selectivity (Cl-/SO42- ), with a value of 34.02, which is much higher than the corresponding values of DA modified AEM (11.59) and results from many other elegant surface modifications (Figure 5b and 5c). The long-term stability of ED with SDA-modified AEM operated for 90 h, indicating the relatively strong adherent strength of SDA layer on the surface of AEMs.
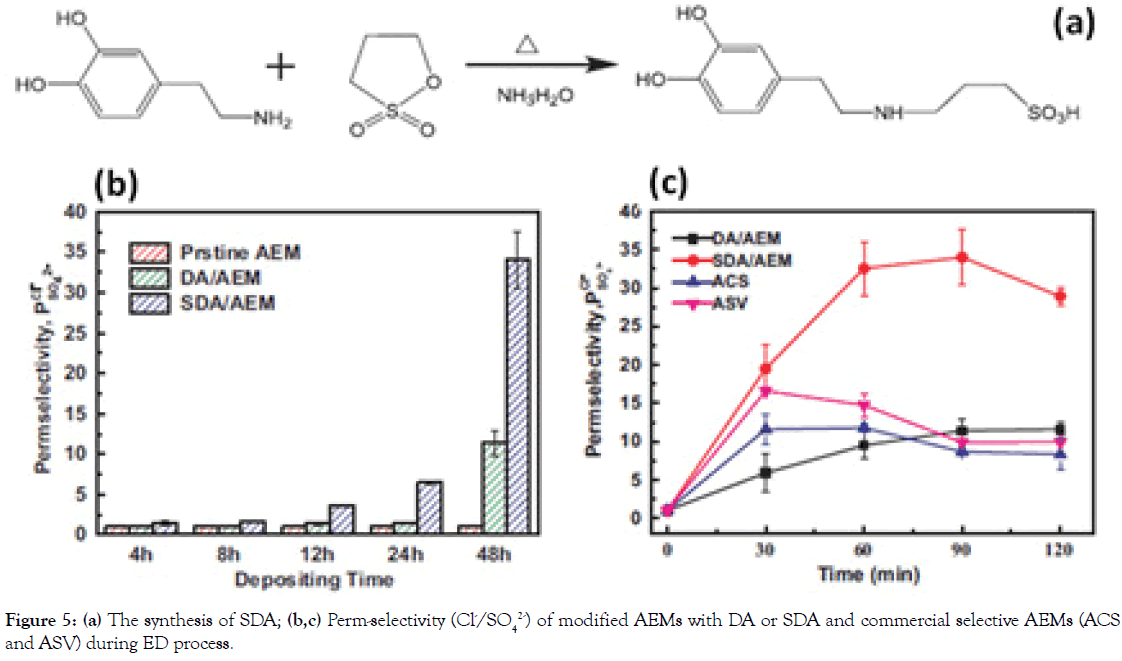
Figure 5. (a) The synthesis of SDA; (b,c) Perm-selectivity (Cl-/SO42-) of modified AEMs with DA or SDA and commercial selective AEMs (ACS and ASV) during ED process.
To firmly anchor the multilayers of PSS and HACC through alternating electro-deposition on AEM surface, a photosensitive molecule of 4,4-Diazostilbene-2,2-disulfonic acid disodium salt (DAS) has been used to bind the multilayers via covalent bonds by UV irradiation (Figure 6a) [19]. The optimized DAS cross-linked (PSS/HACC)5PSS modified AEM has an improved monovalent anion selectivity (Cl-/SO42- system) of 4.36 (Figure 6b) and stable selectivity during the entire duration of testing (76 hrs), relative to a conventional multilayer modified AEMs completely loses its selectivity after 30 hrs, suggesting that cross-linking effectively improved the monovalent anion selectivity and stability.
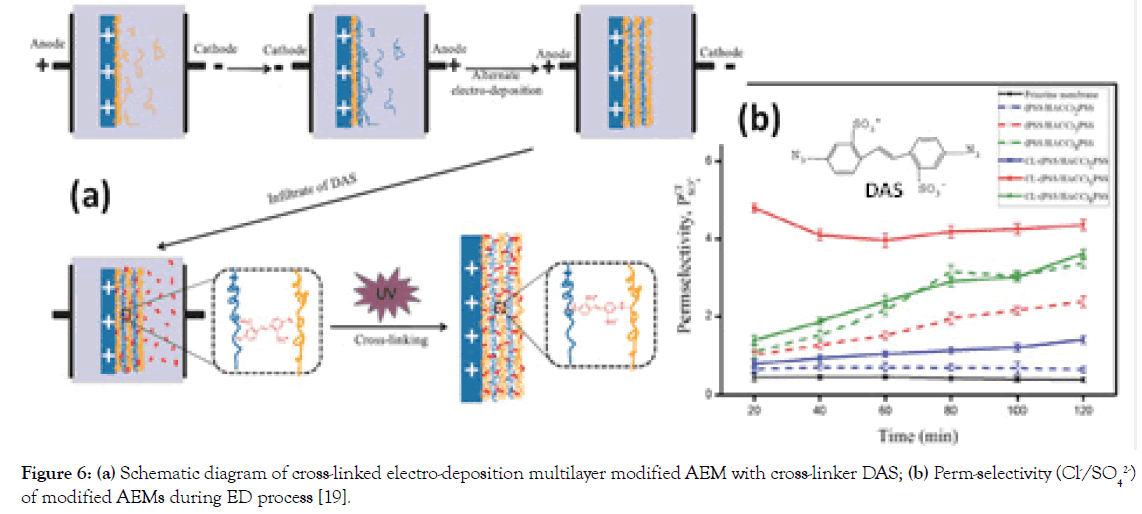
Figure 6. (a) Schematic diagram of cross-linked electro-deposition multilayer modified AEM with cross-linker DAS; (b) Perm-selectivity (Cl-/SO42-) of modified AEMs during ED process [19].
Functional GO layers: Thus far, the perm-selective ion separation of L-B-L modified IEMs still remains a big challenge. A detailed and deep description of ion transport through the multilayer architectures is needed as the results from different studies are controversial [5,20]. In addition, the L-B-L layers via relativelyweak Van der Waals' force or electrostatic interaction under severe condition undergo a gradual loss over the practical ED process. This significantly decays the ion selectivity and thus shortens the life-span during a continuous ED operation process [21,22]. Thus, systematic researches on diversified parameters in L-B-L process should be considered to investigate the mechanism on how the parameters affect the final performance of the membranes [5].
Coating GO with lamellar structure can be another modification for MASMs. To explore this type, Sulfonated reduced Graphene Oxide (S-rGO) nanosheets with negatively charged sulfonic acid groups were synthesized via a facile distillation-precipitation polymerization, followed by hydrazine reduction (Figure 7) [23]. The sulfanilic acid was grafted on the GO sheets to separate GO nanosheets each other, providing anion channels for anions selectivity. As a result, unmodified AEM has no monovalent selectivity, while the perm-selectivity and separation efficiency of S-rGO modified AEMs (reduced by hydrazine hydrate steam in 10 min) increases from 0.65 to 1.80 and from -0.13 to 0.31 (in 40 min), respectively. Another similar work on S-rGO-PDA modified AEMs show the perm-selectivity of 2.5 at 120 min [24].
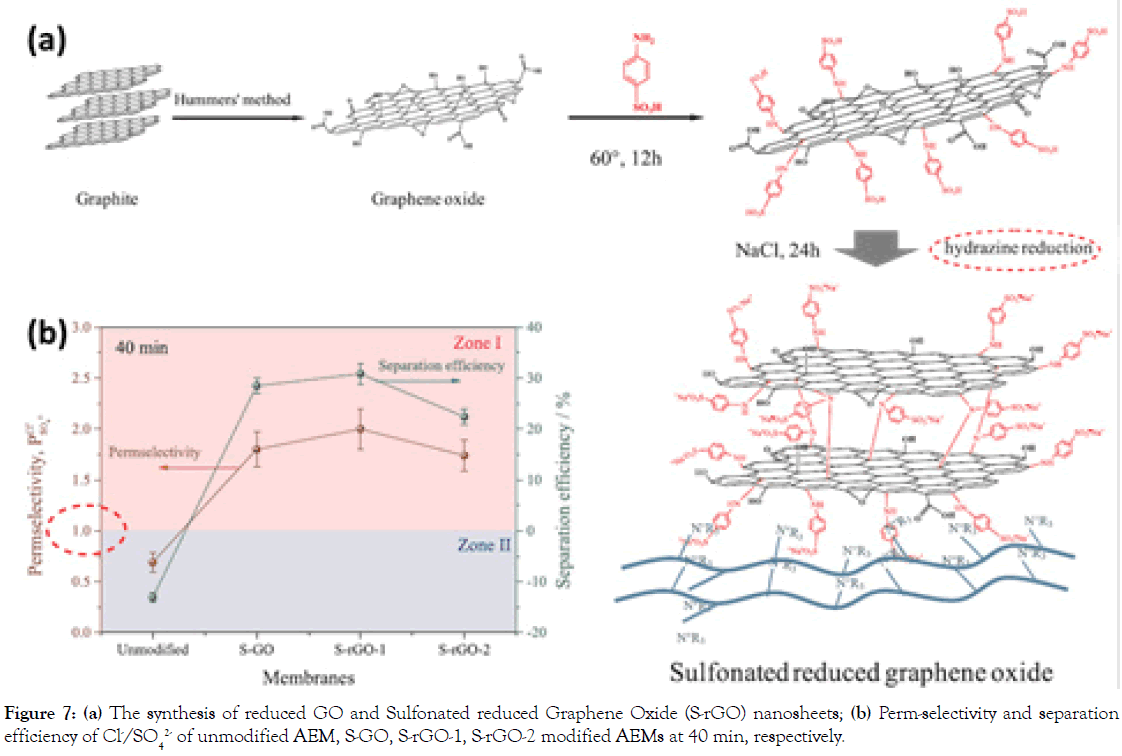
Figure 7. (a) The synthesis of reduced GO and Sulfonated reduced Graphene Oxide (S-rGO) nanosheets; (b) Perm-selectivity and separation efficiency of Cl-/SO42- of unmodified AEM, S-GO, S-rGO-1, S-rGO-2 modified AEMs at 40 min, respectively.
To further explore the functional graphene nanosheets for MASMs, a multilayer architecture of graphene, grafted with Sulfonated 4,4’-Diaminodiphenyl Sulfone (SDDS) was designed [25]. SDDS was mixed with Quaternized Poly(Phenylene Oxide) (QPPO) to form a Multilayer Graphene-Organic Framework (MGOF) having both positive and negative groups. The optimized AEM (rGOSDDS- rGO@QPPO-2) has a low surface electric resistance (2.79 Ω cm2) and shows the selective separation (Cl-/SO42-) of 36.6%.
Surface covalent cross-linking: Though deposition of functional layers on the surface of AEMs is a simple and versatile method that achieves high monovalent anion selectivity, the stability of the polyelectrolyte multilayers can be compromised by the weak interactions formed between the deposited barrier and the pristine membrane surface. To overcome this drawback, covalently chemical modification like cross-linking appears as an efficient method to improve the chemical stability of IEMs by covalent bonding [26,27]. In our case, a photosensitive molecule DAS was used to infiltrate into the AEM surface and immobilize in the structure by cross-linking under UV irradiation (Figure 8a) [28]. The introduced negative charges in the surface layer of AEM without increasing its thickness render high Cl- selectivity with a value of 11.21, significantly superior to the commercial selective membrane Selemion ASV (4.81) as shown in Figure 8b. Furthermore, the newly developed membrane exhibits excellent long-term stability, which maintains constant perm-selectivity during the 80 hrs.
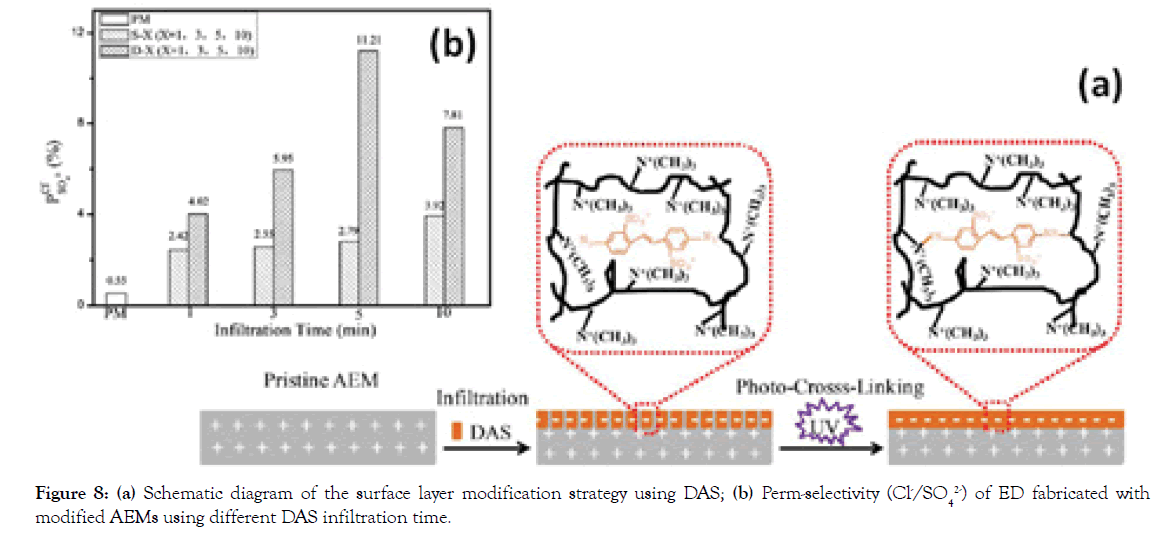
Figure 8. (a) Schematic diagram of the surface layer modification strategy using DAS; (b) Perm-selectivity (Cl-/SO42-) of ED fabricated with modified AEMs using different DAS infiltration time.
Another example is a simple preparative pathway to covalently immobilize the Polyethyleneimine (PEI) onto the surface of partly QPPO (Figure 9a) [29]. As shown in Figure 9a, relative to unmodified QPPO AEMs, the perm-selectivity of PEI modified AEMs increases from 0.79 to 4.27 and the SO42 leakage rate decreases from 39.6-19.4%. The results indicate that this is an effective strategy to fabricate advanced MASMs.
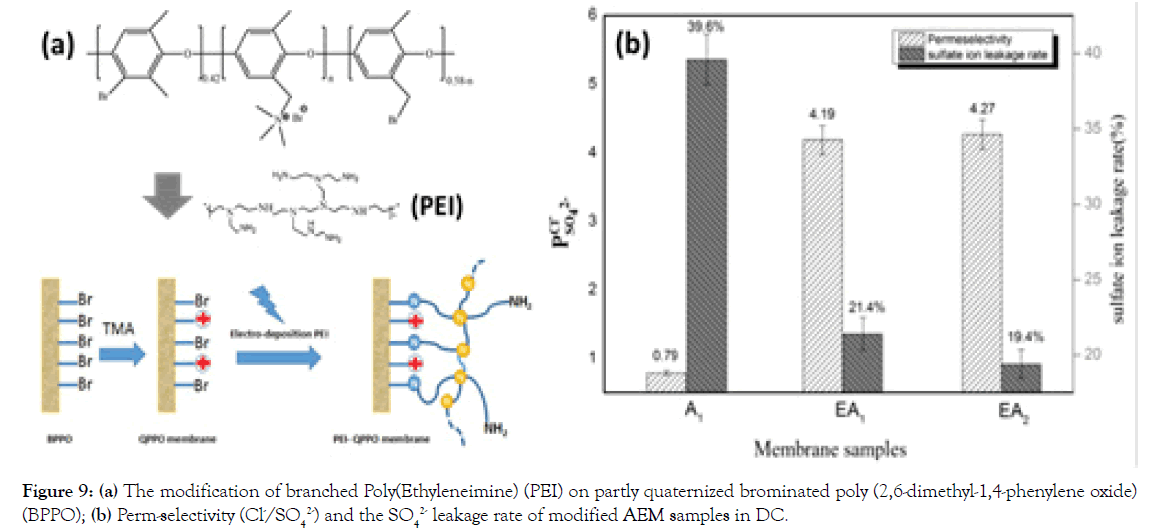
Figure 9. (a) The modification of branched Poly(Ethyleneimine) (PEI) on partly quaternized brominated poly (2,6-dimethyl-1,4-phenylene oxide) (BPPO); (b) Perm-selectivity (Cl-/SO42-) and the SO42 leakage rate of modified AEM samples in DC.
Regulation of micro-phase structure
Nevertheless, surface modification by fabricating a dense ionic layer on the surface of a substrate membrane is the main method for preparing MASMs [4,5]. However, this method has a tradeoff between the ionic flux and perm-selectivity. Fabricating homogeneous MASMs with desirable stability and high permselectivity for ED applications is critical and meaningful. It is reported that covalent cross-linking is a promising strategy to improve both the compactness and chemical stability of MASMs.
Though the improved compactness of AEM matrix might lower the migration speed of anions and thus increase the surface area resistance to a degree, the ion perm-selective separation of AEMs via pore-size sieving effect shows the great possibility upon the difference in hydrated ionic radius of ions (Cl- ion with hydrated radii of 3.32 Å; SO42, 3.79 Å) [30,31].
In our previous work, a series of internally cross-linked monovalent selective AEMs were prepared via a one-pot approach by simply adding different amounts of Sulfamerazine (SF) to partially- Quaternized Chloromethylated Polysulfone (QPSF) [32], as shown in Figure 10a. By tuning the content of conductive cross-linker of sulfamerazine in AEM matrix, the QPSF-SF AEMs show the high monovalent anion perm-selectivity (P Cl−SO42− )s of 3.98–15.90, possibly, due to the increased compactness of AEM matrix (Figure 10b and 10c). In particular, the optimized QPSF-SF-0.09 AEM has a perm-selectivity of 24.55 in alkaline condition (pH=10.0). The facile synthesis procedure and the excellent monovalent ion selectivity firmly confirm the possibility of cross-linked AEMs for the ion separation process.
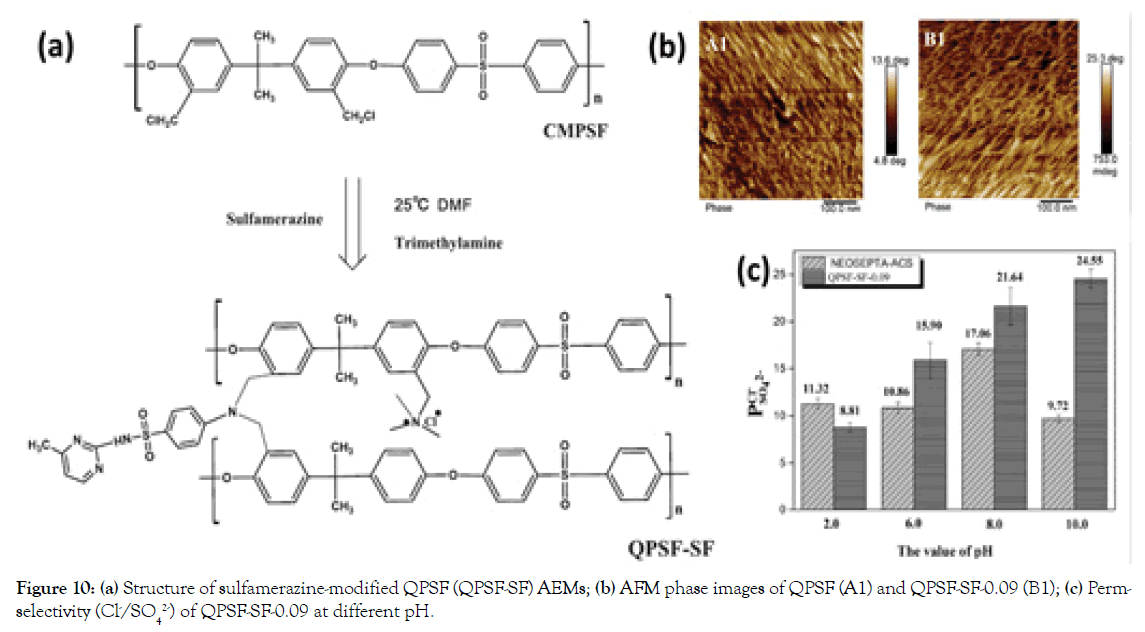
Figure 10. (a) Structure of sulfamerazine-modified QPSF (QPSF-SF) AEMs; (b) AFM phase images of QPSF (A1) and QPSF-SF-0.09 (B1); (c) Permselectivity (Cl-/SO42-) of QPSF-SF-0.09 at different pH.
In earlier years, Sata, et al. [33-35] have demonstrated that the hydrophilicity of the AEMs to hydration energy of anions is important for perm-selectivity for specific anions. In general, hydration energy of anions rather than hydrated ionic size is predominant in changing transport numbers of anions relative to chloride ions through the AEM matrix in some cases. On the other hand, though ions of Cl- and SO42 show the similar hydrated radii, the hydration energy of SO42 ions (-1000 kJ mol-1) is 3 times of that of Cl- ions (-317 kJ mol-1) [31,34]. This suggests that an SO42 ion binds with H2O strongly more, relative to Cl- ion. In addition, SO42 ion has a hydration number of 14, while Cl- having 8. Namely, Cl- ions permeate through the denser IEM matrix more easily under the electric field force through losing their hydration shell, whereas the transfer of SO42 ions tend to be blocked. Considering this difference, we have reported a strategy for fabricating novel AEMs based on a series of fluoro-methyl poly(arylene ether ketone) s having long-side-chain imidazolium groups for ED. Possibly owing to the synergistic effect of pore-size sieving effect resulting from macro-separation morphology (Figure 11a-11c) from flexible side-chain imidazolium groups and rigid hydrophobic backbone, as well as hydration energy difference of SO42 and Cl- ions, PAEK- 60-im shows significantly superior perm-selectivity (Cl-/SO42-) of 7.70 at 30 min, relative to commercial anion-selective Neosepta ACS (5.27) (Figure 11d and 11e). The superior ED performance of the homogeneous AEM is suggestive of its potential separation application.
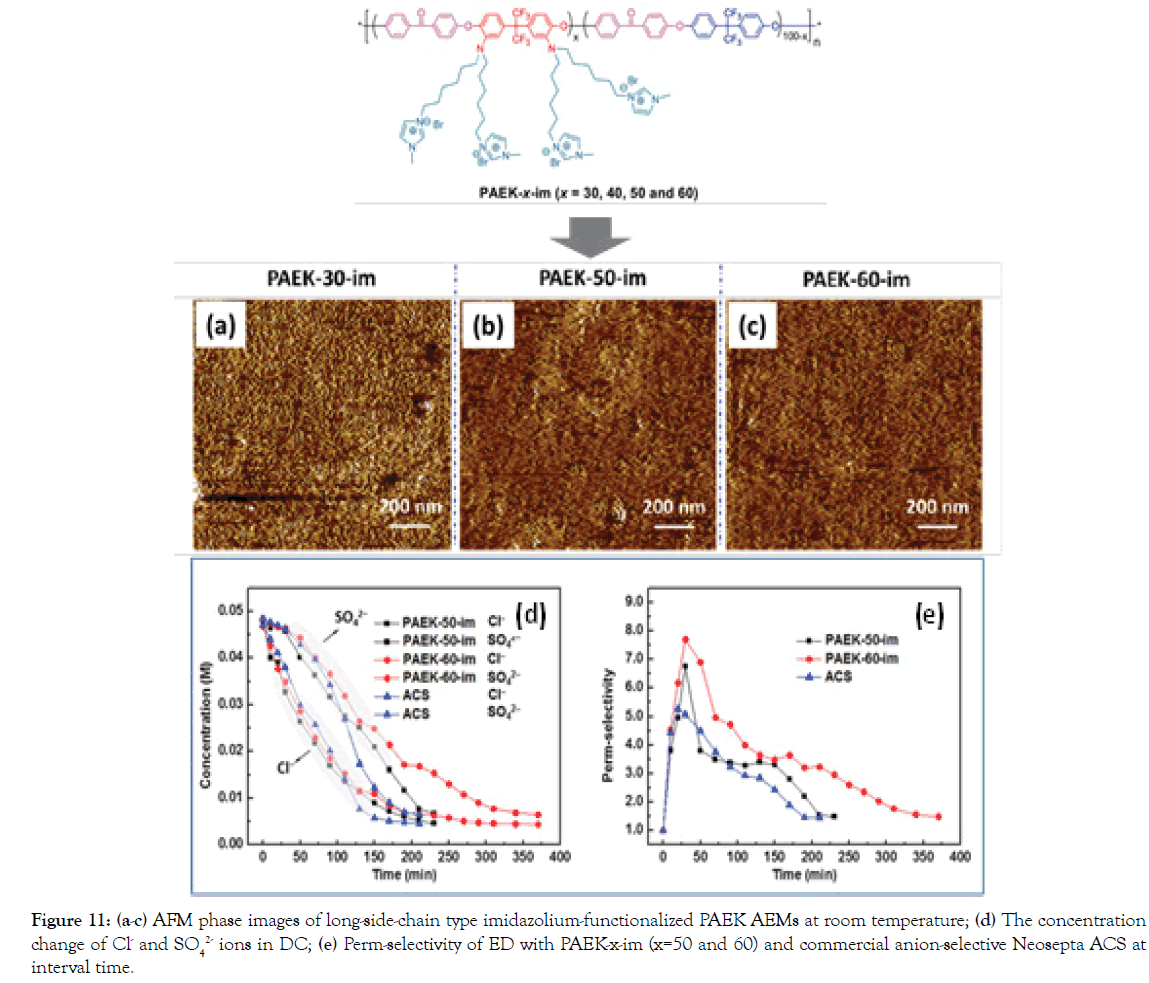
Figure 11. a-c) AFM phase images of long-side-chain type imidazolium-functionalized PAEK AEMs at room temperature; (d) The concentration change of Cl- and SO42- ions in DC; (e) Perm-selectivity of ED with PAEK-x-im (x=50 and 60) and commercial anion-selective Neosepta ACS at interval time.
Summary And Perspective
Though the exploration of high-performance MASMs has been made significant progress, it still remains many challenges. The “trade-off” effect between ionic flux and perm-selectivity is an issue should be improved. In particular, the long-term stability of MASMs in the practical application should be concerned. Possibly, fabricating homogeneous MASMs with desirable long-term stability and anion perm-selectivity is critical and meaningful. It suggests some methods and research direction to develop AEMs having high perm-selectivity to specific anions. It is expected that there should be more novel methods.
Acknowledgment
We thank the funding support from the National Natural Science Foundation of China (No. 21676249), the National Natural Science Foundation of China (No. 21878273), the National Key Research and Development Plan (No. 2017YFC0403701), the 63rd China Postdoctoral Science Foundation (No. 2018M632505), the Project for Assistance of Qinghai from Science Technology Department of Zhejiang Province (No. 2018C26004).
REFERENCES
- Ran J, Wu L, He Y, Yang Z, Wang Y, Jiang C, et al. Ion exchange membranes: New developments and applications. J Membr Sci. 2017;522:267-291.
- Hong JG, Zhang B, Glabman S, Uzal N, Dou X, Zhang H, et al. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J Membr Sci. 2015;486:71-88.
- Parasuraman A, Lim TM, Menictas C, Skyllas-Kazacos M. Review of material research and development for vanadium redox flow battery applications. Electrochim Acta. 2013;101:27-40.
- Luo T, Abdu S, Wessling M. Selectivity of ion exchange membranes: A review. J Membr Sci. 2018;555:429-454.
- Ge L, Wu B, Yu D, Mondal AN, Hou L, Afsar NU, et al. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chinese J Chem Eng. 2017;25:1606-1615.
- Marcus Y. Ionic radii in aqueous solutions. Chem Rev. 1988;88:1475-1498.
- Tansel B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep Purif Technol. 2012;86:119-126.
- Zhang Y, Paepen S, Pinoy L, Meesschaert B, Van der Bruggen B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep Purif Technol. 2012;88:191-201.
- Liao J, Zhu J, Yang S, Pan N, Yu X, Wang C, et al. Long-side-chain type imidazolium-functionalized fluoro-methyl poly (arylene ether ketone) anion exchange membranes with superior electrodialysis performance. J Membr Sci. 2019;574:181-195.
- Khodabakhshi AR, Madaeni SS, Hosseini SM. Preparation and characterization of monovalent ion-selective poly (vinylchloride)-blend-poly (styrene-co-butadiene) heterogeneous anion-exchange membranes. Polym Int. 2011;60:466-474.
- Mulyati S, Takagi R, Fujii A, Ohmukai Y, Matsuyama H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J Membr Sci. 2013;431:113-120.
- White N, Misovich M, Yaroshchuk A, Bruening ML. Coating of Nafion membranes with polyelectrolyte multilayers to achieve high monovalent/divalent cation electrodialysis selectivities. ACS Appl Mater. Interfaces. 2015;7:6620-6628.
- Zhang Y, Van der Bruggen B, Pinoy L, Meesschaert B. Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J Membr Sci. 2009;332:104-112.
- Zhao Y, Tang K, Liu H, Van der Bruggen B, Sotto Díaz A, Shen J, et al. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J Membr Sci. 2016;520:262-271.
- Zhao Y, Zhu J, Ding J, Van der Bruggen B, Shen J, et al. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity. J Membr Sci. 2018;548:81-90.
- Hao L, Liao J, Jiang Y, Zhu J, Li J, Zhao Y, et al. “Sandwich”-like structure modified anion exchange membrane with enhanced monovalent selectivity and fouling resistant. J Membr Sci. 2018;556:98-106.
- Shao Q, Jiang S. Molecular understanding and design of zwitterionic materials. Adv Mater. 2015;27:15-27.
- Ruan H, Zheng Z, Pan J, Gao C, Van der Bruggen B, Shen J, et al. Mussel-inspired sulfonated polydopamine coating on anion exchange membrane for improving permselectivity and anti-fouling property. J Membr Sci. 2018;550:427-435.
- Liu H, Ruan H, Zhao Y, Pan J, Sotto A, Gao C, et al. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J Membr Sci. 2017;543:310-318.
- Femmer R, Mani A, Wessling M. Ion transport through electrolyte/polyelectrolyte multi-layers. Sci Rep. 2015;5:11583.
- Wang M, Liu X, Jia Y, Wang X. The improvement of comprehensive transport properties to heterogeneous cation exchange membrane by the covalent immobilization of polyethyleneimine. Sep Purif Technol. 2015;140:69-76.
- Yao TT, Wang M, Jia YX, Zhou PF. A modified coating method for preparing a monovalent perm-selective cation exchange membrane: I. The evolution of membrane property corresponding to different preparing stages. Desalin Water Treat. 2013;51:2740-2748.
- Zhao Y, Tang K, Ruan H, Xue L, Van der Bruggen B, Gao C, et al. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J Membr Sci. 2017;536:167-175.
- Jin Y, Zhao Y, Liu H, Sotto A, Gao C, Shen J, et al. A durable and antifouling monovalent selective anion exchange membrane modified by polydopamine and sulfonated reduced graphene oxide. Sep Purif Technol. 2018;207:116-123.
- Zhao Y, Zhu J, Li J, Zhao Z, Ochoa SIC, Shen J, et al. Robust multilayer graphene-organic frameworks for selective separation of monovalent anions. ACS Appl Mater Interfaces. 2018;10:18426-18433.
- Sun JQ, Wu T, Sun YP, Wang ZQ, Zhang X, Shen J, et al. Fabrication of a covalently attached multilayer via photolysis of layer-by-layer self-assembled films containing diazo-resins. Chem Commun. 1998:1853-1854.
- Huang XH, Zhang MM, Dou XW, Lu X, Qin YJ, Zhang P, et al. Strengthened graphene oxide/diazoresin multilayer composites from layer-by-layer assembly and cross-linking. Chi Chem Lett. 2015;26:1155-1157.
- Liu H, Jiang Y, Ding J, Shi W, Pan J, Gao C, et al. Surface layer modification of AEMs by infiltration and photo-cross-linking to induce monovalent selectivity. AIChE Journal. 2018;64:993-1000.
- Pan J, Ding J, Tan R, Chen G, Zhao Y, et al. Preparation of a monovalent selective anion exchange membrane through constructing a covalently crosslinked interface by electro-deposition of polyethyleneimine. J Membr Sci. 2017;539:263-272.
- Park CH, Lee SY, Hwang DS, Shin DW, Cho DH, Lee KH, et al. Nanocrack-regulated self-humidifying membranes. Nature. 2016;532:480.
- Miao J, Yao L, Yang Z, Pan J, Qian J, Xu T. Sulfonated poly (2,6-dimethyl-1, 4-phenyleneoxide)/nano silica hybrid membranes for alkali recovery via diffusion dialysis. Sep Purif Technol. 2015;141:307-313.
- Pan J, Ding J, Zheng Y, Gao C, Van der Bruggen B, Shen J. One-pot approach to prepare internally cross-linked monovalent selective anion exchange membranes. J Membr Sci. 2018;553:43-53.
- Sata T, Teshima K, Yamaguchi T. Permselectivity between two anions in anion exchange membranes crosslinked with various diamines in electrodialysis. J Polym Sci A: Polym Chem. 1996;348:1475-1482.
- Sata T, Yamaguchi T, Matsusaki K. Effect of hydrophobicity of ion exchange groups of anion exchange membranes on permselectivity between two anions. J Phys Chem. 1995;99:12875-12882.
- Sata T, Tagami Y, Matsusaki K. Transport properties of anion-exchange membranes having a hydrophobic layer on their surface in electrodialysis. J Phys Chem B. 1998;102:8473-8479.
Citation: Liao J, Gao X, Yu X, Ruan H, Li J, Shen J, et al. (2019) Developments on Monovalent Anion-Selective Membranes (MASMs): A Mini-review of Our Recent Contributions. J Membr Sci Technol 9:192.
Copyright: �© 2019 Liao J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

