Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 12
Detection of Mycotoxins in Aquaculture Feed Ingredients Using a Rapid FT-NIR Method
Sofia Vardali1*, Christina Papadouli2, Myrto Maniaki1, Theodoros Karatzinos1, George Rigos2, Ioannis Nengas2, Panagiota Panagiotaki1 and Eleni Golomazou1*2Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Attiki, Greece
Received: 06-Dec-2023, Manuscript No. JARD-23-24270; Editor assigned: 08-Dec-2023, Pre QC No. JARD-23-24270 (PQ); Reviewed: 22-Dec-2023, QC No. JARD-23-24270; Revised: 29-Dec-2023, Manuscript No. JARD-23-24270 (R); Published: 05-Jan-2024, DOI: 10.35248/2155-9546.24.14.826
Abstract
Mycotoxins are secondary metabolites produced by different types of fungi. They are frequently present in fish feed ingredients and may negatively impact fish farming operations.
In this study aquafeed ingredients collected from suppliers in Greece were spectrally analyzed with Fourier-transform Near Infrared Spectroscopy (FT-NIR). Mycotoxin concentration was indirectly estimated by measuring the spectral absorption from organic compounds present in the samples.
In the examined samples, Fumonisin B1 (FB1), Fumonisin B2 (FB2), Zearalenone (ZEN), and Deoxynivalenol (DON) were measured as the predominant mycotoxins, whereas Aflatoxin B1 (AFB1) and Ochratoxin A (OTA) were not detected. Notably, all mycotoxin concentrations in aquafeed ingredients remained well below the Maximum Permitted Limits (MPL), affirming the safety of aquaculture feeds used in Greece in compliance with the relevant legislation.
This underscores the importance of continuous monitoring of fish feed ingredients, given the presence of mycotoxins at low concentrations that may pose a threat to animal health. Moreover, the application of FT-NIR confirms that it is a valuable analytical tool for contaminant detection, offering distinct advantages compared to traditional analytical methods, including speed, cost-effectiveness, safety, and simultaneous analysis of multiple parameters.
Keywords
Mycotoxins; Detection; Aquaculture; FT-NIR spectroscopy
Introduction
The global expansion of aquaculture has generated an increased demand for fish feeds, playing a pivotal role in ensuring optimal fish nutrition and health. In response to the decreasing availability of fishmeal, there is a growing reliance on cereals as a substitute. Nevertheless, the sustainability of this practice is questionable as fishmeal shortages persist. Consequently, plant ingredients have been included into fish diets, but they frequently harbor different types of mycotoxins, presenting a significant challenge. The pervasive threat of mycotoxins poses a substantial constraint to animal production systems, representing a global concern for both the livestock industry and the safety of the feed supply chain.
Mycotoxins are secondary metabolites that are produced by different types of fungi and are frequently present in agricultural goods intended for animal feed. These chemicals may be harmful for both consumers and livestock. Fungal contamination of agricultural raw material can occur during growth, before harvest, or during storage under unsuitable humidity and/or temperature circumstances [1]. Plant-based feed materials such as soybean meal, rapeseed/canola meal, maize/ corn, wheat bran, wheat, and barley are increasingly included in aquafeeds substituting marine ingredients that are continuously depleting as the result from reduced capture fisheries [2]. The most prevalent contaminants found in animal feed are Aflatoxin B1 (AFB1), Fumonisin B1 (FB1), Zearalenone (ZEN), and Deoxynivalenol (DON) [3]. AFB1 is produced by both Aspergillus flavus and A. parasiticus and it is the only mycotoxin subject to regulatory limits under European Union legislation [4]. Fusarium mycotoxins of great importance include Fumonisins (FB1, FB2, and FB3) and are primarily produced by F. proliferatum and F. verticillioides; DON and ZEN which is classified as a xenoestrogen are primarily synthesized by F. graminearum and F. culmorum [4]. The distinct characteristics and regulatory attention associated with each one underscore the importance of ongoing research and regulatory measures to ensure the safety of food and feed products. FBs and DON are two of the most commonly found mycotoxins in fish feed at elevated concentrations [5]. These mycotoxins have the potential to negatively impact fish farm operations, potentially resulting in large financial losses from increased mortality, decreased production, and increased disease susceptibility [6].
The Directive 2002/32/EC of the European Parliament and European Council (May 7, 2002), on undesirable substances, only regulates the AFB1. For fish species, the maximum permitted concentration in feed materials for AFB1 is 20 µg/kg, and for fish feed for consumption is 10 parts per billion (ppb) [7]. The European Commission (EC) has only set suggested limits for the presence of other significant mycotoxins in feedstuff and feed, such as DON, ZEN, T-2 and HT-2 toxin, FB1 and FB2 [8–10]. Of these suggested limits, fish species are directly referenced only in values for FB1 and FB2. European Commission has suggested a 10000 µg/kg limit for fish feeding material that are complementary and comprehensive to summarize FB1 and FB2. Except for maize byproducts, the recommended maximum concentration for DON in cereal and cereal products is set at 8000 µg/kg; for complementary and complete feeding stuff, the advised limit is 5000 µg/kg. Similarly, excluding maize byproducts, the recommended limits for ZEN stand at 2000 µg/kg for cereal and cereal products. Furthermore, the acceptable limit for Ochratoxin A (OTA) in cereal and cereal products is 250 µg/kg. For T-2 and HT-2 toxins identified in cereal and cereal products (excluding oat bran), the recommended concentration is 500 µg/kg, whereas for ergot alkaloids present in feed containing unground cereal, the advised level is 1000 µg/kg. These regulatory standards serve as crucial benchmarks in ensuring the safety and quality of cereal-based products and feed materials.
Numerous analytical techniques for identifying mycotoxins in aquafeeds and their constituents have been documented. Being the most widely used method for multianalyte analyses, High Performance Liquid Chromatography (HPLC) is extensively used for mycotoxin determination as a highly sensitive, specific, and dependable tool for detecting contaminants in food [11,12]. Several immunological techniques, such as the Enzyme-Linked Immunosorbent Assay (ELISA), have been also documented together with Biosensors used to analyze mycotoxin levels in a range of aquafeed raw materials [13].
Optical techniques like Near Infrared Spectroscopy (NIR) have significantly attributed in the quest for quick procedures for the measurement of components in food samples [14]. These techniques for detecting mycotoxins need small samples and minimal technical know-how. Additionally, unlike conventional chemical analytical methods, these techniques are inexpensive and do not require complicated sample pre-treatment. The most common method for identifying mycotoxin contamination in crops is spectroscopy [15]. This technology relies on indirect measurements due to the complexity of the obtained spectral data. When mycotoxin contamination by fungi and subsequent fungal infection arises, the chemical makeup of food used in the manufacturing sector is changed. NIR has the capacity to examine these variations within particular ranges and develop predictive models using qualitative or quantitative techniques [15–17]. Therefore, in order to retrieve the analytical information from the relevant spectra, methods requiring calibrations using mathematical models and multivariate statistical tools must be used [18].
Consumption of mycotoxin-contaminated feeds by animals may result in adverse health effects, either directly or indirectly. The occurrence and severity of mycotoxicosis are contingent upon mycotoxin concentration and the simultaneous presence of multiple mycotoxins. However, quantifying the precise impact of mycotoxin contamination in fish is challenging due to the lack of specificity regarding mycotoxin-related clinical signs. Understanding the occurrence of mycotoxins, particularly in field conditions, remains crucial even with the implementation of good manufacturing practices. This knowledge gap is particularly pronounced in terrestrial animals, while information regarding mycotoxin presence in fish feeds is limited. The profile of mycotoxin contaminants in fish feeds depends on environmental conditions, geographic origin, and the cultivation practices of plants. This is the first study on Greek aquaculture, a sector that holds the leading position in terms of volume and value in EU aquaculture production. This research will study the prevalence of mycotoxins that could impact Mediterranean aquaculture, guiding the formulation of effective measures to mitigate adverse effects. Tailoring mycobinding strategies to specific mycotoxin types is essential, and this knowledge will enable the development of preventive treatment to reduce fungal prevalence in crops. The insights gained from this study will contribute significantly to the advancement of aquaculture practices and the safeguarding of the Mediterranean aquaculture industry.
The aim of the present study was to assess mycotoxin contamination in Greek aquafeed ingredients and to evaluate FT-NIR spectroscopy as a validated technique for continuous inspection. Specifically, the mycotoxin contamination rates of raw materials used in fish feed production in Greek aquaculture were estimated. The conducted results will lead to conclusion regarding the assurance of farmed fish and consumers’ health. Furthermore, the importance of Fourier Transform-Near Infrared Spectroscopy (FT-NIR) technology, as a useful analytical tool for animal feed ingredient analysis, will be highlighted.
Materials and Methods
Aquafeed ingredient samples
124 aquafeed ingredient samples (approximately 2 kg per sample) comprising of barley (2), soybean meal (32), wheat (46), and corn (44) collected from various Greek suppliers, were analyzed between 2022 and 2023. Following collection, samples were refrigerated for less than 15 days until further analysis.
Pre-treatment of samples
To create a representative laboratory sample, quartering method was applied to ensure representative sampling [19]. Every sample was placed on a spotless, non-absorbent flat surface upon its arrival in the laboratory. A cone-like shape was created from the aggregate sample. Next, using a quartering divider, the top of the cone was flattened and divided into four equal quarters. Following the removal of two opposing quarters (making sure that all dust and other tiny particles are also wiped away each time), the remaining pair was combined and formed into a different cone. This process was repeated until the remaining quarters were down to 100 g. A portion of each sample (100 g) was then ground in an ultra-centrifugal mill (ZM 200 Ultra Centrifugal Mill, Retsch GmbH, Haan, Germany) with 1 mm diameter sieve.
Fourier Transform-Near Infrared (FT-NIR) analysis and acquisition
In the field of mycotoxin detection, Infrared (IR) spectroscopy- based methods stand out as among the most promising due to their ability to work with small samples and requiring limited technical expertise. These techniques not only offer cost-effective solutions but also eliminate the need for extensive sample pre- treatment, streamlining the detection process. Mycotoxin contamination in crops is routinely identified through spectroscopic approaches, with Near-Infrared (NIR) spectroscopy playing a pivotal role. NIR spectroscopy identifies molecular overtones and combined vibrations of chemical bonds. All spectra are challenging to decipher for specific constituents present in a sample. Chemometrics are instrumental in direct information extraction from the data involving three phases: spectral pre-processing, multivariate model construction for calibration, and model transfer. Qualitative and quantitative methodologies are used for NIR spectroscopic model development.
The quantity of 100 g ground sample was transferred to an FT-NIR spectrometer (TANGO FT-NIR spectrometer, Bruker Optics, Ettlingen, Germany) which emitted electromagnetic radiation in the infra-red region to perform spectral acquisition.
The quantity of 100 g ground sample was transferred to an FT-NIR spectrometer (TANGO FT-NIR spectrometer, Bruker Optics, Ettlingen, Germany) which emitted electromagnetic radiation in the infra-red region to perform spectral acquisition.
For recording of FT-NIR spectra with diffuse reflectance, the milled samples were placed in a glass vial (52.0 mm x 22 mm x 1.2 mm, Nipro Diagnostics Germany GmbH, Ratingen,Germany) and positioned at the beam outlet of a TANGO NIR spectrometer (Bruker Optics, Ettlingen, Germany). All measurements were performed at room temperature. FT-NIR spectra were then recorded in total reflectance mode in the wave number range between 12500 and 3600 cm-1 at a resolution of 16 cm-1. The rotating sphere macro sample was the cell type used for reading solid samples; each spectrum was read in approximately 30 s. Background was acquired at the beginning of each measurement series and additionally when indicated by the spectrometer software. The FT-NIR spectrometer was remotely controlled using TANGO software (Bruker Optics, Ettlingen, Germany).
Measured spectra were uploaded to Olimpo platform (Pegasus Science) and the result of mycotoxin concentration in each sample was acquired. The method’s Limits of Detection (LOD) for the acquired mycotoxins were 5 µg/kg for AFB1, 200 µg/kg for the sum of FB1 and FB2, 350 µg/kg for DON, 30 µg/kg for ZEN and 10 µg/kg for OTA.
Mycotoxin occurrence
Mycotoxin occurrence (%) in each aquafeed ingredient was calculated by the ratio of positive samples to all samples analyzed. Aquafeed samples with mycotoxin concentration above method’s LOD were considered as positive whereas mycotoxin concentration below LOD was identified as negative.
Statistical analysis
Statistical analysis of occurrence data for mycotoxins involved the utilization of the χ2 statistical test [20]. Non-normally distributed occurrence data were assessed using the χ2 statistical test to determine independence in the distributions of contaminated samples. The null hypothesis of independence was rejected if the P-value was out of the significance threshold of 0.05, indicating a lack of distinction between positive or negative associations between two species. Contaminated samples were considered associated in the cases that the null hypothesis of independence was rejected. The comparison of mycotoxin rates was conducted at two distinct levels. Evaluation of differences between detectable (positive) and non-detectable (negative) values for each tested mycotoxin was performed, with statistically significant differences indicated in the graph through lowercase letters. Additionally, a comparison was made between the rates of all detected mycotoxins within a specific raw material, with statistically significant differences highlighted in capital letters on the graph. To accurately identify points of statistical significance, the data analysis involved partitioning the initial χ2 into several individual tests (2×2). In the assessment of mycotoxin concentrations in all samples, Student’s t-test was employed to compare mean FB concentrations between corn and soy samples. Furthermore, Analysis of Variance (ANOVA) and Tukey’s post hoc analysis were applied as statistical tests to assess differences in mean concentrations of DON and ZEN among various raw materials. The SPSS Statistics 26 package was utilized in all instances, with a significance level set at P<0.05.
Results
In the analyzed corn samples, the predominant mycotoxins were FB1-FB2, detected in 75% of the samples, followed by ZEN at 47.73% (Figure 1). Both Fumonisins and ZEN occurrence were significantly higher compared to DON (p<0.001) which exhibited a significantly lower occurrence at 9.09%. AFB1 and OTA were not detected in examined samples. All barley samples exhibited contamination with DON and ZEN mycotoxins, while OTA was absent, with concentrations noted below the limit of detection. In wheat samples, a significantly higher rate was observed for DON (78.26%) compared to ZEN-contaminated samples (21.74%), while OTA was not detected in any wheat sample. In soy samples, ZEN was detected in 31.25% and FB1 and FB2 exhibiting lower occurrence (6.25%). AFB1, DON, and OTA were not detected in any soy sample. Mycotoxin occurrence (%) in each aquafeed ingredient is shown in Figure 1.
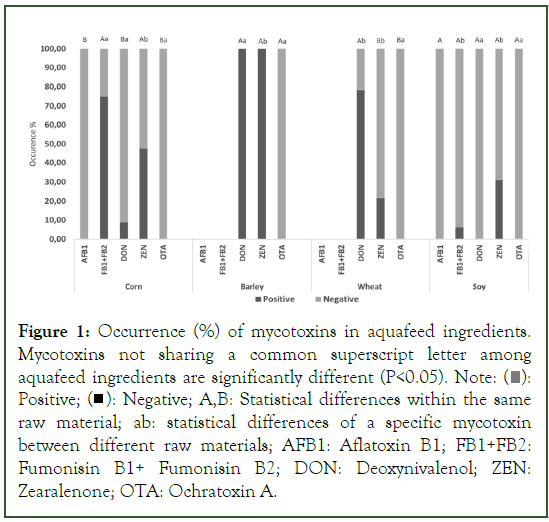
Figure 1: Occurrence (%) of mycotoxins in aquafeed ingredients.
Mycotoxins not sharing a common superscript letter among
aquafeed ingredients are significantly different (P<0.05). Note:  Positive;
Positive;  Negative; A,B: Statistical differences within the same raw material; ab: statistical differences of a specific mycotoxin
between different raw materials; AFB1: Aflatoxin B1; FB1+FB2:
Fumonisin B1+ Fumonisin B2; DON: Deoxynivalenol; ZEN:
Zearalenone; OTA: Ochratoxin A.
Negative; A,B: Statistical differences within the same raw material; ab: statistical differences of a specific mycotoxin
between different raw materials; AFB1: Aflatoxin B1; FB1+FB2:
Fumonisin B1+ Fumonisin B2; DON: Deoxynivalenol; ZEN:
Zearalenone; OTA: Ochratoxin A.
Concerning DON concentrations, the range varied from 463.5 µg/kg in corn to 1007.2 µg/kg in wheat. High concentrations were also identified in barley (894.5 µg/kg), while soy samples concentrations remained below the detection limit. All samples were found to be within recommended limits.
FB1-FB2 were identified in corn and soy, with corn recording significantly higher concentration values (953 µg/kg) compared to soy (288.5 µg/kg). ZEN was detected across all examined samples, with significantly lower concentration values found in corn (36.28 µg/kg) and wheat (37.72 µg/kg). Barley exhibited a mean ZEN concentration of 47 μg/kg, while soy samples displayed a significantly higher concentration of 60.5 µg/kg. All mycotoxin concentrations in aquafeed ingredients detected were well below MPL with several mycotoxins below the LOD. Mycotoxin concentrations (mean, max) in all examined samples and maximum permitted limits are shown in Table 1.
| Corn | Barley | Wheat | Soy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPL | Mean | Max | Mean | Max | Mean | Max | Mean | Max | |
| AFB1 | 20 | <5 | <5 | Na | Na | Na | Na | <5 | <5 |
| FB1,2 | 10,000 | 953 ± 105.7a | 2169 | Na | Na | Na | Na | 288.5 ± 5.5b | 294 |
| DON | 8,000 | 463.5 ± 63.7a | 654 | 894.5 ± 289.5b | 1184 | 1007.2 ± 82.2b | 2338 | <350 | <350 |
| ZEN | 2,000 | 36.28 ± 1.2a | 51 | 47 ± 2.0b | 49 | 37.72 ± 2.0a | 46 | 60.5 ± 5.3c | 83 |
| OTA | 250 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Aw | 0.6 | 0.61 | 0.55 | 0.55 | 0.55 | 0.62 | 0.58 | 0.67 | |
Note: MPL- Maximum Permitted Limits; Na- Non-analyzed mycotoxins; AW: Water Activity; AFB1: Aflatoxin B1; FB1+FB2: Fumonisin B1+ Fumonisin B2; DON: Deoxynivalenol; ZEN: Zearalenone; OTA: Ochratoxin A; a,b: Statistical differences of a specific mycotoxin between different raw materials, Mycotoxins not sharing a common superscript letter among aquafeed ingredients are statistically significant different (p<0.05).
Table 1: Mean mycotoxin concentration ± standard error of positive analyzed samples. Maximum values in aquafeed ingredients (μg/ kg) are presented for corn, barley, wheat, and soy samples, alongside non-analyzed mycotoxins (Na), Maximum Permitted Limits (MPL), and Water Activity (Aw). Mycotoxins not sharing a common superscript letter among aquafeed ingredients are statistically significant different (p<0.05).
According to the survey data, in most samples (49.19%), the co-occurrence of two mycotoxins was noted, while 22.59% of the samples were contaminated with a single mycotoxin, and 0.8%of the samples exhibited contamination with three mycotoxins. Approximately 22.59% of the samples were found to be devoid of mycotoxin contamination. Percentage of mycotoxins co- occurrence is presented in Figure 2.
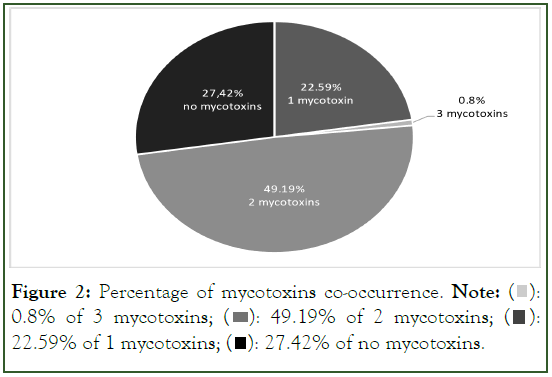
Figure 2: Percentage of mycotoxins co-occurrence. Note: 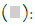 0.8% of 3 mycotoxins;
0.8% of 3 mycotoxins; 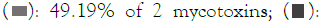 22.59% of 1 mycotoxins;
22.59% of 1 mycotoxins; 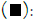 27.42% of no mycotoxins.
27.42% of no mycotoxins.
Discussion
The global issue of mycotoxin contamination in animal production including aquaculture stems from the unpredictable contamination of feed ingredients by fungi, with the inclusion of plant-based protein in fish feed identified as a specific contributing factor. Both consumers and farmed animals are at risk for health problems due to the presence of mycotoxins in agricultural products used to feed animals.
In the current study, AFB1 was found to be below the limit of detection in all examined samples, providing assurance regarding the safety of the samples with respect to public health concerns. Notably, the study identified FB1-FB2 in 44.8% of all samples, with concentrations reaching a maximum of 2169 μg/kg. Additionally, DON was present in 33.9% of the samples, and the maximum concentration observed was 2338 μg/kg. Furthermore, ZEN was detected in 34.7% of the examined samples, with the highest concentration recorded at 83 μg/kg. In Europe, the presence of AFB1 and FB1-FB2 in aquafeeds has previously been documented, with reported occurrence rates of 17% and 30%, respectively. Additionally, a substantial 67% of the tested aquafeed samples exhibited positive results for ZEN and DON [21]. Moreover, raw materials used in aquafeed formulations, including wheat, corn, and soybean meal, displayed positivity for all assessed mycotoxins. Specifically, AFB1 was detected at rates ranging from 2% to 6%, while FB1- FB2 exhibited rates ranging from 26% to 70%. ZEN was identified in raw materials at rates ranging from 5% to 16%, and DON was prevalent at rates varying from 11% to 47% [6]. OTA was consistently identified in all examined samples, falling below the limit of detection of the analytical method (<10 μg/kg). In contrast, other surveys focusing on aquafeeds, and raw materials reported an occurrence of OTA in 67% of the examined aquafeed samples, with occurrence rates in raw materials ranging from 9% to 12%. European fish feed samples exhibited relatively low average concentrations of AF (0.43 μg/kg) and OTA (1.53 μg/kg). However, higher average contamination values were observed in European samples for ZEN (118.01 μg/kg), DON (165.61 μg/kg), and Fumonisins (3415.92 μg/kg) compared to their Asian counterparts [21].
Corn samples exhibited low mycotoxin contamination, with concentrations of various mycotoxins well below the maximum permitted limits. The mean concentration of FB1-FB2 was recorded at 953 μg/kg, reaching a maximum value of 2169 μg/ kg. Notably, the contamination values reported in this study were lower than those previously published for European corn samples, which showed a mean concentration of 2496 μg/kg and a maximum of 49,347 μg/kg for FB1, along with 7944 μg/kg for FB2. Furthermore, ZEN and DON mean concentrations were 36.28 μg/kg with a maximum of 51 μg/kg and 463.5 μg/kg with a maximum of 654 μg/kg, respectively. These values were also lower compared to those reported in other studies, with 165 μg/ kg (maximum 1282 μg/kg) for ZEN and 826 μg/kg (maximum 10,020 μg/kg) for DON [6]. AFB1 and OTA were below the limit of detection in all corn samples, although these mycotoxins have been previously detected at concentrations of 12 μg/kg and 24 μg/kg, respectively [6].
In barley samples, the prevalent mycotoxins were DON and ZEN, with mean concentrations of 894.5 μg/kg (maximum 1184 μg/kg) and 47 μg/kg (maximum 49 μg/kg), respectively, while OTA was below the limit of detection. Unfortunately, these values cannot be directly compared, as data regarding barley samples in aquafeeds are not currently available.
Wheat samples exhibited contamination, particularly with a high mean concentration of DON at 1007.2 μg/kg (maximum 2338 μg/kg) [6]. ZEN showed a mean concentration of 37.72 μg/kg (maximum 46 μg/kg), and OTA was below the limit of detection. Comparative analysis with previously presented European wheat samples revealed higher contamination values for DON (470 μg/kg, maximum 1330.8 μg/kg) and ZEN (64 μg/ kg, maximum 738 μg/kg), while OTA was detected at a mean concentration of 6 μg/kg (maximum 45 μg/kg) [6].
In soybean meal samples, the maximum concentrations of FB1-FB2 and ZEN reached mean concentrations of 288.5 μg/kg (maximum 294 μg/kg for FB1) and 60.5 μg/kg (maximum 83 μg/kg), respectively. Concentrations of DON and OTA were consistently below the limit of detection of the analytical method. It is worth to mention that soybean meal in aquafeeds has been previously reported to be contaminated with these mycotoxins at higher concentrations in earlier studies [5]. Specifically, FB1-FB2 were noted at mean concentrations of 371 μg/kg (maximum 1462 μg/kg) and 83 μg/kg (maximum 424 μg/ kg), respectively. Samples contaminated with ZEN exhibited a mean concentration of 81 μg/kg (maximum 354 μg/kg). Moreover, tested samples were positive for DON and OTA, with mean concentrations of 85 μg/kg (maximum 543 μg/kg) and 3 μg/kg (maximum 7 μg/kg), respectively [6].
Mycotoxins have been emerged as a new research area in aquaculture, while during recent years an effort to elucidate their implications on fish health and welfare is documented. The contamination of plant-based ingredients with mycotoxins presents a global challenge, causing impacts on both animal production and public health. Animal health can be significantly impacted resulting in reduced growth and productivity, toxicity, hepatic problems, immunotoxicity, and functional abnormalities [22–24]. Even at low concentrations, mycotoxins induce a broad spectrum of health issues, underscoring the necessity for early detection and subsequent treatment or rejection of contaminated feeds. To mitigate the economic implications associated with mycotoxin-contaminated fish feeds, increased monitoring practices are recommended to ensure the safety of farmed fish and the proposed techniques herein contribute to this objective.
In addition, this study provides information on the predominant mycotoxins expected in raw materials used in aquafeeds. Gaining knowledge about specific mycotoxin- contaminating feeds becomes crucial for formulating tailored protective strategies, recognizing that different preventive measures must be applied for each individual mycotoxin. AFB1, DON, ZEN, and FB1 are the four predominant mycotoxins found in animal feeds most frequently [3]. As ZEN interferes with animal reproduction through its estrogenic activity, it can cause reproductive issues like hyperestrogenism, sterility, and even abortions [25]. These mycotoxins have the potential to negatively impact fish farm operations, potentially resulting in large financial losses derived from mortality, decreased productivity, and increased disease susceptibility [6]. In all the samples presently examined, the occurrence of various mycotoxins was observed; however, all results remained below the maximum permitted limits. Notably, in Greece, the situation appears more favourable compared to other countries because of the lower mean and maximum concentrations of mycotoxins detected [6,21]. The findings underscore the pervasive nature of mycotoxin contamination in aquafeed ingredients, highlighting the necessity for stringent monitoring and mitigation strategies within aquaculture practices. Moreover, these results highlight the considerable variability in mycotoxin levels in fish feed samples across regions, demanding continual surveillance efforts to ensure the safety and compliance of aquafeed formulations. Similarly, the variability in mycotoxin levels across different grains is underscored, necessitating ongoing monitoring for a comprehensive risk assessment.
Considering that one of the biggest challenges confronting the world today is the safety of food and feed, the European Union (EU) has prioritized this issue, as reflected in numerous legislative documents establishing maximum recommended levels of mycotoxin contamination in food and feed [9,26]. However, attention has been drawn to the observation that EU has set relatively high maximum recommendation levels for mycotoxin detection in aquafeed ingredients [27,28]. This has prompted suggestions from several authors to enforce more stringent legislation in order to ensure the highest quality and safety standards. Such measures are imperative to prevent mycotoxin contamination in food and feed from resulting in severe health problems and financial losses within animal production.
Conclusion
A comprehensive study involving four distinct types of animal feed ingredients (corn, barley, wheat, and soybean meal) from various Greek suppliers was conducted using the FT-NIR analytical technique to detect mycotoxins (AFB1, ZEN, DON, FB1-FB2, and OTA). All identified mycotoxins were found to be within established safe limits, with minor variations observed among the different ingredients. Nevertheless, it is well- established that even at low concentrations, mycotoxins can give rise to significant health and welfare issues in both terrestrial and aquatic farmed animals. This study represents the first research on data concerning the contamination of raw materials used in aquafeeds within the context of Greek Mediterranean aquaculture. The insights obtained from this research will be instrumental for aquafeed industries in monitoring raw materials. The report outlines the most frequently observed mycotoxins in each raw material, providing additional information about the predominant mycotoxins associated with each specific material. This knowledge serves as a valuable resource for enhancing the efficacy of monitoring practices within the aquafeed industry.
Hence, the ongoing monitoring, coupled with a straightforward and rapid mycotoxin detection method, is considered crucial. The application of FT-NIR in mycotoxin determination is facilitated by its established use in measuring the components of food samples in aquafeed industries. This recognition implies the potential for its expanded utilization. Utilizing FT-NIR for mycotoxin analysis offers an accessible, cost-effective, and rapid method for continuous monitoring of raw materials, ensuring supervision and safety in animal feed production, including fish feeds. Its application extends to various aspects of animal feed production, particularly in aquafeed manufacturing.
Notably, in Greece, the fish feeds used in aquaculture conform to the pertinent legislation, displaying lower mean and maximum contamination levels when compared to other countries, finding that underscores the high standards within the aquaculture industry.
Author Contributions
• Eleni Golomazou, George Rigos, Ioannis Nengas, Panagiota Panagiotaki: Conceptualization, Data curation.
• Eleni Golomazou, Sofia Vardali, Christina Papadouli, Myrto Maniaki, Theodoros Karatzinos: Investigation, Methodology.
• Eleni Golomazou and George Rigos: Project administration
• Eleni Golomazou: Supervision, Literature search, Validation, Visualization, Writing - original draft, review and editing revisions.
• Sofia Vardali, Christina Papadouli, Myrto Maniaki, Theodoros Karatzinos, George Rigos, Ioannis Nengas, Panagiota Panagiotaki: Literature search, Writing-original draft, review and editing revisions.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
This research is co-funded by Greece and the European Union under the Fisheries and Maritime Operational Program 2014– 2020 (75% EMFF contribution, 25% National Contribution).
Funding
This research is co-funded by Greece and the European Union under the Fisheries and Maritime Operational Program 2014– 2020 (75% EMFF contribution, 25% National Contribution).
References
- Kokou F, Fountoulaki E. Kokou F, Fountoulaki E. Aquaculture waste production associated with antinutrient presence in common fish feed plant ingredients. Aquaculture. 2018;495:295-310.
- Troell M, Naylor RL, Metian M, Beveridge M, Tyedmers PH, Folke C, et al. Does aquaculture add resilience to the global food system?. Proc Natl Acad Sci USA. 2014;111(37):13257-13263.
[Crossref] [Google Scholar] [PubMed]
- Lee HJ, Ryu D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J Agric Food Chem. 2017;65(33):7034-7051.
[Crossref] [Google Scholar] [PubMed]
- Twarużek M, Skrzydlewski P, Kosicki R, Grajewski J. Mycotoxins survey in feed materials and feedingstuffs in years 2015–2020. Toxicon. 2021;202:27-39.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves RA, Schatzmayr D, Albalat A, Mackenzie S. Mycotoxins in aquaculture: Feed and food. Rev Aquac. 2020;12(1):145-175.
- Koletsi P, Schrama JW, Graat EA, Wiegertjes GF, Lyons P, Pietsch C. The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of deoxynivalenol (don) on the health and growth of farmed fish species—A review. Toxins. 2021;13(6):403.
[Crossref] [Google Scholar] [PubMed]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. 2002.
- Beuerle T, Benford D, Brimer L, Cottrill B, Doerge D, Dusemund B, et al. Scientific Opinion on Ergot alkaloids in food and feed. EFSA Journal. 2012;10(7):2798.
- Recommendation C. 576/EC—on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Off. J. Eur. Union. 2006;229:7-9.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed. EFSA Journal. 2011 ;9(12):2481.
- Krska R, Stubbings G, Macarthur R, Crews C. Simultaneous determination of six major ergot alkaloids and their epimers in cereals and foodstuffs by LC–MS–MS. Analytical and Bioanalytical Chemistry. 2008;391:563-576.
- Soleimany F, Jinap S, Faridah A, Khatib AJ. A UPLC–MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food control. 2012;25(2):647-653.
- Vardali S, Papadouli C, Rigos G, Nengas I, Panagiotaki P, Golomazou E. Recent Advances in Mycotoxin Determination in Fish Feed Ingredients. Molecules. 2023;28(6):2519.
[Crossref] [Google Scholar] [PubMed]
- Bevilacqua M, Bucci R, Materazzi S, Marini F. Application of Near Infrared (NIR) spectroscopy coupled to chemometrics for dried egg-pasta characterization and egg content quantification. Food Chem. 2013;140(4):726-734.
[Crossref] [Google Scholar] [PubMed]
- McMullin D, Mizaikoff B, Krska R. Advancements in IR spectroscopic approaches for the determination of fungal derived contaminations in food crops. Anal Bioanal Chem. 2015;407:653-660.
[Crossref] [Google Scholar] [PubMed]
- Shi H, Yu P. Exploring the potential of applying infrared vibrational (micro) spectroscopy in ergot alkaloids determination: Techniques, current status, and challenges. Anal Bioanal Chem. 2018;53(5):395-419.
[Crossref] [Google Scholar] [PubMed]
- Kos G, Sieger M, McMullin D, Zahradnik C, Sulyok M, Öner T, et al. A novel chemometric classification for FTIR spectra of mycotoxin-contaminated maize and peanuts at regulatory limits. Food Addit Contam Part A. 2016;33(10):1596-1607.
[Crossref] [Google Scholar] [PubMed]
- Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. Journal of pharmaceutical and biomedical analysis. 2007;44(3):683-700.
[Crossref] [Google Scholar] [PubMed]
- Campos-M M, Campos-C R. Applications of quartering method in soils and foods. Int. J. Eng. Res. Appl. 2017;7(1):35-39.
- Albrecht P. On the correct use of the chi-square goodness-of-fit test. Scand Actuar J. 1980;1980(3):149-160.
- Gonçalves RA, Naehrer K, Santos GA. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev Aquac. 2018;10(2):263-280.
- Santos Pereira C, C. Cunha S, Fernandes JO. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins. 2019;11(5):290.
[Crossref] [Google Scholar] [PubMed]
- Oliveira M, Vasconcelos V. Occurrence of mycotoxins in fish feed and its effects: A review. Toxins. 2020;12(3):160.
[Crossref] [Google Scholar] [PubMed]
- Zain ME. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15(2):129-144.
- da Rocha ME, Freire FD, Maia FE, Guedes MI, Rondina D. Mycotoxins and their effects on human and animal health. Food control. 2014;36(1):159-165.
- Commission Recommendation (EU) 2016/1319. 2016. Amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food.
- Yunus AW, Blajet-Kosicka A, Kosicki R, Khan MZ, Rehman H, Böhm J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult Sci. 2012;91(4):852-861.
[Crossref] [Google Scholar] [PubMed]
- Grenier B, Applegate TJ. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins. 2013;5(2):396-430.
[Crossref] [Google Scholar] [PubMed]
Citation: Vardali S, Papadouli C, Maniaki M, Karatzinos T, Rigos G, Nengas I, et al. (2024) Detection of Mycotoxins in Aquaculture Feed Ingredients Using a Rapid FT-NIR Method. J Aquac Res Dev. 14:826.
Copyright: © 2024 Vardali S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research is co-funded by Greece and the European Union under the Fisheries and Maritime Operational Program 2014�??�?�¢??2020 (75% EMFF contribution, 25% National Contribution).

