Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 17, Issue 6
Comparative Bioavailability Study of Ultra Q 300 and COQ300 Coenzyme Q10 in Healthy Adults.
SM Rahman1, Honey Qureshi1, Poorneshwar Sawant2, Sunil Kulkarni2, Ravindra Mote3* and Parikshit Champanerkar32Department of Medicine, Meyer Organics Private Limited, Maharashtra, India
3Department of Bioanalytical, Bio-Prospera Clinical Research Pvt. Ltd, Maharashtra, India
Received: 01-Dec-2025, Manuscript No. JBB-25-30444; Editor assigned: 03-Dec-2025, Pre QC No. JBB-25-30444 (PQ); Reviewed: 17-Dec-2025, QC No. JBB-25-30444; Revised: 24-Dec-2025, Manuscript No. JBB-25-30444 (R); Published: 31-Dec-2025
Abstract
Purpose: In this study, two Coenzyme Q10 (CoQ10) formulations-Ultra Q 300 (test product) and other brand having similar composition (reference product)-were compared for bioavailability in healthy adult volunteers under fasting condition. Evaluating the safety and tolerability of a single oral administration of Ultra Q 300 at a dose of 300 mg was the secondary objective.
Methods: Fourteen healthy male volunteers between the age range of 18 and 45 participated in an open-label, randomized balanced, two- treatment, two- sequence, two- period, two-way crossover bioavailability research. During a 15-day washout interval in between doses, participants received a single 300 mg dosage of either Ultra Q 300 or other brand having similar composition while fasting. To assess Pharmacokinetic (PK) characteristics, such as Cmax and Tmax, 25 blood samples were taken over an interval of 96 hours after the dose. A validated LC-MS/MS method was used to determine the concentrations of plasma CoQ10. Clinical examinations, vital sign monitoring, adverse event reporting, and laboratory testing were all included in the safety assessments.
Results: All 14 participants completed the study without protocol deviations or dropouts. The mean Cmax for Ultra Q 300 was 1345.129 ng/mL versus 1181.796 ng/mL for other brand having similar composition, with Ultra Q 300 showing an average 12.93% higher plasma concentration. Tmax ranged from 5.5 to 7.5 hours for both formulations, indicating comparable absorption rates. The 90% confidence interval of 80%-125% consisted of all significant pharmacokinetic parameters, meeting the regulatory standards for bioavailability. No adverse events or safety concerns were reported during the trial.
Conclusion: Ultra Q 300 demonstrated improved bioavailability over the other brand having similar composition, shown by a higher mean Cmax and similar Tmax, with no additional safety concerns. This suggests a pharmacokinetic advantage and positions Ultra Q 300 as a potentially more effective and safer option for daily Coenzyme Q10 supplementation.
Keywords
Coenzyme Q10; Bioavailability; Safety; Healthy volunteers; Pharmacokinetic parameters
Introduction
Every human cell contains Coenzyme Q10 (CoQ10), also known as ubiquinone or ubidecarenone, a fat-soluble, vitamin-like molecule which emphasizes its widespread distribution. Although it is mostly found in the mitochondria, it is also found in cell membranes and is transported into the bloodstream via lipoproteins [1]. CoQ10 is a strong antioxidant that protects cells from oxidative damage when it is reduced to ubiquinol [2]. It functions as a coenzyme for mitochondrial complexes I, II, and III, which are in the role of synthesizing Adenosine Triphosphate (ATP), which is responsible for around 95% of the energy produced by the cell. Because free radicals are commonly produced in the inner mitochondrial membrane, CoQ10's antioxidant activity is crucial there. It also promotes antioxidant protection in plasma membranes [3].
In the United States, CoQ10 is available over the counter as a dietary supplement, as it may help boost the production of key antioxidants like superoxide dismutase-an enzyme that plays an important role in reducing vascular oxidative stress, particularly in people with high blood pressure. Additionally, CoQ10 helps decrease lipid peroxidation by reducing the presence of harmful pro-oxidant substances. By improving blood circulation and safeguarding blood vessels by preserving nitric oxide, it also promotes cardiovascular health [4,5].
The body naturally produces CoQ10, and it is found in highest concentrations in organs that require significant energy, including the heart, kidneys, liver, muscles, pancreas as well as thyroid gland. However, CoQ10 levels in these tissues tend to decrease with age [6]. Although it is found in some foods, such as meat and fish, the dietary amounts are relatively limited. Therefore, many nutrition experts consider CoQ10 a valuable option for supplementation [7].
CoQ10 is a fat-soluble compound with a large molecular weight, which leads to slow and limited absorption. However, its uptake improves when consumed with fatty foods. After ingestion, the levels in the blood are usually reached between 5.8 and 8.1 hours, and it has a long half-life of over 30 hours. The bioavailability of CoQ10 depends on its formulation-solubilized forms like ubiquinol, liposomes, nano-capsules, and nanoemulsions are absorbed more efficiently. Once absorbed in the small intestine, CoQ10 is converted into its active form, ubiquinol, transported to the liver, and then released into the bloodstream via VLDL and LDL particles. It tends to accumulate in energy-demanding tissues such as the heart, liver, and muscles, highlighting its essential role in mitochondrial energy production. A second peak in blood levels may occur due to enterohepatic recycling, and the compound is mainly eliminated through bile and feces [4,8]
Coenzyme Q10 (CoQ10) supplementation underscores its essential role in supporting cellular energy production and protecting against oxidative stress. Numerous illnesses, such as metabolic, mitochondrial, neurological, and cardiovascular problems, have been connected to low levels of CoQ10. Taking CoQ10 as a supplement is generally regarded as safe and may help improve symptoms in several conditions, such as fibromyalgia, diabetes, cancer, and age-related diseases [9].
While CoQ10 is naturally found in the human body and is typically well-tolerated, occasional mild side effects have been observed, such as loss of appetite, nausea, vomiting, diarrhea, indigestion, dizziness, and headaches. Less common side effects include light sensitivity, irritability, heartburn, increased involuntary movements, and fatigue. In some cases, individuals taking 300 mg or more per day may experience elevated liver enzymes, though no evidence of liver toxicity has been reported. An early study suggested a potential interaction with warfarin; however, subsequent controlled research did not support this finding. People with bile duct obstruction should use CoQ10 with caution [4,8].
CoQ10 deficiency is associated with cardiomyopathies as well as degenerative diseases affecting muscles and nerve cells. Key clinical manifestations of CoQ10 deficiency include encephalomyopathy, severe infantile multisystem disorders, cerebellar ataxia, and leigh syndrome accompanied by growth delays, ataxia with hearing loss, and isolated muscle disorders. The most severe forms of CoQ10 deficiency in humans result from autosomal recessive mutations. These deficiencies are categorized as primary when the mutations directly impact genes involved in CoQ10 biosynthesis, or as secondary when they arise from other underlying genetic abnormalities [10].
Supplementation with Coenzyme Q10 (CoQ10) has been shown to consistently reduce oxidative stress and muscle damage, which promotes a quicker recovery from exercise. Nevertheless, it usually has a limited and erratic effect in enhancing aerobic performance. CoQ10 seems to be more helpful for recuperation than for improving overall athletic performance, even if some benefits have been observed in anaerobic or high-intensity activity [11].
Studies on Coenzyme Q10 (CoQ10) and its effects on exercise suggest that supplementation reliably lowers oxidative stress and muscle damage, which supports faster recovery. However, its impact on boosting aerobic performance is limited and varies between individuals. Although some improvements have been noted in anaerobic or high-intensity exercises, CoQ10 is generally more beneficial for recovery than for directly enhancing athletic performance [12].
Coenzyme Q10 (CoQ10) protects against oxidative damage and increases mitochondrial generation of energy, which helps control neurological disorders. It works most effectively in cases of primary CoQ10 deficiencies and is generally safe and welltolerated. Clinical outcomes have been inconsistent, despite experimental research suggesting potential benefits for diseases like Parkinson's, Alzheimer's, and ALS. Consequently, CoQ10 is regarded as a promising supportive therapy; nevertheless, further research is required to verify its efficacy in humans [13].
A meta-analysis of six studies including 1,529 women with diminished ovarian reserve revealed that CoQ10 supplementation prior to IVF/ICSI enhanced pregnancy rates, increased the number of retrieved oocytes, improved embryo quality, and positively influenced hormone levels. Additionally, it was associated with lower miscarriage rates and reduced medication usage. However, CoQ10 did not impact endometrial thickness [14].
Review of 11 small studies examining Coenzyme Q10 in heart failure revealed limited and inconsistent evidence. CoQ10 might help lower mortality rates, reduce hospitalizations, and improve certain symptoms such as NYHA classification and 6-minute walk distance. However, its impact on heart function Left Ventricular Ejection Fraction (LVEF), exercise capacity, and overall symptom scores was minimal or uncertain. Due to significant variability in the data, results could not be combined, and most studies had short follow-up periods and small participant numbers. Overall, more rigorous, high-quality research is necessary to confirm the potential benefits of CoQ10 in heart failure [15].
As oxidative stress, inflammation, and mitochondrial function are significant contributors in autoimmune illnesses, Coenzyme Q10 (CoQ10) has shown promise in managing these medical conditions. According to studies, CoQ10 may help reduce the symptoms of conditions like multiple sclerosis, fibromyalgia, lupus, and rheumatoid arthritis. Although the results are promising larger, more reliable clinical trials are required to confirm its efficacy, as the majority of current research is based on small-scale investigations [16].
A six-month study involving 60 patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MDASLD) demonstrated that high-dose CoQ10 decreased liver fat accumulation and enhanced vascular, endothelial, and heart functions, while also reducing blood pressure. These findings indicate that CoQ10 could help lower cardiovascular risk in MASLD patients with few side effects [17].
The objective of this study was to compare and evaluate the absorption rate and total bioavailability of the test formulation of Ultra Q 300 (Coenzyme Q10) capsules with the reference formulation of other brand having similar composition (Coenzyme Q10) capsules. Evaluating the safety and tolerability of a single Ultra Q 300 dosage in healthy adult volunteers while they were fasting was a secondary goal.
Materials and Methodology
Study materials
The test formulation, Ultra Q 300 (Co-enzyme Q10) capsules, was supplied by Meyer Organics Pvt. Ltd., 300 mg, while the reference formulation, other brand having similar composition (Co-enzyme Q10) capsule, and 300 mg. All study drugs were offered by Meyer Organics Pvt. Ltd.
Subjects
The study recruited fourteen (14) healthy adult volunteers. Routine laboratory tests, clinical examinations, vital sign assessments, ECG recordings, and urinalysis results were all normal for each subject. The following individuals were not permitted to participate: (i) subjects who have a history or are currently suffering from of any condition affecting the hematopoietic, renal, hepatic, endocrine, pulmonary, central nervous, cardiovascular, immune, dermatological, gastrointestinal, or other body systems; (ii) people who have taken any medication, whether prescribed, over-the-counter, or herbal remedies, within 14 days prior to dosing in period I, or who have received any vaccinations during that time; (iv) people who have a history or present of asthma (including aspirininduced asthma), nasal polyps, or NSAID-induced urticarial; (v) participants who were enrolled in current investigations at the beginning of the experiment or who had taken part in other clinical trials within the previous ninety days; and (vi) women who were pregnant or expected to become pregnant. The Shah Lifeline Hospital and Heart Institute Pvt. Ltd. Ethics Committee in Thane, Maharashtra, granted its approval to the study protocol.
Study Design
The declaration of helsinki and good clinical practice criteria were followed in the study protocol. Participants received comprehensive information about the study's objectives, methods, possible risks, advantages, and potential adverse drug reactions prior to recruitment. Every participant provided their written informed consent and voluntarily consented to participate in study. Every participant has undergone a thorough screening procedure. Anyone who met the exclusion criteria was not allowed to participate in the study, but those who met the criteria for inclusion were.
Healthy adults between the ages of 18 and 45 who had a BMI between 18.5 and 29.9 kg/m² participated in the bioavailability trial. Participants that were male weighed at least 50 kg. Two equal groups of participants were assigned to receive the test medication, Ultra Q 300, and the reference medication, other brand having similar composition. According to the schedule, patients received a 300 mg dosage of either Ultra Q 300 or other brand having similar composition on the first day of each dosing period after fasting for at least 10 hours the night before. The dose intervals were separated by a 15-day washout period.
All subjects fasted for at least 10 hours before intake of Coenzyme Q10 and for an additional 4 hours following dosing throughout each dosing interval. 240 milliliters of water were administered to take the doses. Water consumption was restricted for one hour prior to and one hour following dosage, but otherwise unrestricted. After receiving their doses at the scheduled times, participants were instructed to remain seated for the first two hours.
Sample size
The bioavailability of Ultra Q 300 and other brand having similar composition was compared in an open-label, randomized, balanced, two-treatment, two-sequence, two-period crossover, single-dose oral bioavailability study. The Coefficient of Variation (CV%) for Coenzyme Q10 Cmax was calculated to be between 28% and 32% based on data from earlier studies [18,19]. The study's estimated sample size was 14 participants, based on a β value of 20% (power=80%), an α level of 0.05, and a bioequivalence margin (θ) of 0.95-1.05.
Pharmacokinetic analysis
Blood samples were drawn from participants at 25 specific time intervals for Pharmacokinetic (PK) study. Five milliliters (5 ml) of blood were drawn within two minutes of the scheduled time for pre-dose samples (at -1.00 and -0.50 hours), and five minutes before the drug was administered in each research period for the 0.00-hour pre-dose sample. Post-dose samples were collected at the following intervals: 1.00, 2.00, 3.00, 4.00, 5.00, 5.25, 5.50, 5.75, 6.00, 6.25, 6.50, 6.75, 7.00, 7.50, 8.00, 10.00, 12.00, 16.00, 24.00, 48.00, 72.00, and 96.00 hours after dosing.
Five milliliters of blood were drawn into Tripotassium Ethylenediaminetetraacetic Acid (K3EDTA) anticoagulant tubes for each sample. To maintain sample stability, the tubes were submerged in freezing water immediately after collection. Samples were centrifuged at 4000 rpm for 5 minutes at a controlled temperature of 5°C ± 3°C within an hour of the final blood draw. For subsequent study, the resultant plasma was thereafter kept in a low-temperature freezer at -55°C.
Using Coenzyme Q9 as the internal standard, a validated bioanalytical method developed to quantify the analyte, Coenzyme Q10, in human plasma. Since the body naturally has Coenzyme Q10, a conventional addition procedure was used to ensure precise quantification. The analyte concentration in the biological matrix was determined using this method for both the analysis of research samples and the preparation of calibration standards and quality control samples.
An AB Sciex Triple Quad 4500 mass spectrometer performing in Multiple Reaction Monitoring (MRM) mode with positive ionization was used for the analysis. Liquid-Liquid Extraction (LLE) from plasma samples treated with K3EDTA as an anticoagulant was a component of the sample preparation process. A chromatographic apparatus with a HyPURITY™ C8 column (100 × 4.6 mm) and an autosampler stored at 10°C was used for analyzing the extracted samples.
Acetonitrile, 2-propenol, and 0.1% formic acid were the components of the mobile phase, which was provided isocratically at a flow rate of 1.000 mL/min in a 90:10 (v/v) ratio. The injection volume was 2 μL, and the run time was 6.0 minutes in total. An 80:20 (v/v) ratio of acetonitrile to water was used as the rinse solution.
Coenzyme Q10 and Coenzyme Q9 had retention periods of approx 4.70 and 3.80 minutes, respectively. The peak area ratio was used for quantification, and Analyst Software version 1.7.3 was used for analysis. For a concentration range of 50.000 ng/mL to 8000.000 ng/mL, the method demonstrated linearity using a 1/X2 (1/X*X) weighting factor for calibration. While stock and working solutions were stored in polypropylene tubes at temperatures ranging from 2°C to 8°C, matrix samples were kept at -70°C ± 10°C. There was no need for any extra specific conditions beyond those mentioned, and the method was not light-sensitive. It's crucial to remember that retention times are not considered specific method requirements, but rather assay properties.
Safety analysis
Vital signs, clinical observations, and Adverse Events (AEs) were continuously tracked during the course of the study. From the moment the drug was administered until 24 hours after the last blood sample had been collected, safety laboratory testing was carried out. Throughout every aspect of study, clinical investigators kept a close eye out for any adverse events.
Statistical analysis
Phoenix® WinNonLin® software version 8.4 or higher was used to assess major pharmacokinetic parameters and plasma drug concentrations. SAS® software (version 9.4 or higher, SAS® Institute Inc., USA) was used to statistically evaluate pharmacokinetic parameters including Cmax and Tmax.
The pharmacokinetic properties of the supplied formulations were evaluated using descriptive statistics, such as sample size (N), mean, Standard Deviation (SD), median, minimum, maximum, coefficient of variation (%CV), and geometric mean. The assessment of safety outcomes also made use of quantitative data, including mean, SD, median, minimum, and maximum values.
Results
Overview of baseline participant characteristics
Out of the 25 volunteers that underwent screening for the study, 11 had been excluded in accordance with the specified exclusion criteria. Finally, a total of 14 males were recruited (Figure 1). In Table 1, comprehensive demographic data is displayed (Table 1). The individuals were 35.29 ± 5.3 years old on average, 168 ± 7.1 cm tall, 71.63 ± 6.7 kg in weight, and had an average Body Mass Index (BMI) of 25.55 ± 2.5 kg/m2. The inclusion and exclusion criteria of the study were entirely satisfied by every participant, and there were no deviations from the protocol. (Figure 1).
Figure 1: Participant flow diagram. N: number of participants.
| Characteristics | N = 14 |
|---|---|
| Age (in Years) | |
| N (N miss) | 14 (0) |
| Mean ± Std | 35.29 ± 5.3 |
| Median (Q1, Q3) | 37 |
| Min-Max | 24-43 |
| Gender (M/F) | |
| Male (M) | 14 (100.00) |
| Female (F) | 0 (0.00) |
| Total | 14 (100.00) |
| Weight (kg) | |
| N (N miss) | 14 (0) |
| Mean ± Std | 71.63 ± 6.7 |
| Median (Q1, Q3) | 70.95 |
| Min-Max | 56.8-82.2 |
| Height (cm) | |
| N (N miss) | 14 (0) |
| Mean ± Std | 168 ± 7.1 |
| Median (Q1, Q3) | 167 |
| Min-Max | 160-185 |
| BMI (kg/m2 ) | |
| N (N miss) | 14 (0) |
| Mean ± Std | 25.55 ± 2.5 |
| Median (Q1, Q3) | 25.85 |
| Min-Max | 19.89-25.85 |
| Note: N: Number of Participants; Std: Standard Deviation; BMI: Body Mass Index. | |
Table 1: Baseline Demographic Characteristics
Pharmacokinetic analysis data
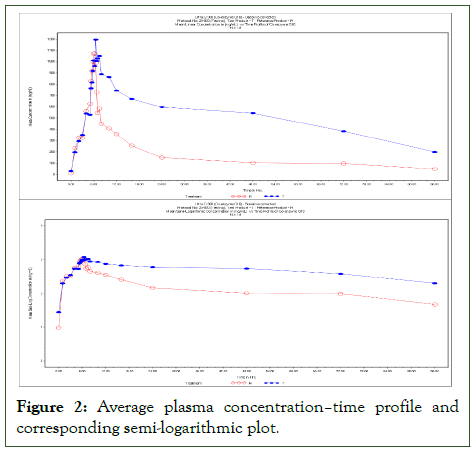
During each study period, samples were collected from every subject at 25 distinct times, and the plasma concentrations of Ultra Q 300 and other brand having similar composition were subsequently analyzed (Figure 2).
The mean plasma concentration–time profile and associated semi-logarithmic curve are shown in Figure 2.
Figure 2: Average plasma concentration–time profile and corresponding semi-logarithmic plot.
The data showed that the plasma concentration curves of Ultra Q 300 and other brand having similar composition differed noticeably. The Test formulation (Ultra Q 300) typically had somewhat higher Cmax values in the majority of patients, even though the Cmax values for both formulations showed a comparable pattern across participants-indicating similar absorption characteristics. This implies that some people may experience increased systemic exposure as a result of the Test product. Cmax's adjusted geometric mean ratio (90% CI) ranged from 102.27% to 118.47%, with a value of 110.07%.
The average Cmax values for Ultra Q 300 and other brand having similar composition were 1345.129 ng/mL and 1181.796 ng/mL, respectively (Tables 2 and 3).
| Parameters | Mean ± SD | |
|---|---|---|
| Test Product (T) | Reference Product (R) | |
| Tmax (h)# | 6.500 (5.750-7.500) | 6.250 (5.500-6.500) |
| Cmax (ng/mL) | 1345.129 ± 621.982 | 1181.796 ± 406.663 |
| Note: Cmax: Highest observed drug concentration in plasma; Tmax: Time taken to reach the peak plasma concentration of the analyte after administration. | ||
Table 2: Overview of Pharmacokinetic (PK) Parameters.
| Parameters | GLS Means | 90% Confidence Interval (CI) | Intra-Subject CVw (%) | Power (%) | ||
|---|---|---|---|---|---|---|
| Test Product (T) | Reference Product (R) | Ratio (T/R) % | ||||
| lnCmax | 1222.871 | 1110.974 | 110.07 | 102.27 - 118.47 | 10.94 | 99.64 |
| Note: CI: Confidence Interval; GLS Means: Geometric Least Squares Means; Cmax: Peak observed concentration of the drug in plasma; CVw: Within-subject coefficient of variation for the differences between the test and reference formulations. | ||||||
Table 3: Bioequivalence statistics of pharmacokinetic parameters.
Safety results
No significant adverse responses or serious adverse events were noted, and all subjects maintained stable vital signs and good general health throughout the research. These results show that in healthy individuals, both Ultra Q 300 and other brand having similar composition capsules have good safety profiles and are well-tolerated.
Discussion
Regulatory bodies in the US and Europe have approved Coenzyme Q10 as a dietary supplement. The bioavailability and safety profiles of Ultra Q 300 and other brand having similar composition were evaluated and compared in this single-center bioavailability study using a randomized, open-label, crossover design.
Earlier studies on co-enzyme Q10 have highlighted the high inter-individual variability among subjects [18]. A study examining the effects of age and sex on serum CoQ10 levels in 860 European adults aged 18 to 82 found an inverse U-shaped pattern between CoQ10 concentration and age. Women consistently had lower cholesterol-adjusted CoQ10 levels than men, regardless of age. In both sexes, CoQ10 levels declined in older individuals [20]. Notable racial differences have been observed in coenzyme Q10 levels, with Black individuals showing significantly higher CoQ10 values compared to white individuals. However, the reference ranges for total CoQ10 and ubiquinol-10 in Blacks, Chinese, and Indians still fall within the established reference range for white populations [21].
Given these factors, this study specifically enrolled healthy male participants aged 18 to 45 years to maintain consistency and reduce variability.
Coenzyme Q10 has been shown to improve New York Heart Association (NYHA) functional class, lower Brain Natriuretic Peptide (BNP) levels, decrease overall mortality, and decrease hospitalization rates in heart failure patients. Additionally, it raises the left ventricular ejection fraction and increases 6- minute walk test scores, both of which are beneficial without causing any noticeable adverse effects [22]. Even at higher doses, CoQ10 is generally well tolerated and has demonstrated promise in preventing migraines [23].
Furthermore, by substantially lowering levels of Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL-6), and C-Reactive Protein (CRP), CoQ10 has shown anti-inflammatory properties. However, additional well-controlled clinical trials are required to prove its therapeutic efficacy and provide optimal dose guidelines because of the unpredictability and limitations in the current research [24].
CoQ10 has been found to significantly alleviate fatigue, with greater effects observed at higher doses and with longer treatment periods. However, additional research is needed to estimate the long-term benefits once supplementation is discontinued [25]. CoQ10 has demonstrated promise in enhancing glucose metabolism, reducing inflammation, and balancing hormone levels in patients with PCOS, with significant reductions in Fasting Plasma Glucose (FPG) and HOMA-IR. Nevertheless, larger and well-designed randomized controlled studies are required to validate its effectiveness and safety [26]. GF Bacillus antioxidative enzyme SOD (GF101) demonstrated similar effectiveness to CoQ10 in addressing female infertility and significantly lowered total cholesterol and LDL levels. It may be a valuable antioxidant alternative, especially for women with dyslipidemia [27]. CoQ10 has been shown to significantly enhance biomarkers of oxidative damage such as Total Antioxidant Defense (TAC), Malondialdehyde Concentration (MDA), and Superoxide Dismutase Enzyme Activity (SOD), when compared to a placebo. However, additional long-term studies involving uniform patient populations are necessary to better understand its overall effectiveness and variability in individual responses [28].
By lowering the synthesis of metalloproteinases and interleukin-6, CoQ10 has been shown to inhibit the development of wrinkles caused by UVB radiation in both lab and real-world models. Collagenase, which degrades collagen fibers, is especially significant among these enzymes. CoQ10 contributes to wrinkle reduction and skin renewal by preserving the integrity of dermal fibers through the inhibition of collagenase.
Furthermore, CoQ10 has demonstrated encouraging results when administered to treat oxidative stress associated with Down syndrome in children. The antiangiogenic and cholesterol-lowering properties of CoQ10 have been emphasized in several research, especially in patients with breast cancer receiving tamoxifen treatment. Further, a 2008 case report detailed a patient who experienced chronic intestinal pseudoobstruction and improved after taking CoQ10 medication. The patient had maternally inherited diabetes and deafness, a hereditary form of diabetes that affects insulin secretion [29].
CoQ10 is initially absorbed by the liver, where it is incorporated into lipoproteins and then distributed throughout the bloodstream. This process likely contributes to its extended elimination phase, with a half-life of approximately 33 hours. In terms of metabolism, CoQ10 is broken down by cytochrome P450 enzymes [30]. To prevent any carryover effects from the previous treatment cycle. In this study, the washout period was intended to be longer than five times the half-life of the medication. This approach ensured that drug concentrations in all individuals were undetectable by bioassays prior to the start of the next dosing phase. Each participant's pre-dose plasma levels of coenzyme Q10 were actually below the detectable limit, indicating that the trial protocols specified washout time was sufficient.
In this study, pharmacokinetic data from individual subjects will be excluded if outliers are detected to maintain the integrity and accuracy of the analysis. Participants were excluded if they had known hypersensitivity to CoQ10 or its components, significant medical conditions affecting major body systems, recent use of medications or vaccines (within 14 days), history of asthma, nasal polyps, or NSAID-induced reactions, recent consumption of grapefruit, xanthine-containing foods, tobacco, or alcohol, or were smokers within the last 6 months. A history of psychiatric disorders or seizures, substance abuse, abnormal laboratory results, blood donation within the last 90 days, participation in other clinical trials, swallowing difficulties, positive hepatitis B, hepatitis C, or HIV tests, atypical diets, and a history of dystonic reactions were among the other exclusion criteria. Crucially, there were no outliers among the 14 subjects who finished both research cycles. Thus, these 14 participants are included in the safety analysis set, bioavailability analysis set, and whole analysis set.
In this bioavailability estimation, key parameters including Tmax, and Cmax were analyzed as primary measures [31]. Additionally, the pharmacokinetic parameter ratios comparing the generic drug to the reference drug are required to fall within a 90% confidence interval ranging from 80% to 125% [32].
The pharmacokinetic analysis of Ultra Q 300 (Test) and other brand having similar composition (Reference) demonstrated that both formulations had comparable absorption patterns, with very slight subject-to-subject differences evident in the Cmax values. The Test formulation had a plasma concentration that was roughly 12.93% higher than the Reference, with an average Cmax of 1181.796 ng/mL and 1345.129 ng/mL for the Test product. Comparable absorption rates were indicated by Tmax values for both formulations, which varied between 5.5 and 7.5 hours. A statistical power of above 99.0% and a variability rate of 10.94% were found in the trial's evaluation of individual variability and accuracy for Cmax. These findings verify that there was a sufficient sample size to evaluate the Ultra Q 300 and other brand having similar composition equivalency.
Overall, Ultra Q 300 and other brand having similar composition are closely aligned in systemic exposure, with Ultra Q 300 showing a modest increase. Importantly, both formulations were well tolerated with no adverse events or safety concerns reported during the study, indicating that the increased systemic exposure from Ultra Q 300 also did not compromise safety. The improved bioavailability may be attributed to better formulation characteristics of Ultra Q 300, such as enhanced solubilization or absorption-promoting excipients. The current data, obtained from only 14 healthy individuals, supports the interpretation of similar systemic exposure between the formulations.
Conclusion
This study demonstrated that Ultra Q 300 has improved bioavailability, while maintaining a similar absorption profile and excellent safety in healthy adults. Both formulations were well tolerated, with no adverse events reported. The improved systemic exposure observed with Ultra Q 300 may translate to better therapeutic effectiveness in clinical practice. These findings support Ultra Q 300 as a safe, effective, and beneficial for individuals seeking enhanced energy metabolism or antioxidant support.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by the Shah Lifeline Hospital and Heart Institute Ethics Committee reviewed the protocol and gave final approval for the trial to proceed (approval number: SLH-IEC/2025/026). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
Dr. S. M. Rahman, Dr. Honey Qureshi: Manuscript review; Dr. Poorneshwar Sawant, Mr. Sunil Kulkarni: Conceptualization, Study design. Dr. Ravindra Mote: Clinical study conduct, Data collection, Statistical analysis, Manuscript drafting. Dr. Parikshit Champanerkar: Bioanalytical method development, Sample analysis, Data interpretation, Manuscript editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Meyer Organics Private Limited, Thane, and Mumbai. The founder helps pay for this study and has no role in study design, data collection and analysis, or preparation of the manuscript.
Acknowledgments
We thank all enrolled participants, investigators, and people who contributed to this study.
Conflict of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publishers Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Rodick TC, Seibels DR, Babu JR, Huggins KW, Ren G, Mathews ST. Potential role of coenzyme Q10 in health and disease conditions. Nutrit Diet Suppl. 2018;10:1-11.
- Petrangolini G, Ronchi M, Frattini E, De Combarieu E, Allegrini P, Riva A. A new food-grade coenzyme Q10 formulation improves bioavailability: single and repeated pharmacokinetic studies in healthy volunteers. Curr Drug Deliv. 2019;16(8):759-767.
[Crossref] [Google Scholar] [PubMed]
- Langsjoen PH, Langsjoen AM. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014;3(1):13-17.
- Sood B, Patel P, Keenaghan M. Coenzyme Q10 In StatPearls [Internet]. StatPearls.
- Miles MV, Horn P, Miles L, Tang P, Steele P, DeGrauw, T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutrition Research. 2002;22(8):919-929.
- Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579-584.
[Crossref] [Google Scholar] [PubMed]
- Ikematsu H, Nakamura K, Harashima SI, Fujii K, Fukutomi N. Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regul Toxicol Pharmacol. 2006;44(3):212-218.
[Crossref] [Google Scholar] [PubMed]
- Raizner AE. Coenzyme Q10. Methodist DeBakey Cardiov J. 2019;15(3):185.
- Garrido-Maraver J, Cordero MD, Oropesa-Avila M, Fernández Vega A, De La Mata M, Delgado Pavon A, et, al. Coenzyme q10 therapy. Molecul Syndromol. 2014;5(3-4):187-197.
- Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs. 2010;19(4):535-554.
[Crossref] [Google Scholar] [PubMed]
- Drobnic F, Lizarraga MA, Caballero-García A, Cordova A. Coenzyme Q10 supplementation and its impact on exercise and sport performance in humans: a recovery or a performance-enhancing molecule? Nutrient. 2022;14(9):1811.
[Crossref] [Google Scholar] [PubMed]
- Castro-Marrero J, Segundo MJ, Lacasa M, Martinez-Martinez A, Sentañes RS, Alegre-Martin J. Effect of dietary coenzyme Q10 plus NADH supplementation on fatigue perception and health-related quality of life in individuals with myalgic encephalomyelitis/chronic fatigue syndrome: a prospective, randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13(8):2658.
[Crossref] [Google Scholar] [PubMed]
- Rauchova H. Coenzyme Q10 effects in neurological diseases. Physiol Res. 2021;70:S683-714.
[Crossref] [Google Scholar] [PubMed]
- Lin G, Li X, Jin Yie, SL, Xu L. Clinical evidence of coenzyme Q10 pretreatment for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Ann Med. 2024;56(1):2389469.
[Crossref] [Google Scholar] [PubMed]
- Al Saadi T, Assaf Y, Farwati M, Turkmani K, Al-Mouakeh A, Shebli B, et, al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2021;2.
[Crossref] [Google Scholar] [PubMed]
- Mantle D, Hargreaves IP. Coenzyme Q10 and autoimmune disorders: an overview. Int J Mol Sci. 2024;25(8):4576.
[Crossref] [Google Scholar] [PubMed]
- Vrentzos E, Ikonomidis I, Pavlidis G, Katogiannis K, Korakas E, Kountouri A, et, al. Six-month supplementation with high dose coenzyme Q10 improves liver steatosis, endothelial, vascular and myocardial function in patients with metabolic-dysfunction associated steatotic liver disease: A randomized double-blind, placebo-controlled trial. Cardiovasc Diabetol. 2024;23(1):245.
- Martucci A, Reurean-Pintilei D, Manole A. Bioavailability and sustained plasma concentrations of CoQ10 in healthy volunteers by a novel oral timed-release preparation. Nutrients. 2019;11(3):527.
[Crossref] [Google Scholar] [PubMed]
- Pravst I, Rodriguez Aguilera JC, Cortes Rodriguez AB, Jazbar J, Locatelli I, Hristov K, et al. Comparative bioavailability of different coenzyme Q10 formulations in healthy elderly individuals. Nutrients. 2020;12(3):784.
[Crossref] [Google Scholar] [PubMed]
- Niklowitz P, Onur S, Fischer A, Laudes M, Palussen M, Menke T, Doring F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J Clin Biochem Nutr. 2016;58(3):240-245.
[Crossref] [Google Scholar] [PubMed]
- Miles MV, Horn PS, Morrison JA, Tang PH, DeGrauw T, Pesce AJ. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta. 2003;332(1-2):123-132.
- Xu J, Xiang L, Yin X, Song H, Chen C, Yang B, et al. Efficacy and safety of coenzyme Q10 in heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2024;24(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Rozen TD, Oshinsky ML, Gebeline CA, Bradley KC, Young WB, Shechter AL, et al. Open label trial of coenzyme Q10 as a migraine preventive. BMC Cardiovasc Disord. 2002;22(2): 137-141.
[Crossref] [Google Scholar] [PubMed]
- Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128-136.
[Crossref][Google Scholar] [PubMed]
- Tsai I, Hsu CW, Chang CH, Tseng PT, Chang KV. Effectiveness of coenzyme Q10 supplementation for reducing fatigue: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:883251.
[Crossref] [Google Scholar] [PubMed]
- Liu M, Zhu H, Hu X, Zhu Y, Chen H. Efficacy of coenzyme Q10 supplementation on glucose metabolism, lipid profiles, and biomarkers of inflammation in women with polycystic ovary syndrome: A protocol for a systematic review and meta-analysis. Medicine. 2020;99(46):e23130.
[Crossref] [Google Scholar] [PubMed]
- Shin SY, Yoon HK, Kim JH, Kim JH, Park C, Choi DH, et al. The efficacy and safety of gf101 and its antioxidant effect on in vitro fertilization outcomes: a double-blind, non-inferiority, randomized, controlled trial with coenzyme q10. Antioxidants. 2024;13(3):321.
[Crossref] [Google Scholar] [PubMed]
- Akbari A, Mobini GR, Agah S, Morvaridzadeh M, Omidi A, Potter E, et al. Coenzyme Q10 supplementation and oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Eur J Clin Pharmaco. 2020;76:1483-1499.
[Crossref] [Google Scholar] [PubMed]
- Littarru GP, Tiano. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26(3):250-254.
[Crossref] [Google Scholar] [PubMed]
- Arenasâ?ÂÂJal M, Sunéâ?ÂÂNegre JM, Garcíaâ?ÂÂMontoya E. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf. 2020; 19(2):574-594.
[Crossref] [Google Scholar] [PubMed]
- U.S. Food and Drug Administration (USFDA). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. U.S. Food and Drug Administration. 2025.
- Li X, Liu L, Deng Y, Li Y, Zhang P, Wang Y, et al. Pharmacokinetics and bioequivalence of 2 immediateâ?ÂÂrelease tofacitinib tablet formulations in Chinese healthy volunteers under fasting and fed conditions. Clin Pharmacol Drug Dev. 2021;10(5):535-541
[Crossref] [Google Scholar] [PubMed]
Citation: Mote R, et al. (2025). Comparative Bioavailability Study of Ultra Q300 and COQ300 Coenzyme Q10 in Healthy Adults. J Bioequiv Availab. 17:644.
Copyright: © 2025 Mote R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.