Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 0, Issue 0
Characterization, Associated Risk Factors and Possible Treatment of Healthcare Associated Methicillin-Resistant Staphylococcus aureus (HA-MRSA) and Community-Associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA)
Rabeya Nahar Ferdous1, Rashed Zaman2, Shahedur Rahman3, Oliullah Rafi3, Shuvra Kanti Dey4, Abdul Khaleque5 and Anowar Khasru Parvez4*2Department of Genetic Engineering and Biotechnology Rajshahi University, Rajshahi, Bangladesh
3Department of Genetic Engineering and Biotechnology, Jashore University of Science and Technology, Jashore, Bangladesh
4Department of Microbiology, Jahangirnagar University, Bangladesh
5Department of Biochemistry and Microbiology, North South University, Dhaka, Bangladesh
Received: 02-Aug-2021 Published: 23-Aug-2021, DOI: 10.35248/2155-9597.21.S11.002
Abstract
Methicillin-Resistant Staphylococcus aureus (MRSA) has long been a common pathogen in healthcare facilities, but now, it has emerged as a problematic pathogen in the community setting as well. Healthcare-Associated Methicillin- Resistant S. aureus (HA-MRSA) and Community-Associated Methicillin-Resistant S. aureus (CA-MRSA) strains have appeared as a significant pathogen in healthcare and community-associated settings. The CA-MRSA used to be susceptible to mostly used antibiotics, but the criteria have been changed for past decade. Although HA-MRSA most commonly found in urine but CA-MRSA responsible for causing UTI. So Polymerase Chain Reaction (PCR) can be used as gold standard to characterize S. aureus (nuc gene), MRSA (mecA gene), CA-MRSA (PVL gene in SCCmec types IV). On the other hand, HA-MRSA can be detected by the detection of SCCmec types I, II, or III. But detection of PVL gene may reduce cost and time to screen CA-MRSA and HA-MRSA. After identifying targeting gene, sequencing can be carried out to know amino acid changes or any mutation that may occur in PVL gene and may change the characteristics of CA-MRSA. Whole genome sequencing can play a vital role for shaping the future and identifying transmission of MRSA in outbreak or endemic settings. Another way to control infection associated with HA-MRSA and CA-MRSA is to control risk factors and important to identify the antibiotics before prescribing to the infected person. Though vancomycin has susceptibility to most of MRSA but resistant pattern has also been found. Development of vaccines against MRSA may have dramatic impacts upon morbidity and mortality caused by a number of infection associated with HA-MRSA and CA-MRSA. However, further work is required to assess their long-term roles in controlling infection associated with MRSA
Keywords
MRSA; HA-MRSA; CA-MRSA; Detection; Prevention; Risk factors; Staphylococcus aureus
Introduction
The pervasiveness of the gram positive bacteria in nature makes the exposition of their recovery from patient specimens occasionally difficult, unless the clinical manifestations of an infectious disease are apparent. Among other gram positive bacteria Staphylococcus aureus is the most important and commonly isolated human bacterial pathogens. S. aureus isolates are resistant to methicillin, termed Methicillin-Resistant S. aureus (MRSA), first identified in the 1960s [1,2]. They are usually found to be resistant to other b-lactam antimicrobial drugs [3]. Methicillin-Resistant S. aureus (MRSA) is one of the most frequent cases of Hospital-Associated (HA) infection [4,5]. But it reported in community settings in the year 1980s, they are phenotypically, and genetically distinct from healthcareassociated Methicillin-Resistant S. aureus (HA-MRSA) and there has chance to replace HA-MRSA [6-9]. Meticillin-resistance in all of these clones was due to the same mechanism, with the responsible gene, mecA. Expression of mecA conferred resistance to all available beta-lactam antibiotics, while resistance to non-beta-lactam antibiotics commonly accumulated in HAMRSA due to a variety of mechanisms.
Literature Review
HA-MRSA, which reached almost 50% of all S. aureus isolated in some countries like in Bangladesh, Australia because of using high level of antibiotic in healthcare facilities [3,10]. Prolonged length-of-stay in hospital settings increase the morbidity and mortality rate associated with HA-MRSA.
Despite of this HA-MRSA, Community-associated methicillinresistant S. aureus (CA-MRSA) also been observed as emerging pathogen. In Bangladesh, the percentages of CA-MRSA is noticeable which is 57.89% [3]. Cluster of CA-MRSA infection have been described among aboriginals in rural Native American communities in the United States, prisoners, children, and Injecting Drug Users (IDUs) [6,11-13]. Most of the CA-MRSA associated with Skin and Soft Tissue Infection (SSTI) and bacteremia respectively. But study showed that CA-MRSA responsible for 25% of UTI infection as well [14]. CA-MRSA has also been found to be composed of more-diverse clonal groups than HA-MRSA and to usually contain an unique SCC mec type IV element and Panton Valentine Leukocidin (PVL) encoding genes LukS-PV and LukF-PV [3,15]. On the other hand, HA-MRSA strains carry SCCmec types I, II, or III [16,17].
Methicillin-Resistant S.aureus (MRSA)
S. aureus isolates became resistant to methicillin termed as Methicillin-Resistant S. aureus (MRSA) [4]. According to NCCLs, (2013) S. aureus isolates with zone of inhibition of cefoxitin disk ≤ 21mm were phenotypically considered as MRSA worldwide [3].
Healthcare-Associated Methicillin-Resistant S.aureus (HA-MRSA)
According to CDC, an MRSA isolate as HA associated if the original entry criteria of hospitalization for >72 hours before culture acquisition was met and if in the year before the present hospitalization, the patient had any 1 of the following: hospitalization, surgery, residency in a long-term care facility, and hemodialysis or peritoneal dialysis, or at the present admission had indwelling percutaneous devices or catheters [18].
Community-associated Methicillin-Resistant S.aureus (HA-MRSA)
The definition of CA-MRSA includes cases where MRSA is isolated ≤ 72 h after hospital admission and there is no history in the previous 12 months of hospitalization or surgery, permanent indwelling catheters or percutaneous medical devices, residence in a long-term care facility, dialysis or prior culture of MRSA [18].
Of note, in the CDC definition, an infection is considered HA if it occurs >48 hours after admission and CA if it occurs <48 hours after admission [18]. But evidence showed that >72 hours and ≤ 72 hours as a cut-off to more conservatively capture HA infections and CA infections respectively, that may minimize the miscategorization of HA-MRSA infections as CA-MRSA infections.
Antibiotic susceptibility pattern of HA-MRSA and CA-MRSA
A study found that, in Bangladesh prevalence of MRSA was 72% and United States is 60% [3,19]. In 2007, CA-MRSA found more susceptible to mostly common antibiotic than HAMRSA [20]. But in 2018, CA-MRSA showed highest pattern of resistant to Cefoxitin, Oxacillin, Ceftazidime, Chloramphenicol, Tetracyclin and HA-MRSA showed resistant to Cefoxitin, Oxacillin, Tetracyclin which is an alarming resistant pattern. A Minimal Inhibitory Concentration (MIC) was also studied against Ceftriaxone and Gentamicin and almost 35% isolates showed 2048 MIC value against both antibiotic [20]. Only Vancomycin showed susceptible pattern for both HA-MRSA and CA-MRSA in that study. But there also evidence of Vancomycin Resistance S. aureus (VRSA) which may be a Vancomycin treatment failure [21].
Characterization of HA-MRSA and CA-MRSA
Detection pattern can be categorized into conventional detection (such as biochemical identification, antibiogram) and molecular detection (such as PCR, sequencing). Antibiotic susceptibility pattern is a valuable tool for the detection of methicillin resistance pattern by using cefoxitin disk (30 μg) (≤ 21 zone diameter known as MRSA) [3]. But it is not well enough for the characterization of HA-MRSA and CA-MRSA. Most study showed HA-MRSA highly resistant to beta-lactam antibiotic and as well non-beta-lactam antibiotics due to inadequate and extensive use of antibiotic in hospital settings [11]. We also found in our statistical analysis that urine could be suitable for detecting HA-MRSA. But recent study showed that CA-MRSA became more resistant to commonly used antibiotics and along with skin, soft-tissue infection they also responsible for causing UTI [3]. So, antibiotic profile and clinical manifestation are not quite enough to detect HA-MRSA and CA-MRSA.
Molecular method (polymerase chain reaction, Sequencing) could be an extensive way to classified them easily and we can also detect S. aureus and MRSA by detect nuc and mecA gene individually [3]. By using Polymerase Chain Reaction (PCR) CAMRSA is distinguishable from HA-MRSA based on the production of Panton-Valentine leukocidin (PVL), a powerful cytotoxin in human mononuclear cells or SCCmec type IV in the variable genome CA clones [3,8,22]. On the other hand, HA-MRSA can be detected by the detection of SCCmec types I, II, or III using PCR as shown (Figure 1) [22]. After detection of gene, sequencing of identified gene will add a promising result to combat the infection associated with HA-MRSA and CAMRSA [23].
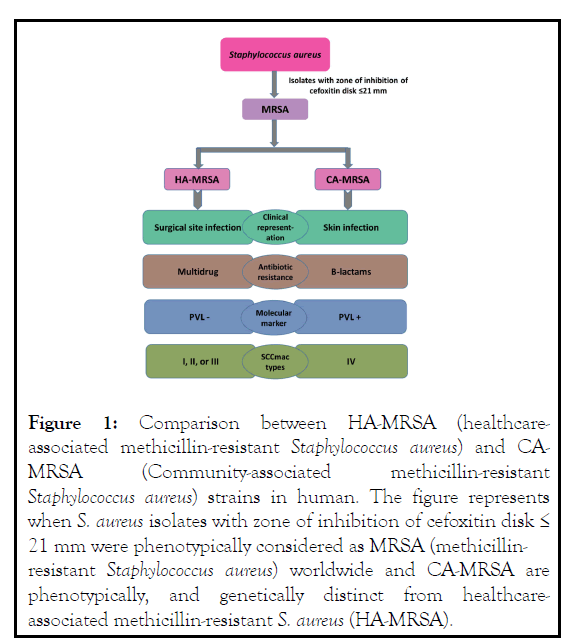
Figure 1: Comparison between HA-MRSA (healthcareassociated methicillin-resistant Staphylococcus aureus) and CAMRSA (Community-associated methicillin-resistant Staphylococcus aureus) strains in human. The figure represents when S. aureus isolates with zone of inhibition of cefoxitin disk ≤ 21 mm were phenotypically considered as MRSA (methicillinresistant Staphylococcus aureus) worldwide and CA-MRSA are phenotypically, and genetically distinct from healthcareassociated methicillin-resistant S. aureus (HA-MRSA).
However, whole genome sequencing in identifying transmission of HA-MRSA and CA-MRSA in outbreak or endemic settings need to be applying. Further studies are required to determine its long-term impact on infection control programmes. In addition, real-time sequencing and analysis remains problematic for many institutions, despite signi cant reductions in costs. Further work is required in this area, though results to date are promising [24-27].
Risk factors
Patients with CA-MRSA infections have often lacked risk factors [28]. For example, skin infections due to S. aureus in general and CA-MRSA in particular are well known to be in uenced by climate, having higher incidence in warmer and more humid months in temperate regions but are generally more common in tropical climates [29,30]. Younger people have frequently been found to be at greater risk of CA-MRSA. Socio-economic deprivation and overcrowding also act as risk factors [31-34]. Other factors associated with social disadvantage have been implicated including higher levels of fomite and environmental contamination, diabetes, obesity, smoking in the household and skin infections in household contacts. Antibiotic use in the previous 12 months has been associated with increased risk in both the general population and in prison populations [35-38].
The commonly associated risk factors for HA-MRSA infection are prolonged hospitalization, intensive care admission, recent hospitalization, recent antibiotic use, invasive procedures, HIV infection, and admission to nursing homes, open wounds, hemodialysis, and discharge with long-term central venous access or long-term indwelling urinary catheter. A higher incidence of HA-MRSA infection is also seen among healthcare workers who come in direct contact with patients infected with this organism.
Although advancing age by itself is not considered a risk factor for HA-MRSA infection, age more than 65 years is a significant risk factor for hospitalization. Hence, advancing age is indirectly linked to HA-MRSA acquisition. Living in an area with a high prevalence of CA-MRSA or admission to a hospital with a high prevalence of HA-MRSA also is considered a significant risk factor for HA- MRSA colonization [39].
Prevention strategy for HA-MRSA
Many different strategies have been assessed for their impact in controlling HA-MRSA, but signicant controversy still exists as to which infection control practices are most effective. Often when studied or employed in healthcare settings, these practices make up part of a bundle of care, and therefore it is often difficult to assess for each strategy as a stand-alone intervention [40-42]. An example of this, is the utility of contact precautions and isolation, which in the absence of effective hand hygiene, are unlikely to demonstrate a signi cant reduction in the transmission of HA-MRSA.
Significant disagreement still remains due to large costs, potential for interference in patient care and an inability to eradicate endemic transmission. It worthy to note that the comparison of data within different settings may be difficult due to inconsistencies, varied study populations and differences in baseline prevalence rates of MRSA. Despite national guidelines in many countries mandating prospective surveillance, the focus of this reporting is to guide institutions on benchmarking and monitoring of control strategies, not necessarily to demonstrate signi cant success from a single intervention employed amongst a bundle of healthcare activities. Therefore, although the following discussion focuses on interventions individually, the greatest reduction in MRSA acquisition and infection is likely to be achieved through a multi-faceted approach like as source identification, contact precaution, decolonization, and last but not least antibiotic stewardship will be the key interventions to reduce MRSA within the hospital and region [43-45]. A retrospective ecological study with time series analysis in Scotland suggested an association between the restriction of Coamoxyclav, Cephalosporins, Clindamycin, Fouroquinolones and macrolides and a reduction in the acquisition of HA-MRSA in hospital and the community [46].
Prevention strategy for CA-MRSA
Control of CA-MRSA is in compared to that of HA-MRSA is difficult due to lack of information and reports. Efforts at prevention and control strategy require an understanding of the epidemiology and of risk factors that are amenable to modi cation. While those for HA-MRSA have been well established, but data of CA-MRSA is not studied well. It will be obvious that some risk factors apply generally while others apply to particular geographic and demographic conditions.
In the United States a high incidence of CA-MRSA in incarcerated populations has been associated with risk factors including previous skin infection, nasal colonization, lower educational level, less frequent showering, sharing soap and less healthcare contact [38]. When it comes for Asian country like Bangladesh, nasal colonization, geographical differences in typical antimicrobial resistance profiles became the risk factor in community people [47]. Population-based studies have generally found very low levels of nasal carriage of MRSA including CAMRSA. An agent-based model of Chicago CA-MRSA data indicated that contact with colonized individuals was probably the major source of acquisition and that this was most likely to occur in households with school/daycare, hospital, sport and jails being of much less importance in descending order [48]. A whole genome sequencing study of household transmission of USA300 CA-MRSA in Los Angeles and Chicago demonstrated that single strains were transmitted within households and could persist for 2.5–8.5 years long [49]. A screening and eradication of carriage of MRSA infection associated with patients has been a mainstay of control in HA-MRSA, a similar approach should be attempted with CA-MRSA.
Furthermore, the generalizability of that nding to other HAMRSA clones and indeed to CA-MRSA has yet to be established. While vaccination has proven successful in controlling a number of bacterial pathogens and could be of great bene t in the control of all invasive infection due to S. aureus. Study suggested that efforts to provoke protective immunity specfic to invasive staphylococcal infection have proven more difficult and complex than those for other pathogens. An effective vaccine is still awaited and issues related to achievement of that have been reviewed elsewhere [50,51].
Discussion
Diagnosis and treatment
Healthcare providers are encouraged to obtain appropriate specimens for culture and susceptibility testing. Incision and drainage are useful in the treatment of localized skin abscesses only [51]. Empiric therapy should be based on local susceptibility patterns and the clinical evaluation of the patient.
Treatment option for HA-MRSA and CA-MRSA is not absolute due to lack of proper data and information associated with MRSA infection. Vancomycin remains the drug of choice for treatment of infections caused by MRSA in Bangladesh [3,52]. But S. aureus isolates were found resistance to vancomycin in Bangladesh and has been increasing in various parts of the world like in India, United States, Brazil, France and so on [53-57]. Treatment should be modified depending on the culture and susceptibility results and on the clinical course of the patient.
A very recent review has structured to know the role of antibody and animal models in the design of staphylococcal vaccines. So vaccination can be another possible way to treat HA-MRSA and CA-MRSA with proper understanding of clinical medicine and basic epidemiology of HA-MRSA and CA-MRSA [58,59].
Conclusion
This review aimed that prevalence of MRSA increasing day by day and remains a major healthcare challenge. CA-MRSA infections appear to be an emerging phenomenon and has possibility to replace HA-MRSA. Antibiotic susceptibility pattern, clinical features of HA-MRSA and CA-MRSA has been changed from past decade. So far, for characterization of them PCR and sequencing should be the prime concern as identification strategy. PCR is found to be the gold standard for the detection of MRSA, CA-MRSA and HA-MRSA. Detection of PVL gene and sequencing of PVL gene can be widely used to detect the prevalence, amino acid pattern, or mutation of CAMRSA worldwide. In addition to cost of Whole genome sequencing, it can play a vital role for shaping the future and identifying transmission of MRSA in outbreak or endemic settings. The pragmatic use of bundles of interventions to long term control of HA-MRSA and CA-MRSA can be achieved by controlling factors associated with MRSA infection.
Every strain of bacteria is susceptible to a specific antibiotic; hence, it is important to identify the antibiotics before prescribing to the infected person. Removal of unnecessary antibiotic selective pressure by curtailing inappropriate use as mandated in objective 4 of the WHO Global Action Plan on Antimicrobial Resistance should be achievable. Though results to date are promising, further study is required for clear image of infection associated with HA-MRSA and CA-MRSA and to delineate optimum control strategies.
Conflict of Interest
No financial and other conflict of interest related to this article.
REFERENCES
- Mahy BWJ, Meulen V, Borriello SP, Murray PR, Funke G, Merz W, et al. Topley & Wilson's Microbiology and Microbial Infections (10th edn), Hodder Arnold, London, United Kingdom, 2006.
- Hsiao CH, Ong SJ, Chuang CC, Ma DHK, Huang YC. A Comparison of Clinical Features between Community-Associated and Healthcare-Associated Methicillin-Resistant Staphylococcus aureus Keratitis. J Ophthalmol. 2015;2015(1):923941.
- Parvez MAK, Ferdous RN, Rahman MS, Islam S. Healthcare-associated (HA) and community-associated (CA) methicillin resistant Staphylococcus aureus (MRSA) in Bangladesh – Source, diagnosis and treatment. J Genet Eng Biotechnol. 2018;16(2):473-478.
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5(5):275-286.
- Jarvis WR, Schlosser J, Chinn RY, Tweeten S, Jackson M. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities. Am J Infect Control. 2012;40(3):194-200.
- Saravolatz LD, Markowitz N, Arking L, Pohlod D, Fisher E. Methicillin-resistant Staphylococcus aureus: Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96(1):11-16.
- Riley TV, Pearman JW, Rouse IL. Changing epidemiology of methicillin-resistant Staphylococcus aureus in Western Australia. Med J Aust. 1995;163(8):412-414.
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureuscarrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9(8):978-984.
- David MZ, Cadilla A, Boyle-Vavra S, Daum RS. Replacement of HA-MRSA by CA-MRSA infections at an academic medical center in the midwestern United States, 2004-5 to 2008. PLoS One. 2014;9(4):e92760.
- Henderson A, Nimmo GR. Control of healthcare-and community-associated MRSA: recent progress and persisting challenges. Br Med Bull. 2018;125(1):25-41.
- Groom AV, Wolsey DH, Naimi TS, Smith K, Johnson S, Boxrud D, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA. 2001;286(10):1201-1205.
- Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections--Los Angeles County, California, 2002-2003. Centers for Disease Control and Prevention. 2003.
- Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureusin southern New England children. Pediatrics. 2004;113(4):e347-e352.
- Lqbal J, Rahman M, Kabir MS. Ciprofloxacin resistance among community-derived methicillin-resistant Staphylococcus aureus (MRSA) strains. Southeast Asian J Trop Med Public Health. 1999;30(4):779-780.
- Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47(1):196-203.
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616-687.
- Kong EF, Johnson JK, Jabra-Rizk MA. Community-associated methicillin-resistant Staphylococcus aureus: An enemy amidst us. PLoS Pathog. 2016;12(10):e1005837.
- Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13(2):236-242.
- Haq JA, Rahman MM, Asna SMZH, Hossain MA, Ahmed I, Haq T, et al. Methicillin-resistant Staphylococcus aureus in Bangladesh--a multicentre study. Int J Antimicrob Agents. 2005;25(3):276-277.
- Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007;87(1):3-9.
- Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10(1):12689.
- Asghar AH. Molecular characterization of methicillin-resistant Staphylococcus aureus isolated from tertiary care hospitals. Pak J Med Sci. 2014;30(4):698-702.
- Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schäffer AA. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24(16):1757-1764.
- Harrison EM, Ludden C, Brodrick HJ, Blane B, Brennan G, Morris D, et al. Transmission of methicillin-resistant Staphylococcus aureus in long-term care facilities and their related healthcare networks. Genome Med. 2016;8(6):102.
- Kinnevey PM, Shore AC, Aogáin MM, Creamer E, Brennan GI, Humphreys H, et al. enhanced tracking of nosocomial transmission of endemic sequence type 22 methicillin-resistant Staphylococcus aureustype IV isolates among patients and environmental sites by use of whole-genome sequencing. J Clin Microbiol. 2016;54(2):445-448.
- Kong Z, Zhao P, Liu H, Yu X, Qin Y, Su Z, et al. Whole-genome sequencing for the investigation of a hospital outbreak of MRSA in China. PLoS One. 2016;11(1):e0149844.
- Tong SYC, Holden MTG, Nickerson EK, Cooper BS, Köser CU, Cori A, et al. Genome sequencing defines phylogeny and spread of methicillin-resistant Staphylococcus aureus in a high transmission setting. Genome Res. 2015;25(1):111-118.
- Huang H, Flynn NM, King JH, Monchaud C, Morita M, Cohen SH. Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J Clin Microbiol. 2006;44(7):2423-2427.
- Wang X, Towers S, Panchanathan S, Chowell G. A population based study of seasonality of skin and soft tissue infections: implications for the spread of CA-MRSA. PLoS One. 2013;8(4):e60872.
- Mermel LA, Machan JT, Parenteau S. Seasonality of MRSA infections. PLoS One. 2011;6(3):e17925.
- Casey JA, Cosgrove SE, Stewart WF, Pollak J, Schwartz BS. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001-2010. Epidemiol Infect. 2013;141(6):1166-1179.
- Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission. Arch Intern Med. 2007;167(10):1026-1033.
- Knox J, Uhlemann AC, Miller M, Hafer C, Vasquez G, Vavagiakis P, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureustransmission. PLoS One. 2012;7(11):e49900.
- Tong SYC, Bishop EJ, Lilliebridge RA, Cheng AC, Spasova-Penkova Z, Holt DC, et al. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J Infect Dis. 2009;199(10):1461-1470.
- Uhlemann AC, Knox J, Miller M, Hafer C, Vasquez G, Ryan M, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureusUSA300: a case-control study. PLoS One. 2011;6(7):e22407.
- Early GJ, Seifried SE. Risk factors for community-associated Staphylococcus aureus skin infection in children of Maui. Hawaii J Med Public Health. 2012;71(8):218-223.
- Lee GC, Hall RG, Boyd NK, Dallas SD, Du LC, Treviño LB, et al. Predictors of community-associated Staphylococcus aureus, methicillin-resistant and methicillin-susceptible Staphylococcus aureus skin and soft tissue infections in primary-care settings. Epidemiol Infect. 2016;144(15):3198-3204.
- Maree CL, Eells SJ, Tan J, Bancroft EA, Malek M, Harawa NT, et al. Risk factors for infection and colonization with community-associated methicillin-resistant Staphylococcus aureus in the Los Angeles County jail: A case-control study. Clin Infect Dis. 2010;51(11):1248-1257.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1990-May 1999, issued June 1999. A report from the NNIS system. Science Direct. 1999.
- Welsh CA, Flanagan ME, Kiess C, Doebbeling BN. Implementing the MRSA bundle in ICUs: One citywide collaborative’s key lessons learned. Infect Control Hosp Epidemiol. 2011;32(1):918-921.
- Fisher D, Tambyah PA, Lin RTP, Jureen R, Cook AR, Lim A, et al. Sustained meticillin-resistant Staphylococcus aureus control in a hyper-endemic tertiary acute care hospital with infrastructure challenges in Singapore. J Hosp Infect. 2013;85(2):141-148.
- Perencevich EN. Deconstructing the veterans affairs MRSA prevention bundle. Clin Infect Dis. 2012;54(11):1621-1623.
- Harbarth S, Fankhauser C, Schrenzel J, Christenson J, Gervaz P, Bandiera-Clerc C, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299(10):1149-1157.
- Robicsek A, Beaumont JL, Paule SM, Hacek DM, Thomson RBJ, Kaul KL, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148(6):409-418.
- Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus(MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61(8):26-38.
- Lawes T, Lopez-Lozano JM, Nebot CA, Macartney G, Subbarao-Sharma R, Dare CR, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis. 2015;15(12):1438-1449.
- Duza SS. Evaluation of virulence properties of Staphylococcus aureus isolated from a tertiary level hospital of Dhaka city. University of Dhaka. 2021.
- Macal CM, North MJ, Collier N, Dukic VM, Wegener DT, David MZ, et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: a dynamic agent-based simulation. J Transl Med. 2014;12(1):124.
- Alam MT, Read TD, Petit RA, Boyle-Vavra S, Miller LG, Eells SJ, et al. Transmission and microevolution of USA300 MRSA in U.S. households: evidence from whole-genome sequencing. mBio. 2015;6(2):e00054.
- Missiakas D, Schneewind O. Staphylococcus aureus vaccines: Deviating from the carol. J Exp Med. 2016;213(9):1645-1653.
- Proctor RA. Recent developments for Staphylococcus aureus vaccines: clinical and basic science challenges. Eur Cell Mater. 2015;30(1):315-326.
- Haque ME, Shahriar M, Haq A, Gomes BC, Hossain MM, Razzak MA, et al. Prevalence of-lactamase-producing and non-producing methicillin resistant Staphylococcus aureus in clinical samples in Bangladesh. J Microbiol Antimicrob. 2011;3(1):112-118.
- Khanam S, Haq JA, Shamsuzzaman SM, Rahman MM, Mamun KZ. Emergence of vancomycin resistant Staphylococcus aureus during hospital admission at a tertiary care hospital in Bangladesh. J Infect Dis. 2016;3(1):11-16.
- Assadullah S, Kakru DK, Thoker MA, Bhat FA, Hussain N, Shah A. Emergence of low level vancomycin resistance in MRSA. Indian J Med Microbiol. 2003;21(3):196-198.
- Staphylococcus aureus resistant to vancomycin--United States, 2002. Centers for Disease Control and Prevention (CDC). 2002.
- Oliveira GA, Dell’Aquila AM, Masiero RL, Levy CE, Gomes MS, Cui L, et al. Isolation in Brazil of nosocomial Staphylococcus aureus with reduced susceptibility to vancomycin. Infect Control Hosp Epidemiol. 2001;22(7):443-448.
- Ploy MC, Grélaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351(9110):1212.
- Pozzi C, Lofano G, Mancini F, Soldaini E, Speziale P, Gregorio ED, et al. Phagocyte subsets and lymphocyte clonal deletion behind ineffective immune response to Staphylococcus aureus. FEMS Microbiol Rev. 2015;39(5):750-763.
- Antimicrobial resistance: a manual for developing national action plans. World Health Organization. 2016.
Citation: Ferdous RN, Zaman R, Rahman S, Rafi O, Dey SK, Khaleque A, et al. (2021) Characterization, Associated Risk Factors and Possible Treatment of Healthcare-Associated Methicillin-Resistant Staphylococcus aureus (HA-MRSA) and Community-Associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA). J Bacteriol Parasitol. S11: 002.
Copyright: © 2021 Ferdous RN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

