Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 10, Issue 9
Cellular Toxicity Mechanisms of TiCN-Cp-Ti Screw on Tooth Gum Cells in Rat
Parvaneh Naserzadeh1, Abbas Razmi2, Ruhi Yesildal2 and Behnaz Ashtari1,3,4*2Department of Mechanical Engineering, Ataturk University, Erzurum, Iran
3Department of Medical Nanotechnology, Iran University of Medical Sciences, Tehran, Iran
4Department of Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran
Received: 03-Sep-2021 Published: 24-Sep-2021, DOI: 10.35248/2161-1009.21.10.404
Abstract
Cellular toxicity mechanism of TiCN film deposited on Cp-Ti substrate by Cathodic Arc Physical Vapor Deposition (CAPVD) method has yet to be clarified. The present study is aimed at finding out the possible effects of synthesized TiCN on the isolated tooth gum cells and realizing its cytotoxicity mechanism under experimental conditions compared to that of Au screws (standard in medicine). Results of the XRD analysis showed that TiCN film was formed in coating. The SEM image of TiCN film displayed a rough and irregular morphology and fine-grained surface. In addition, some other features such as cell viability, the level of Reactive Oxygen Species (ROS), lipid peroxidation (MDA), Glutathione Count (GSH and GSSG), Adenosine Triphosphate (ATP) were examined. On the other hand, activity of succinate dehydrogenase (complex II), NADH dehydrogenase (complex I), Coenzyme Q-cytochrome c reductase/Cytochrome b (complex III) and cytochrome c oxidase (complex IV) were also determined. Finally, it was also determined that level of Alkaline Phosphatase (ALP), Aspartate Amino Transferase (AST), Alanine Aminotransferase (ALP), Urea, Creatinine Clearance (CR) in rat (tooth gum cells) could also change. The results of the study revealed that TiCN coating did not incur an extremely change on cellular toxicity biomarker compared to Au standard screw in animal study. Our study provides the first evidence that TiCN coating is a biocompatible material in the cell. This paper suggests that the use of the TiCN in human dental applications need further examinations in a wide range of cellular-death signaling.
Keywords
Cathodic arc; Physical vapor deposition; TiCN coating; Cp-Ti substrate; Reactive oxygen species; Tooth gum
Introduction
Titanium, gold, nickel, palladium, platinum, copper and silver are common materials for dental applications, such as screw, filling and restoration [1]. The properties of oral environment, such as PH and temperature, vary due to intake of drinks and food as well as due to the components of saliva [2]. This variation contributes to the corrosion of metallic dental applications, and ionization and release of metal ions from dental alloys [3]. Oral mucosal epithelium layer is exposed to metals in the oral environment and dental screw, restorations have been reported to induce cellular toxicity and genotoxicity to oral epithelium cells such as fibroblast and keratinocytes. Furthermore, contact with metals can induce immune reactions in the surrounding oral mucosa, which may produce oral mucosal diseases such as cancer or chronic inflammatory mucocutaneous disease (Oral Lichen Planus (OLP) [4].
Titanium alloys are preferable in dental applications for they offer excellent biocompatibility as well as other superior mechanical properties such as high corrosion resistance and fatigue life, lower density and elastic modulus [5-7]. Previous studies showed titanium is more useable in medicine than other metals. On the other hand, titanium and stainless steel implants were biocompatible materials in bone damage and surgery but researcher showed the latter may induce adverse response in adolescent patients which eventually require a second intervention for implant removal [8]. The great corrosion resistance property is ensured by a titanium oxide film layer of about 1-10 nm formed on titanium surface [9]. However, commercially pure titanium alloys have some disadvantages for use in biomedical applications, such as poor wear resistance, higher Young modulus and comparatively low mechanical strength [10]. TiN and TiCN are well-known as hard ceramic coatings that exhibit excellent wear resistance, high hardness, low friction coefficient, good corrosion resistance and biocompatibility [11,12].Various deposition techniques, such as Chemical Vapor Deposition (CVD), magnetron sputtering, Plasma Enhanced Chemical Vapor Deposition (PECVD), thermal spraying and cathodic arc physical vapor deposition (CAPVD), have been used to improve surface properties of titanium alloys [13-18]. Assessment of biocompatibility of implantable devices involves complex experiments, both in-vitro and in-vivo, on cell culture, tissue sections, and the whole body in order to study the effects of a particular material on the local and systemic response [8]. The International Organization for Standardization (ISO) (10993-Part 1) (2009) has listed several tests for biological evaluation of medical devices, including cytotoxicity and genotoxicity testing [8]. Our hypothesis is that a TiCN coating synthesized on Cp-Ti screw substrates may give better initial responses to blood and cells. In this study, a CAPVD technique was used to create a TiCN coating on Cp-Ti dental screw substrates.
The study described herein focused on the cell signaling in animals model and related surface properties of the TiCN coating synthesized on Cp-Ti. The study had two aims: (1) to understand how the cell response time affects the viability of cell and (2) Compare the effectiveness between TiCN screw shape and Au standard screw shape.
Materials and Methods
We used 3-[4,5-Dimethylthiazol-2-yl]-2,5-Diphenyltetrazolium Bromide (MTT), Dimethyl Sulfide (DMSO), 2′,7′-Dichlorofluorescein Diacetate (DCFH-DA), Malondialdehyde (MDA), Thiobar-Butiric Acid (TBA), n-Butanol, Tetramethoxypropane (TEP), Rotenone, Myxotiazole (Myx) and Antimycin A (AA), Tris-HCl, Acridine orange, Sucrose, MgCl2, KCl, MnCl2 potassium phosphate 2-aminoethy- lether- N, N, N′, N′-Tetraacetic acid (EGTA), Ethylenediamine Tetraacetic Acid (EDTA), O-Phthalaldehyde (OPA), N-Ethylmaleimide (NEM), 2,4-dinitrophenylhydrazine, Dulbecco's Modified Eagle Medium (DMEM), Trypsin II-S, Collagenase IA,Cytochrome c oxidase kit and ATP assay kit were purchased from Sigma Chemical Co (St. Louis, MO). Quantikine Rat/Mouse cytochrome c Immunoassay kit (Minneapolis ,U.S.A.), Xylocaine, Dentsply- Sankin was were purchased from Tokyo, Japan, Percoll was purchased from GE Healthcare Bio-Science AB, Uppsala, Sweden, Bovine serum albumin (BSA) and Fetal bovine serum (FBS) was purchased from Nichirei Bioscience, Tokyo, Japan.
Instruments
Quanta 250 FEG model scanning electron microscope GNR X-Ray; MCO-17AI CO2 Incubator, Japan; Eliwell EWPC 800, UKA; Refrigerated centrifuge, Harrier 18/80 laboratory,Japan; Floremetry ,Shimadzu RF-5000, Japan; Digital Scale ,DSJ; Shimadzu 20 E8 330H, Japan; Shaker, REAX2000, Iran; ELISA reader, In finite 200 M, Tecan; Flowcytometry, BD Biosciences FACS Calibur TM flowcytometer; Sonicator, Sonics & Materials, Newton, CT, USA; Luminometer ,Berthold Detection System, Germany and auto analyzer ,Orphee mythic 22 hematology analyzer; diamond diagnostic; USA were used in this study.
Preparation of TiCN screw
As substrate materials, commercially pure titanium (Cp-Ti) Grade 4 sheet with thickness of 3 mm were cut in 25 × 25 mm2 dimensions by using abrasive water jet method. These specimens were grinded by abrasive emery papers of SiC with grit sizes of 220–1200 mesh, and then polished with alumina powder with 1 μm grain size. Afterwards, samples were cleaned ultrasonically with ethanol for 10 min. Chemical composition of Cp-Ti samples provided by supplier was given in Table 1. The TiCN coating was coated on Cp-Ti substrate using a voestalpine eifeler coating machine. The coating process was performed at BARLOK PVD Co. in Istanbul/ Turkey. The deposition was carried out at a bias voltage of -100 V, a cathode current of 100 A and substrate temperature of 450°C. After put samples into chamber, it was evacuated to a base pressure of 3 × 10-2 mbar. The surface microstructure of the TiCN film deposited on Cp-Ti was observed by Quanta 250 FEG model scanning electron microscope. The crystalline structure of the film was investigated by the GNR X-Ray Explorer diffractometer using CuKα radiation (λ=1.5406 Å) with a Bragg-Brentano configuration (θ/2θ) and the scan range was from 20° to 90° at a scan speed of 2°/min.
Experimental design
The 15 male Sprague-dawley rats with weighing 150–200 g and 8 weeks old were purchased from Institute Pasteur, Tehran, Iran. Animals were homed in cages with a 12 h light/12 h dark cycle at 25 ± 3°C under normal environmental conditions and had free availability to tap water and pellet diet. All rats randomly divided into three groups, group 1; control group give normal saline injection, group 2; treatment group give Au standard implant (Au) and group 3; treatment group give TiCN coated implant (TiCN). Exposure time were 1, 15 and 30 days (n=5).
Surgical procedure
The rat root of the nose was shaved and dis-infected with 70% ethanol. Then, local anesthesia was implemented with 2% xylocaine/epinephrine and a skin flap was also made by linear minimum cutting to expose laterally the tooth gum. Dental holes made at the position of the eyes angel and 7 mm lateral towards the midline in which there were a tooth gum area and inside drilled (dental drill) with diameter of 1 mm and length of 1cm, with varying angles to the tooth gum under sterile saline flow. Au standard implant and TiCN coated implant were then inserted into the hole using a hand driver [19].
Blood histological analysis
Animals were euthanized with an intracranial injection of 1 mg/ kg gallamine triethiodide under general anesthesia. Blood sample of about 2 ml was collected into two polypropylene tubes (EDTA tube). First step, the peripheral blood samples were stained with H&E staining method for further evaluation. Moreover, the hemo-toxicity of Au standard and TiCN implant were evaluated in peripheral blood of animals. A hematological auto analyzer was utilized to distinguish various hematological parameters such as White Blood Cells (WBCs), neutrophils (%), lymphocytes (%), monocytes (%), eosinophils (%), basophils (%). Second step, the serum was accumulated by permitting the blood to clot at 37°C and then centrifuged at 3,000 rpm for 10 min and we determined Alkaline Phosphatase (ALP), Aspartate Amino transferase (AST), Alanine Aminotransferase (ALP), Urea, Creatinine Clearance (CR).
Fibroblast cells isolation
Rat tissue were extracted and immediately rinsed with boric acid for fungal infections prevention. Under a local anesthetic, a gingiva small section (2 × 1 × 1 mm) was removed by scalpel. Subsequently, the gingiva tissue was put in a DMEM. We obtained samples wash in a sterile solution (with PBS, pH=7.4) and thereafter transferred to a petri dish containing DMEM, where mechanically was minced by sterile scissors and a scalpel. Tissue suspension was centrifuged 200 × g for 5min. The re-suspended pellet placed in aplastic bottle containing DMEM with 10% FCS penicillin/streptomycin and amphotericin 100 μg/mL. Resulted monolayer cell cultures were incubated at 37ºC and humidified air 5% CO2 [20].
Osteoblastic cells isolation
Osteoblastic cells were isolated either enzymatically bone jaw male adult rat. The bone pieces were digested sequentially in a trypsin II-S (25 mg) and collagenase IA (70 mg) in 50 ml DMEM media culture at 37°C for 70 minutes. Bone was cut into pieces of approximately 2 × 2 mm2 by normal saline washed to remove blood and bone marrow. Bone pieces were incubated for 15 min at 37°C on the bottom of a standing tissue culture dish to let them adhere to the culture dish before standard DMEM was added. They were cultured until confluence in a controlled atmosphere (5% CO2/95% air, 37°C) [21].
Cell viability
Cell viability was evaluated by MTT staining. Briefly, the dehydrogenases can convert the molten form of MTT salt into crystalline propellant violet formulas using the co-enzyme NADH or NADPH. Formazan crystals dissolved in DMSO and fibroblast and Osteoblastic cells (1 × 104cells) incubated for 4h, 37°C and the absorbance was measured at 570 nm using ELISA reader [22].
Reactive oxygen species assay
Osteoblastic cells (1 × 106 cells) were treated with the Au standard and TiCN screw for 1,15 and 30 day. After treatment, cells washed with PBS. H2DCFD (10 mM) were measured ROS. These agents diffuse into the cells causing de-esterification. Subsequent reactions with peroxides generate fluorescent 5-chloromethyl-2′,7′ dichlorofluorescein. ROS was determined using a Shimadzu RF5000U fluorescence spectrophotometer set for at 495 nm excitation and 530 nm emission wavelengths [23].
Lipid peroxidation
Osteoblastic cells (1 × 106 cells) were incubated with Au standard and TiCN screw for 1,15 and 30 day at 30 °C. Then, 0.25 ml sulfuric acid was added to 0.2 mL cell fractions. Afterwards, 0.3 ml of a solution containing 0.2% TBA was added. All the samples were placed in a boiling water bath for 30 min. Samples were shifted to an ice-bath and 0.4 mL n-butanol was added to each tube. The amount of MDA formed in each of the sample was assessed through measuring the absorbance of the supernatant at 532 nm with an ELISA reader [24].
Glutathione disulfide and oxidized glutathione content assay
Therefore, intracellular GSH and GSSG was determined based OPA and NEM probe. Aliquots of the cell (1 × 106 cells) stained with OPA and NEM probe (5 μM) was separated from the incubation medium by 1 min centrifugation at 1000 rpm. Each sample was measured in cuvettes using a fluorescence spectrophotometer set for at 495 nm excitation and 530 nm emission wavelengths [23].
Adenosine triphosphate assay
Base on kit protocol, osteoblastic cells (1 × 106 cell) ATP levels were measured using Luciferin/Luciferase Enzyme system. Bioluminescence intensity was measured using Sirius tube luminometer [25].
Cytochrome c release assay
The cytochrome c Immunoassay kit was used to determining of cytochrome c level in cell by spectrophotometric method at 450 nm.
Lysosome membrane integrity assay
Constancy of lysosomal membrane was distinguished from the fluorescent dye redistribution and acridine orange; hence, allocated osteoblastic cells (1 × 106 cell) were stained by acridine orange (5 μM) in which cells separated from the incubation plate by centrifugation with 1 min at 1000 rpm. As a result, the osteoblastic cells pellet replaced in 2 mL of fresh DMEM. Acridine orange redistribution in the cells suspension was then measured spectrophotometer at the excitation and emission wavelength of 490 and 535 nm [23].
Osteoblastic mitochondria isolation
Osteoblastic cells (1 × 106 cells) were lysed by sonication in a medium consisting of 0.23 M mannitol, 0.07 M sucrose, 10 mM Tris-HCl, 1 mM EDTA, pH 7.4. Cells were centrifuged at 500 g for 10 min to discard nuclei and cell debris. The sediment was discarded and the supernatant was centrifuged at 11000 g for 10 min to obtain the enriched mitochondrial fraction. Mitochondrial protein concentration was determined by the Coomassie blue protein-binding method using BSA as the standard. To keep the uniformity of experimental condition, the mitochondrial samples (0.5 mg protein/mL) were incubated on 1h in all experiments [26].
Estimation of complex II activity
The activity of mitochondrial complex II (succinate dehydrogenase) was assayed through the measurement of MTT reduction. Briefly, mitochondrial suspensions and then 0.4% of MTT was added to the medium and incubated at 37°C. The product of purple formazan crystals was dissolved in 100 mL DMSO, and the absorbance at 570 nm was measured with an ELISA reader [22].
Evaluation of the role of complexes I and III
Au and TiCN screw induced ROS (H2O2) Formation. The role of complexes I and/or III in Au standard and TiCN screw induced H2O production was determined using a fluorescence spectrophotometer at 312 nm excitation and 420 nm emission wavelengths [27].
Measurement of cytochrome c oxidase
The colorimetric assay based on the observation that a decrease in the absorbance of ferricytochrome c at 550 nm was caused by its oxidation to ferricytochrome c by cytochrome c oxidase. Cytochrome c oxidase activities were calculated and normalized for protein per reaction, and the results were expressed as units per milligram mitochondrial protein.
Statistical analysis
Results are presented as mean ± SD. All statistical analyses were performed using the Prism software, version 6. Statistical significance was determined using the one-way ANOVA test, followed by the post hoc Tukey test. In some experiments, the two-way ANOVA test followed by the post hoc Bonferroni test was performed.
Results and Discussion
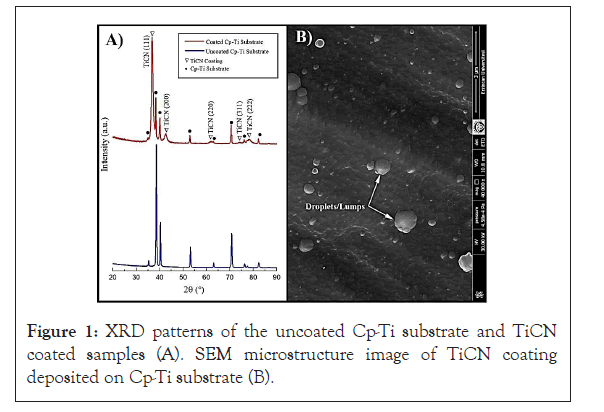
When biomaterial is implanted into the human body; it is unavoidable that blood will contact the implant surface [28]. Therefore, the implant surface can be covered with a layer of plasma proteins that will mediate the cellular responses and thereby lead to a better biocompatibility [29,30]. In our study, the coating process was successfully completed and it can be observed that new peaks related to the (111), (200), (220), (311) and (222) plane of the TiCN phases were formed on Cp-Ti substrates [31-33]. The microstructure and morphology of the TiCN coating deposited on Cp-Ti exhibited rough and irregular morphology and fine-grained surface without voids or pores. Nevertheless, there were some small macro particles, namely droplets/lumps or grains, on coating surface as shown in Figures 1A and 1B [34].
Figure 1: XRD patterns of the uncoated Cp-Ti substrate and TiCN coated samples (A). SEM microstructure image of TiCN coating deposited on Cp-Ti substrate (B).
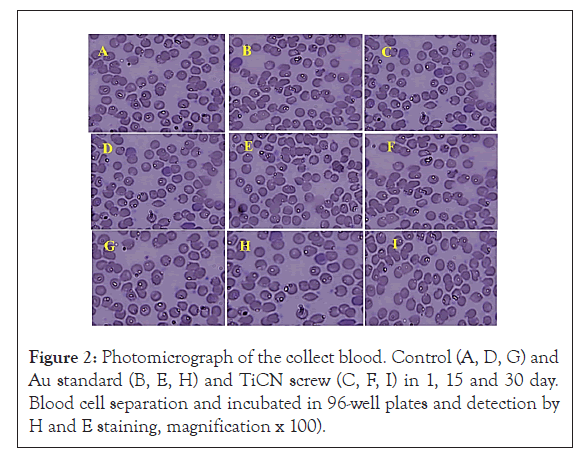
It is known that the characteristics of the interactions between blood and TiCN screw may affect cellular mechanisms Along with other studies focusing on the subject, Researchers studied the blood interactions with Ti implant surfaces of different roughness values [29,32]. No side effects of TiCN and Au standard screw compare to control group in morphology lymphocytes, neutrophil, monocytes and basophil, however an increase in the count of WBC cell that were standard level in all days were observed (Figure 2 and Table 1). ALP increases in TiCN coated screw (*p<0.05) but in Au standard screw there were no significant change compared to control group. AST and CR enzyme marker in Au standard and TiCN screw were not any different compared to control group after 1, 15 and 30 day periods as shown in Figures 3A-3E. ALT enzyme marker created difference (*p<0.05) on Au standard screw and TiCN screw compared to control group after15 and 30 day (Figure 3C) and finally BUN level have changed (*p<0.05) in Au standard and TiCN screw after 30 day period (Figure 3D).
| Count of WBC ( count; cells 106) | Control | Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Â Â Â Groups/day | Au | TiCN | |||||||
| 1 | 15 | 30 | 1 | 15 | 30 | 1 | 15 | 30 | |
| Lymphocytes | 22 | 24 | 22 | 25ns | 43* | 48* | 33* | 39* | 44* |
| Monocytes | 3 | 4 | 6 | 4ns | 6ns | 8ns | 4ns | 5ns | 9ns |
| neutrophil | 45 | 46 | 42 | 47ns | 50ns | 58* | 46ns | 53
|
58* |
| basophil | 2 | 2 | 0 | 2ns | 2ns | 2ns | 2ns | 2ns | 1ns |
The values are expressed as means ± SD (n=5). ns= non-significant and *P<0.05 compared with control group
Table 1: A hematological autoanalyzer was utilized to distinguish various hematological parameters such as WBCs, neutrophils (%), lymphocytes (%), monocytes (%).
Figure 2:Photomicrograph of the collect blood. Control (A, D, G) and Au standard (B, E, H) and TiCN screw (C, F, I) in 1, 15 and 30 day. Blood cell separation and incubated in 96-well plates and detection by H and E staining, magnification x 100).
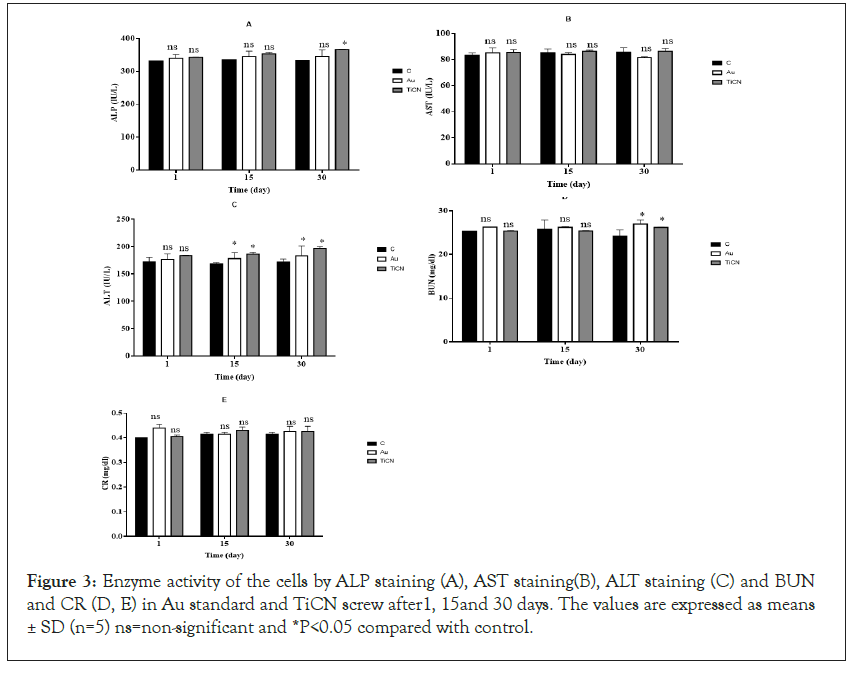
Figure 3: Enzyme activity of the cells by ALP staining (A), AST staining(B), ALT staining (C) and BUN and CR (D, E) in Au standard and TiCN screw after1, 15and 30 days. The values are expressed as means ± SD (n=5) ns=non-significant and *P<0.05 compared with control.
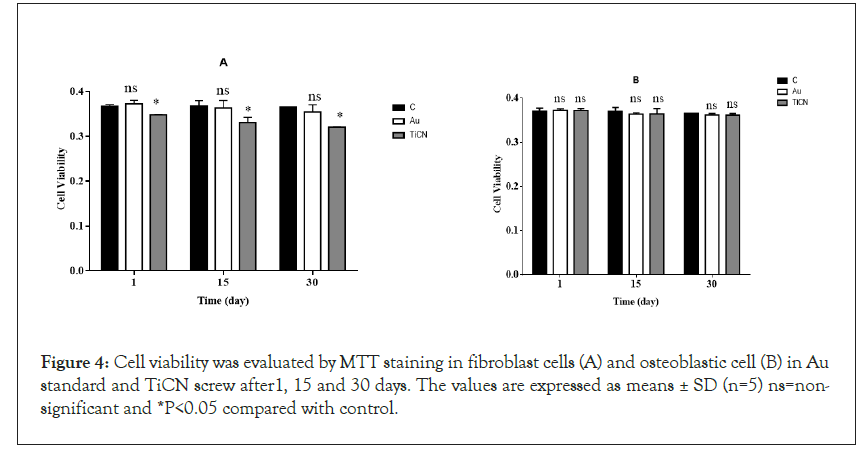
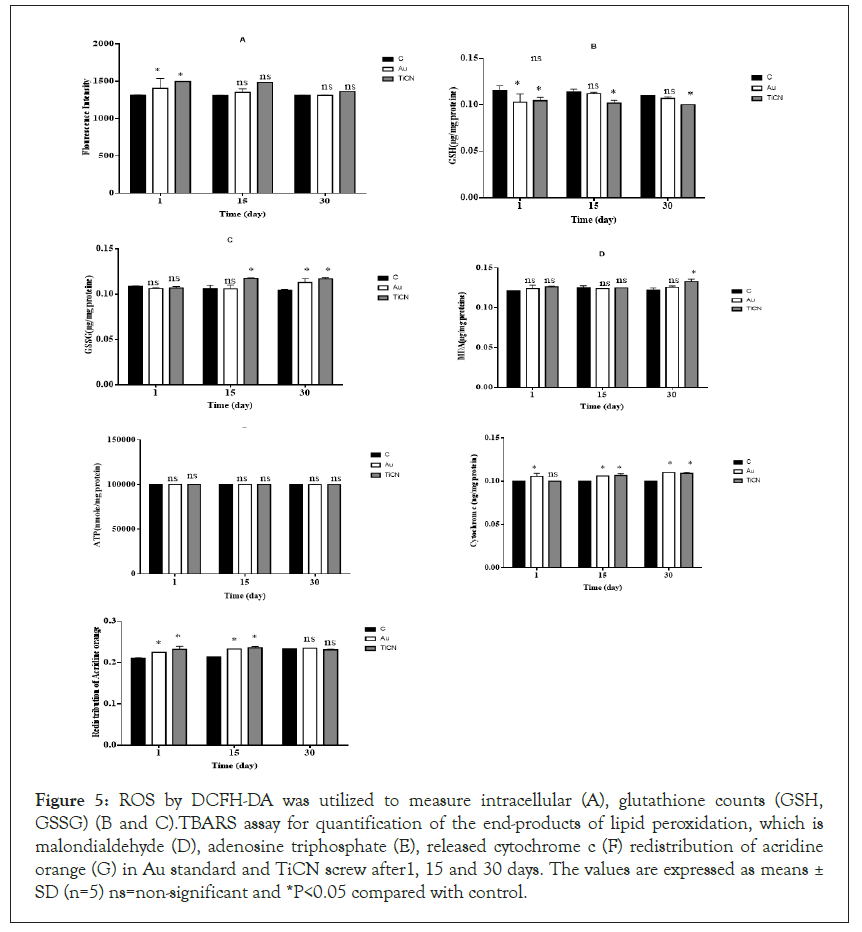
Apoptosis is the process of programmed cell death that occurs routinely in multicellular organisms during organ development, particularly during development of the immune system [34,35]. Apoptosis has been frequently noticed on implant surfaces; it may be induced by specific signals from other cells (death signals, Fas ligands, TNFa and NO) but it also occurs under the influence of inadequate environmental conditions, in particular by toxic substances [36-38]. Apoptosis occurs in several steps, beginning with membrane blabbing, it progresses with loss of cell membrane asymmetry and exposure of phosphatidylserine (outer cell surface), mitochondrial swelling through the formation of membrane pores, or increase the permeability of the mitochondrial membrane which causes apoptotic factors to leak out into the cytoplasm [39]. In our projects, cell viability, as can be seen in Figure 4A, in the groups (Au standard and TiCN screw) exposed to osteoblastic and fibroblastic cells, viability was evaluated by MTT staining in isolated cells minimal decreased compared to the control group (*p<0.05) in TiCN screw but in Au standard screw was not changed enzyme activity compared to control group in 1, 15 and 30 day. The enzyme activity was normal in fibroblast cells in the case of Au standard and TiCN screw compared to control group after all period time (Figure 4B). Mitochondrial toxicity often occurs in stress oxidative process and plays an important role in various diseases and generated of ROS in cells [35]. Thiol groups as a primary defense mechanism against cytotoxicity [36,37]. Rapid oxidation of the cysteine thiol (GSSG) in MPT pore region, opening of the contact material to mitochondria generated a collapse of the mitochondrial membrane potential and disruption of Electron Transport Chain (ETC) which is independent of the cytosolic ROS generated by redox cycling [39]. In our study, ROS detection by DCFH-DA was utilized to osteoblastic cells, the results showed in the groups (Au and TiCN screw), have not made any difference compared to the control group in 15 and 30 day and ROS low level increased after 1 day on two treatment groups (Figure 5A). As can be seen in the groups (Au standard and TiCN screws) exposed to cells, the GSH decreased in TiCN screw (*p<0.05) after 1, 15, 30 day in Figures 5B and 5C. Au standard screw did not have any change in 15 1nd 30 day. We showed GSSG level have no change in Au standard screw after 1, 15 day and TiCN after 1 day. GSSG was increased (*p<0.05) after 15, 30 day in cells on TiCN treatment group. Thiobarbituric acid reactive substances assay for quantification of the end-products of lipid peroxidation, malondialdehyde, as can be seen from osteoblastic cells exposure to Au standard and TiCN coated screws, the peroxidation of lipid increased (*p<0.05) in TiCN coated screw after 30 day compared to the control group and was not increased in the peroxidation of lipid on Au standard (after 1, 15, 30 day) and TiCN (1, 15 day) screw (Figure 5D). In Figure 5E, ATP level did not change in Au standard and TiCN screw groups in osteoblastic cells compared to control group. Cytochrome c in osteoblastic cells in Au standard and TiCN (except 1 day) screw (*p<0.05) did not have release after all of day (Figure 5F). Redistribution of acridine orange increased (*p<0.05) in osteoblastic cells that contacted Au standard and TiCN screws on 15 and 30 day periods (Figure 5G). Complex II was not change in the mitochondrial structure in all of time (Figure 6A).
Figure 4: Cell viability was evaluated by MTT staining in fibroblast cells (A) and osteoblastic cell (B) in Au standard and TiCN screw after1, 15 and 30 days. The values are expressed as means ± SD (n=5) ns=nonsignificant and *P<0.05 compared with control.
Figure 5: ROS by DCFH-DA was utilized to measure intracellular (A), glutathione counts (GSH, GSSG) (B and C).TBARS assay for quantification of the end-products of lipid peroxidation, which is malondialdehyde (D), adenosine triphosphate (E), released cytochrome c (F) redistribution of acridine orange (G) in Au standard and TiCN screw after1, 15 and 30 days. The values are expressed as means ± SD (n=5) ns=non-significant and *P<0.05 compared with control.
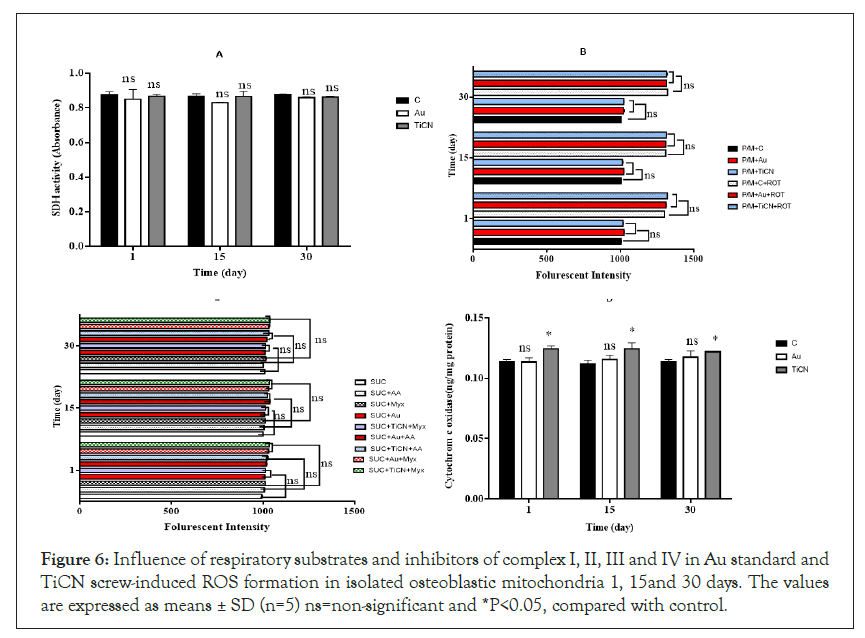
Figure 6: Influence of respiratory substrates and inhibitors of complex I, II, III and IV in Au standard and TiCN screw-induced ROS formation in isolated osteoblastic mitochondria 1, 15and 30 days. The values are expressed as means ± SD (n=5) ns=non-significant and *P<0.05, compared with control.
To assess the involvement of complex I and III on Au standard and TiCN coated screw induced low level H2O2 (a member of the ROS family) production in osteoblastic mitochondria. There was an elevation in low level H2O2 production following the addition of complex I inhibitor rotenone (complex I inhibitor) into the incubation medium containing complex I substrate (malate and pyruvate) (Figures 6B and 6C), compared to that observed in the presence of sole complex I substrate. We did not see significantly production of H2O2 following contact with Au standard and TiCN screws plus rotenone (Figure 6B). There was also a minimal increase in H2O2 production following the addition of Complex III linked substrate succinate into the incubation DMEM (Figure 6C). Whereas contact of TiCN screw plus the respiratory complex III inhibitor, Antimycin A in complex III linked substrate succinate increases H2O2 generation in isolated mitochondria compared to Antimycin A plus succinate (Figure 6B). Activity of c0079 tochrome c oxidase (complex IV), one of the most important enzymes in the mitochondrial respiratory complex, was also determined in the isolated osteoblastic mitochondria after contact with TiCN screw except Au screw and it minimal increased activity of complex IV compared to control group in all of time (*p<0.05) (Figure 6D). We could easily produce a new dental screw and tested in animal. This TiCN coating synthesized on Cp-Ti compared to standard medical screw did not significantly cellular toxicity in whole blood, fibroblast and osteoblastic cell.
Conclusion
Rat tissue was examined for the cellular toxicity of TiCN compare with Au standard group. Results showed that fibroblast and osteoblastic cell treated with TiCN low level changed oxidative stress factor (cell viability, ROS formation, GSH, GSSG, MDA level, release of cytochrome c and ATP) and mitochondrial complex (complex I, II, III, IV). The present study hereby provides the first evidence for the stability of TiCN that improved stress oxidative pathway. Consequently, low-level cytotoxicity effects of TiCN (compared Au standard) that use in dental application.
Acknowledgements
The results presented in this paper were partly extracted from project of Parvaneh Naserzadeh, researcher from Radiation Biology Research Center, Iran University of Medical Sciences, Tehran, Iran and Abbas Razmi, PhD from Faculty of Engineering, Mechanical Engineering, Department, Construction and Manufacturing Division, Ataturk University, Erzurum, Turkey that performed their project under the supervision of Dr Behnaz Ashtari and Dr Ruhi Yesildal. The investigation was carried out in Dr Behnaz Ashtari laboratory at Iran University of Medical Sciences, Ruhi Yesildal laboratory at Engineering, Mechanical, Ataturk University. The authors received no funding from national or international sources.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Roberts HW, Berzins DW, Moore BK, Charlton DG. Metal‐ceramic alloys in dentistry: a review. Journal of Prosthodontics: Implant, J Esthet Restor Dent. 2009;18(2):188-194.
- Duffo GS, Castillo EQ. Development of an artificial saliva solution for studying the corrosion behavior of dental alloys. Corros. 2004;60(6):594-602.
- Souza JC, Henriques M, Teughels W, Ponthiaux P, Celis JP, Rocha LA. Wear and corrosion interactions on titanium in oral environment: literature review. J Bio- Tribo-Corros. 2015;1(2):13.
- Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000;83(2):223-234.
- Ataibis V, Taktak S. Characteristics and growth kinetics of plasma paste borided Cp–Ti and Ti6Al4V alloy. Surf Coat Technol. 2015;279:65-71.
- Lautenschlager EP, Monaghan P. Titanium and titanium alloys as dental materials. Int. Dent. J. 1993;43(3):245-253.
- Sivaprakasam P, Hariharan P, Gowri S. Modeling and analysis of micro-WEDM process of titanium alloy (Ti–6Al–4V) using response surface approach. Int J Eng Sci Technol. 2014;17(4):227-235.
- Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006 Mar 1;27(9):1728-1734.
- Jinlong L, Huasheng Z, Yongxin W. Dynamic tribochemical behavior of TiN/TiCN coated Ti6Al4V in artificial seawater. RSC Adv. 2016;6(107):105854-105861.
- Elias, C.N., et al., Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J Mater Res Technol. 2019; 8(1):1060-1069
- Antunes RA, Rodas AC, Lima NB, Higa OZ, Costa I. Study of the corrosion resistance and in vitro biocompatibility of PVD TiCN-coated AISI 316 L austenitic stainless steel for orthopedic applications. Surf Coat Technol. 2010;205(7):2074-2081002E
- Uddin GM, Jawad M, Ghufran M, Saleem MW, Raza MA, Rehman ZU, et al.Experimental investigation of tribo-mechanical and chemical properties of TiN PVD coating on titanium substrate for biomedical implants manufacturing. Int J Adv Manuf Syst. 2019;102(5):1391-1404.
- Bao M, Xu X, Zhang H, Liu X, Tian L, Zeng Z, et al. Tribological behavior at elevated temperature of multilayer TiCN/TiC/TiN hard coatings produced by chemical vapor deposition. Thin Solid Films. 2011 Nov 1;520(2):833-836.
- Saoula N, Madaoui N, Tadjine R, Erasmus RM, Shrivastava S, Comins JD. Influence of substrate bias on the structure and properties of TiCN films deposited by radio-frequency magnetron sputtering. Thin Solid Films 2016;616:521-529.
- Wang HL, He JL, Hon MH. Sliding wear resistance of TiCN coatings on tool steel made by plasma-enhanced chemical vapour deposition. Wear. 1993;169(2):195-200.
- Mi P, He J, Qin Y, Chen K. Nanostructure reactive plasma sprayed TiCN coating. Surf Coat Technol. 2017;309:1-5.
- Zhu L, He J, Yan D, Xiao L, Dong Y, Zhang J, et al. Synthesis and microstructure observation of titanium carbonitride nanostructured coatings using reactive plasma spraying in atmosphere. Appl Surf Sci. 2011;257(20):8722-8727.
- Hacisalihoglu I, Yildiz F, Alsaran A. Wear performance of different nitride-based coatings on plasma nitrided AISI M2 tool steel in dry and lubricated conditions. Wear. 2017;384:159-168.
- Jia WT, Zhang X, Luo SH, Liu X, Huang WH, Rahaman MN, Day DE, Zhang CQ, Xie ZP, Wang JQ. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010;6(3):812-819.
- Simain‐Sato F, Lahmouzi J, Heinen E, Defresne MP, De Pauw‐Gillet MC, Grisar T, Legros JJ, Legrand R. Graft of autologous fibroblasts in gingival tissue in vivo after culture in vitro preliminary study on rats. J Periodontal Res. 1999;34(6):323-328.
- Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas NP, Raschke M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32(5):521-531.
- Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem.2002;13(5):273-281.
- Eskandari MR, Fard JK, Hosseini MJ, Pourahmad J. Glutathione mediated reductive activation and mitochondrial dysfunction play key roles in lithium induced oxidative stress and cytotoxicity in liver. Biometals. 2012;25(5):863-873.
- Mashayekhi V, Tehrani KH, Hashemzaei M, Tabrizian K, Shahraki J, Hosseini MJ. Mechanistic approach for the toxic effects of perfluorooctanoic acid on isolated rat liver and brain mitochondria. Hum Exp Toxicol. 2015;34(10):985-996.
- Tafreshi NK, Hosseinkhani S, Sadeghizadeh M, Sadeghi M, Ranjbar B, Naderi-Manesh H. The influence of insertion of a critical residue (Arg356) in structure and bioluminescence spectra of firefly luciferase. J Biol Chem. 2007;282(12):8641-8647.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-254.
- Barja G. Minireview: The Quantitative Measurement of H 2 O 2 Generation in Isolated Mitochondria. J. Bioenerg. 2002;34(3):227-333.
- Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000;11(6):530-539.
- Abrahamsson I, Zitzmann NU, Berglundh T, Wennerberg A, Lindhe J. Bone and soft tissue integration to titanium implants with different surface topography: an experimental study in the dog. Int J Oral Maxillofac Implants. 2001;16(3).
- Zheng J, Hao J, Liu X, Gong Q, Liu W. A thick TiN/TiCN multilayer film by DC magnetron sputtering. Surf Coat Technol. 2012;209:110-116.
- Kumar TS, Jebaraj AV, Shankar E, Tamiloli N, Sivakumar K. Metallurgical and mechanical characterization of TiCN/TiAlN and TiAlN/TiCN bilayer nitride coatings. Surf Interfaces. 2019;15:256-264.
- Park JY, Gemmell CH, Davies JE. Platelet interactions with titanium: modulation of platelet activity by surface topography. Biomaterials. 2001 Oct 1;22(19):2671-82.
- Thor A, Rasmusson L, Wennerberg A, Thomsen P, Hirsch JM, Nilsson B, Hong J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials. 2007;28(6):966-974.
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495-516.
- Wyllie AH. Apoptosis: an overview. British medical bulletin. 1997;53(3):451-465.
- Park J, Bauer S, Schmuki P, Von Der Mark K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009;9(9):3157-3164.
- Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, et al. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. PNAS. 2002;99(16):10287-10292.
- Secchi AG, Grigoriou V, Shapiro IM, Cavalcanti‐Adam EA, Composto RJ, Ducheyne P, et al. RGDS peptides immobilized on titanium alloy stimulate bone cell attachment, differentiation and confer resistance to apoptosis. J Biomed Mater Res. A: J Biomed Mater Res B, JBMRGL, and J Biomed Mater Res B Appl Biomater. 2007;83(3):577-584.
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205-219.
Citation: Naserzadeh P, Razmi A, Yesildal R, Ashtari B (2021) Cellular Toxicity Mechanisms of TiCN-Cp-Ti Screw on Tooth Gum Cells in Rat.Biochem Anal Biochem. 10:404.
Copyright: © 2021 Naserzadeh P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.