Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 2
Brominated Organic Biocides Control Lactic Acid-Producing Bacteria in Bioethanol Fermentation Matrices
Christopher L. Wiatr1*, Julie Bazzell2 and Rita deCassia Bortolo Porto32Department of Microbiology, Southern Illinois University, Illinois, USA
3Buckman Laboratories International, Inc., Brazil, South America
Received: 10-Apr-2023, Manuscript No. JMBT-23-20909; Editor assigned: 15-Apr-2023, Pre QC No. JMBT-23-20909 (PQ); Reviewed: 28-Apr-2023, QC No. JMBT-23-20909; Revised: 05-May-2023, Manuscript No. JMBT-23-20909 (R); Published: 12-May-2023, DOI: 10.35248/1948-5948.23.15.549
Abstract
Over a million barrels of bioethanol are made every day by fermenting plant-based biomass. Since bioethanol manufacturing is not aseptic, addition of antimicrobial agents is of paramount importance to protect ethanol fraction from Lactic Acid Bacteria (LAB) that can grow in the acid environment of the process, contaminate the system, and convert ethanol to unsalable organic acids. Antibiotics are typically applied. Unfortunately, antibiotics are chemically stable and carry through the course of the process and contaminate the solids, called Dried Distiller Grains with Solubles (DDGS) that are collected post-distillation and sold as animal feed for cattle, pigs and poultry. This research evaluated the effectiveness of alternative antimicrobial methods. Quick-killing brominated biocides, 2,2-DiBromo-3-Nitrilo-PropionAmide (DBNPA) and 2-Bromo-2-Nitrilo-Propane-1,3-Diol (BNPD) were investigated as antimicrobial agents to control viable acid-producing bacteria Lactobacillus plantarum and Acetobacter cerevisiae that commonly infect bioethanol fermentation. In a pilot plant study fermenting corn to ethanol using yeast, DBNPA was found effective against these bacteria at a stepwise dose-response from 25 mg/L to 200 mg/L, with an optimal dose reaching 200 mg/L. However, BNPD was not effective at 25 mg/L, but it was effective at 100 mg/L and 200 mg/L. The organic bromicides were then advanced to field trials in a corn-to-ethanol industrial plant. DBNPA killed 3 log10 LAB and nearly 3 log10 total heterotrophic bacteria at a dosage of 100 mg/L, whereas BNPD had a kill that approached 2 log10 for LAB and total heterotrophic bacteria at the same dosage. At a cane sugar plant, the organic bromicides at 100 mg/L were effective in cane syrup with DBNPA outperforming BNPD; however, lower dosages of both biocides were not. During the trials, antibiotics employed at typical application dosages resulted in comparatively unsatisfactory effects, decreasing LAB or total population of bacteria only one log10 (vs. controls). Moreover, during biochemical tests of fermentation corn mashes infected by LAB growing 62 hours, DBNPA doses at ≥ 100 mg/L significantly reduced the final lactic acid level 14-fold, and it completely eliminated the effect of bacterial infection on ethanol yield. DBNPA had no adverse effects on the ethanol production rate, increasing the ethanol yield 2%. An addition of only 0.5% in bioethanol yield is valued at approximately a $4 million additional output at a 50 MGY plant. Furthermore, since DBNPA was previously found analytically to degrade in fermentation co-products and not reach DDGS, this microbicidally effective organic bromicide may also provide a successful alternative to antibiotics for controlling bacterial infections in fuel-ethanol production and thereby help remove antibiotics from the food chain, obviating antibiotic resistance development.
Keywords
2,2-Dibromo-3-nitrilopropionamide; 2-Bromo-2nitro-propane-1,3-diol; Antibacterial resistance; Microbicides; Bioethanol; Dried Distiller Grains with Solubles (DDGS); Lactic Acid Bacteria (LAB)
Introduction
Bioethanol is a clean, renewable, energy source created by fermenting plant-based materials. During the corn-to-ethanol process, two salable products are made: Bioethanol and Dried Distiller Grains with Solubles (DDGS). Bioethanol is fermented from corn biomass, distilled off, and is sold as an admixture with gasoline. After distillation and centrifugation, DDGS also is collected as a nutrient-rich residue and is sold as animal feed or feed supplement for cattle, pigs and poultry.
At the start of 2022, bioethanol capacity reached 22.1 billion gal/ yr in the USA [1]. At the beginning of 2023 ethanol production is expected to average 1.02 million barrels per day [2]. The transportation grade bioethanol market is forecasted at $66.7 billion. Globally, North America, Europe, and a few Asian countries continue to convert to greener, eco-friendly, technologies, such as bioethanol, to achieve zero carbon emissions. Thus, the global bioethanol market size is expected to expand to reach $91,365.82 million by 2028 [3]. With that, DDGS is also growing as a major byproduct.
DDGS consists of no fermentable components of the corn kernel (e.g., yeast, biomass and glycerol) [4]. During corn fermentation, bacterial contamination and spoilage of products are common phenomena since biological and chemical processing are not performed under aseptic conditions [5]. For example, the equipment used and its food content are subjected to infection from bacteria entering from various sources such as water, raw materials, equipment, plant personnel, insects and sometimes even stray birds. Lactic Acid Bacteria (LAB) are particularly a problem as they compete with the yeast for nutrients, oxidize ethanol to lactic acid or acetic acid, decreasing the ethanol yield and causing loss of salable products. Costs of subsequent clean- up and sanitization are staggering in terms of labor, loss of raw materials, purified water, containers, labels, finished products and packaging. Prevention of contamination is obviously of paramount importance. To prevent and treat bacterial infections, antibiotics, such as penicillin and virginiamycin, are often added to fermentations [6-8]. However, these antibiotics are chemically and thermally stable and carry over into the DDGS. DDGS is then sold to make animal feeds and in turn can lead to antibiotic resistance in the food chain [9-13]. Recently, the FDA has become more concerned about antibiotic residues in DDGS [9,10]. As a result, interest in alternative strategies for controlling bacterial infections in the ethanol industry has increased. One such strategy is the application of organic bromicides such as 2,2-Dibromo-3- Nitrilopropionamide (DBNPA; Figure 1a) and 2-Bromo-2-Nitro- Propane-1,3-Diol (BNPD; Figure 1b) during fermentation [11-13]. Using either one of these successfully to control acid-producing bacteria such as Lactobacillus and Acetobacter would help circumvent antibiotic resistance problems. Unfortunately, application of brominated organic biocides in fermentation is underrepresented in the literature (Figures 1a and 1b).
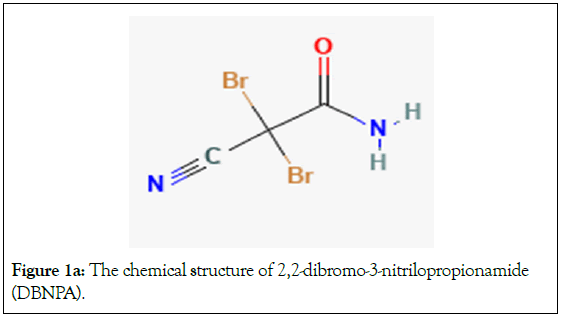
Figure 1a: The chemical structure of 2,2-dibromo-3-nitrilopropionamide (DBNPA).
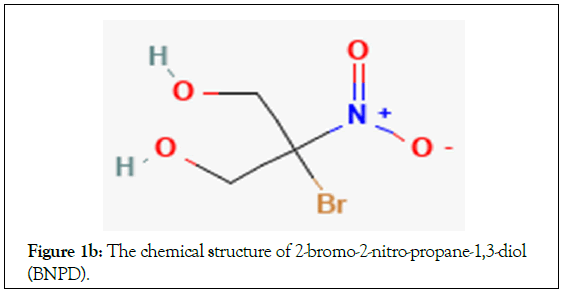
Figure 1b: The chemical structure of 2-bromo-2-nitro-propane-1,3-diol (BNPD).
DBNPA is of special interest because it was recently found to break down in fermentation coproducts and not carry over into DDGS [14]. DBNPA is an acetamide biocide originally applied as a seed fungicide [15,16]. Currently DBNPA is used effectively to limit bacterial growth in different water applications, such as cooling tower water, paper processing, and coatings [17-19]. In these applications, DBNPA is a favorable microbicide because of its instantaneous antimicrobial activity and its quick breakdown to nontoxic byproducts [11,20]. Its characteristics of quick-kill plus the fact that DBNPA subsequently breaks down would be beneficial to another water application, bioethanol production.
Whole stillage is comprised of nonvolatile residues produced by removal of ethanol from corn-based fermentation beer by distillation. It typically contains 95% water and 5% residual material. This includes fermentation byproducts, residual fermentable sugars and nonfermentable components of corn such as protein, triglycerides, and free fatty acids and corn fiber [20]. Because whole stillage is a critical intermediate product of the operations that eventually results in the production of DDGS, it was selected as a sample matrix for this study. To achieve our microbiological goals of this study, we investigated how well DBNPA controlled acid-producing bacteria in fermentation matrix. For that we used Lactobacillus plantarum (renamed recently Lactoplantibacillus plantarum) and Acetobacter cerevisiae because these bacteria were reported to be chief contaminants of multidrug resistance in bioethanol systems [21-23], frequently infect ethanol systems, and oxidize ethanol to lactic acid and acetic acid [24]. We challenged these bacteria and measured the performance of DBNPA vis-à-vis another brominated organic biocide, 2-Bromo-2-Nitro-Propane-1,3-Diol (BNPD) which is also frequently applied to water treatment systems. We then expanded our study to assess how well the brominated organic biocides would perform in the field against antibiotics in practice by measuring both the control of LAB and the general bacterial population in bioethanol fermentation coproducts made in two bioethanol plants. One plant produced bioethanol from corn; the other from sugar cane. Pursuant to those results, we selected the best candidate and elucidated its effective biocide range. In comparing the toxicology of each biocidal active, we tested the best antimicrobial further in process by measuring the biochemistry of the byproduct yields post application. The findings led us to make recommendations on using the most successful antimicrobial fermentation applications to control LAB and heterotrophic bacteria and would also consequentially combat antibiotic resistance.
Materials and Methods
Chemicals and biochemicals
The biocides used in this study were 2,2-Dibromo-3-Nitrilo- propionamide (DBNPA) as 20% active (Mw 241.87 Da, CAS#10222-01-2) and 2-Bromo-2-Nitro-Propane-1,3-Diol (BNPD) as 20% active (Mw 199.99 Da, CAS#52-51-7) and were obtained from Dow Chemical Co. (Midland, MI) and Sigma-Aldrich (St. Louis, MO). Both are EPA approved pesticides under Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) and are used widely in industrial water applications. DBNPA is also represented as Bronam® 20 or Busan® 94 in technical literature and in field work previously reported [14]. It is important to note that for this project other brominated biocides frequently used in water treatment applications, such as 1,3-Dibromo-5,5-Dimethyl Hydantoin (DBDMH), 1-Bromo-3-Chloro-5,5-Dimethyl Hydantoin (BCDMH), similar halogenated isocyanurates and HOBr were purposely omitted because these are oxidizing biocides that would quickly destroy yeast cells that are needed to ferment the corn into bioethanol. As biocides, oxidizers are also poor performers in the presence of high solid systems. Furthermore, hydrations are also known to degrade chemically into formaldehyde, and isocyanurates are stable organic rings that are difficult to eliminate from water systems, both of which should be avoided in a food system. Microbiological media were obtained from Fisher Scientific (Memphis, TN) and Sigma-Aldrich (St. Louis, MO). Yeast nutrient AYF1177 was obtained from Ethanol Technology (Milwaukee, WI). Cycloheximide was obtained from the latter. Enzyme alpha amylase (Liquozyme SC DS) and glucoamylase (Spirozyme Fuel) were obtained from NovozymesTM (Franklinton, NC).
Microorganisms
Homofermentative Lactobacillus plantarum ATCC 149179 (now called Lactiplantibacillus 202195) [21], and Acetobacter cerevisiae ATCC 23765 were obtained in lyophilized form from the American Type Culture Collection (Manassas, VA) and were reconstituted according to ATCC instructions. A.cerevisiae was chosen because it appeared to be successfully grow in fermentation broth. The yeast, Saccharomyces cerevisiae Ethanol Red, was obtained from Fermentis (Marq-en-Baroeul, France). This yeast strain was used because it performs well in both anaerobic and aerobic conditions [25].
Preparation of bacteria and yeast
The amount of bacterial culture needed to achieve an initial concentration of ~107 CFU/ml in the corn mash was estimated from growth curve data for both bacteria. The bacteria were reconstituted from freezer stocks. For Lactobacillus a standard 1:100 dilution was made in DeMan, Rogosa-Sharpe (MRS) broth and grown overnight (24 h). However, for Acetobacter 1 ml of freezer stock was diluted into 10 ml YPM broth overnight (24 h) and then transferred 90 ml of YPM broth overnight. Flasks were incubated at the respective temperatures and shaken at 150 rpm. The A600 nm of the 100 ml broth cultures was checked at 25 h. All suspensions were plated using 200 µl and incubated 48 h before enumeration; at 37°C. (L. plantarum) and 26°C (A. cerevisiae) before colony counting was attempted. A 0.2 g/ml suspension of Saccharomyces cerevisiae was prepared in a sterile 250 ml flask. This suspension was incubated and mixed for 20 min at 40°C before inoculation into the fermentation flasks. Each fermentation flask was inoculated with 160 µl of the yeast suspension to attain an initial concentration of 107 yeast cells/ml. After the initial 2 h incubation with bacteria, the flasks were inoculated with yeast and dosed immediately with antimicrobials such as DBNPA or BNPD. The performance of DBNPA and BNPD in the presence of yeast was tested according to the experimental design given in Table S1. These experiments were conducted in the presence of dry solids concentration of 30% (w/w) in 4 h fermentation.
Fermentation-preparation corn slurry
Corn (Zea mays) was prepared very similarly to that in reference [14]. The moisture of the ground maize was determined gravimetrically using a moisture balance by measuring the mass loss that occurred during drying (LMET 145.5.50-Moisture-Mettler Toledo HR83. doc). Amounts of corn, Deionized (DI) water and enzyme needed to prepare 160 g of corn slurry at a total dry solids concentration of 30% (w/w) for each replicate was determined using a proprietary mash-calculator spreadsheet. For each treatment, three independent replicate slurries were prepared by weighing the required amount of DI water into labeled Labomat beakers, followed by the addition of the required mass of corn flour. Alpha-amylase enzyme was diluted to ensure precise delivery of enzyme to each flask. A 0.13 g/ml working solution of alpha-amylase was used and added at a dose rate of 0.02% (w/w) based on the wet weight of the corn. The slurries were hand-swirled after all the components were in the Labomat beakers. Sealed beakers were attached to a vertically mounted wheel in the Labomat (Model BFL 12 805, Mathis Switzerland), which rotated at 50 rpm during the incubation. The wheel was programmed to reverse direction every 50 sec to improve the mixing efficiency. Samples were liquefied by incubating at 83°C for 90 min, after which the samples were cooled to 40°C in the Labomat.
Fermentation-inoculation and measurements
Once the mash was cooled, the entire contents (approximately 160 g) of each Labomat beaker were transferred to a sterile 250 ml Erlenmeyer flask. The masses of the mash and the flasks were recorded, and the mass of mash transferred to the fermentation flasks was calculated. The pH of the mass was adjusted to <5.2 by the addition of 150 µl of 10 N sulfuric acid. The flasks were shaken at 170 rpm on an incubator-shaker (Sartorius, Certomat BS-1) at 32°C until preparation of all mashes was complete. All enzymes, nutrients, and other amendments added to the fermentation flasks were freshly prepared before use. The yeast nutrients were prepared as a 0.2 g/ml solution, and a dose of 1500 mg/L (w/w, based on the wet weight of corn) was used. Urea was added as a sterile 0.2 g/ml solution to a final concentration of 500 mg/L as nitrogen (w/w, based on the total mass of mash). The glucoamylase enzyme (Spirizyme Fuel, Novozymes) was prepared as a 0.25 g/ml solution and added at a dose of 0.015% (w/w, based on the wet weight of corn).
L. plantarum culture was prepared for inoculation by growing throughout the day in 100 ml of MRS broth. The amount of culture needed to achieve an initial concentration of 107 CFU/ ml in the corn mash was estimated from growth curve data for L. plantarum in MRS broth. The initial concentration of the bacteria was determined by plating serial dilutions of the culture containing cycloheximide and incubating at 32°C for 48 h before counting colonies. Fermentation flasks were inoculated with 0.5 ml of this L. plantarum culture, which had a bacterial cell concentration of 2 × 109 CFU/ml. This gave an initial concentration of 6 × 106 CFU/ ml.
After inoculating with lactobacilli, the fermentation flasks were fitted with sanitized fermentation traps and incubated at 32°C with shaking at 170 rpm for 1 h. This simulated the typical time between the beginning of a fermenter fill and inoculation with yeast in full scale, fuel-ethanol facilities.
A 0.2 g/ml suspension of Saccharomyces cerevisiae was prepared in a sterile 250 ml flask. This suspension was incubated and mixed for 20 min at 40°C before inoculation into the fermentation flasks. Each fermentation flask was inoculated with 160 µl of the yeast suspension to attain an initial concentration of 107 yeast cells/ ml. After the initial 1 h incubation with bacteria, the flasks were inoculated with yeast and dosed with DBNPA (Bronam®20). See Table S2.
The mass of each flask was recorded after all additions were made, and the sanitized fermentation traps were reinserted into each flask. The flasks were incubated at 32°C with shaking at 170 rpm for 61 h. Periodically the mass of each flask (with the traps in place) was measured to estimate the rate of fermentation (the mass of the fermentation flasks decreases when CO2 is lost by bubbling out of the fermentation traps).
After that incubation period, the mass of each flask was measured before and after removing the trap. While hand swirling, 1.0 ml of mash was pipetted from each sample and transferred to a test tube containing 9.0 ml of 0.05 M phosphate buffer. These samples were then serially diluted to achieve dilution factors of 10-5 to 10-7. One hundred microliters of each of the highest three dilutions was then plated on MRS agar to estimate the final concentration of L. plantarum in each flask. The plates were incubated for 2 days at 32°C, and then the bacterial colonies were counted. Each flask was mixed with an overhead agitator, and samples were collected for the following measurements: Yeast cell counts, sugar, final concentrations of fermentation products ethanol, lactic acid, total dry solids, dissolved dry solids and the density of the beer liquid phase. Samples were prepared for yeast cell counts by diluting by a factor of 100 in DI water, subsequently staining with methylene blue to estimate viability and then counted microscopically using a hemocytometer. The final concentrations of substrates and fermentation products were determined by HPLC. In preparation for HPLC samples were centrifuged (8,000 × g, 3 min), filtered, acidified to 0.01 N, dried, and then measured gravimetrically based on mass loss during drying for 3 h at 105°C. The density of the beer liquid was measured using an Anto-Parr densitometer. Lactic acid and ethanol concentrations were measured by HPLC.
Field trials
A schematic of a corn-to-bioethanol plant was previously described [11-14,26]. Proprietary field trials were conducted in a corn- to-ethanol plant using maize (Zea mays) in midwestern United States. There, the bacteria were indigenous to the system process, fermentation matrix. Before the trial, since the systems were thoroughly cleaned and then rinsed with chlorine-free permeate RO water as done in previous trials [14], the indigenous bacteria most likely originated from corn and additives introduced to the process. Exposure of DBNPA, BNPD, or antibiotic (Table 1), in corn-to-ethanol fermentation coproducts, was carried out as slug doses for 4 h exposure prior to plating (Table 1). The thermochemical conditions were 34 ± 2°C for 48 h., pH 4.5. Yeast was the same as in the laboratory experiments described above. Samples were drawn into sterile containers. LAB was measured on MRS and total (primarily) heterotrophic, facultative anaerobes also were determined using TGE medium. Similarly, a cane sugar syrup- matrix was challenged (Table 2).
| Bacteria post DBNPA and BNPD treatment of corn-to-ethanol fermentation | |||
|---|---|---|---|
| Antimicrobial | Dose(mg/L) | TPC | LAB |
| BNPD | 30 | N.D. | N.D. |
| 50 | 5.632 | 5.592 | |
| 100 | 4.342 | 4.445 | |
| 200 | 3.556 | 3.079 | |
| DBNPA | 30 | N.D. | N.D. |
| 50 | 5.634 | 5.519 | |
| 100 | 3.832 | 3.256 | |
| 200 | 2.716 | 2.278 | |
| Antibiotic standard | 5 | 4.995 | 4.939 |
| No treatment control | 0 | 6.532 | 6.291 |
Note: TPC: Total bacteria; LAB: Lactic Acid Bacteria; N.D.: Not Determined
Table 1: Total bacteria and LAB in corn-to-ethanol fermentation before and after treatment with DBNPA, BNPD, or an antibiotic. In every case, the antimicrobial contact time was 4 h. (See Methods.) Total bacteria (TPC) were determined on TGE agar. Lactic Acid Bacteria (LAB) were determined on MRS. The counts are represented as log10 of CFU/mL. N.D.=Not Determined.
| Bacteria post DBNPA and BNPD treatment of sugar cane syrup | |||
|---|---|---|---|
| Antimicrobial | Dose (mg/L) | TPC | LAB |
| BNPD | 30 | 5.542 | 5.513 |
| 50 | 4.857 | 4.835 | |
| 100 | 3.591 | 3.518 | |
| 200 | N.D. | N.D. | |
| DBNPA | 30 | 4.741 | 4.204 |
| 50 | 4.303 | 4.217 | |
| 100 | 3.475 | 2.819 | |
| 200 | N.D. | N.D. | |
| Antibiotic Standard | 3.5 | 4.897 | 4.878 |
| No treatment-Control | 0 | 5.324 | 5.518 |
Note: TPC: Total bacteria; LAB: Lactic Acid Bacteria; N.D.: Not Determined
Table 2: Total bacteria and LAB in sugar can syrup before and after treatment with DBNPA, BNPD, or an antibiotic. Total bacteria (TPC) were determined on total plate count agar. Lactic Acid Bacteria (LAB) were determined on MRS. In every case, the antimicrobial contact time was 4 h in sugar cane syrup. (See Methods.) The counts are represented as log10 CFU/mL. N.D.=Not Determined.
Sugar cane syrup was obtained from and tested at a cane (Saccharum officianum) sugar-to-ethanol plant in Sao Paulo, Brazil, South America. Fermentation temperature on sugar cane syrup was maintained at 35°C for 48 h, the pH was 4.5-4.7. Microbiological work was performed doing the challenges similar to the corn-to- ethanol procedures where antimicrobial exposure was carried out for 4 h. LAB were plated on MRS; however, total (primarily) heterotrophic bacteria were determined on PCA. Doses of DBNPA, BNPD, and antibiotic were determined from the advice of production plant engineering per their operation costs and affordability (where 200 mg/L was too expensive) and are listed in Table 2. In each trial, the plant was legally bound by contract with the antibiotic supplier to maintain their name and type of antibiotic confidential.
Results and Discussion
Brominated biocides in laboratory testing
DBNPA and BNPD (Figures 1a and 1b) were used as the test microbicides. Previous studies in the laboratory and in field industrial water applications, using typical dosages (50-100 mg/L), made us well aware of the quick-killing ability of both DBNPA and BNPD against a type strain such as Pseudomonas aeruginosa 13388, and field pseudomonads isolated from cooling tower water (>6 log10 kill within 30 min and 60 min, respectively, pH 7.0-8.0; P. Song, 1984, unpublished results). Similar results were found for DBNPA recently [27]. However, how well these microbicides performed against LAB in an acidic environment and in the presence of yeast and corn fermentation biomass was hitherto unknown. This is especially important because most biocides are ineffective in the presence of high concentrations of solids [28]. Thus, in this project DBNPA was compared with BNPD in tests that were scaled up in the laboratory using a fermentation matrix prepared in a corn- to-ethanol pilot plant, similar to what was done previously [14]. The acid-producing bacteria tested were two different species: Homofermentative Lactobacillus plantarum and Acetobacter cerevisiae. These bacteria were grown and challenged in the presence of a dry solids concentration of 30% (w/w) in 4 h fermentation. The results are presented in Figures 2a-2b.
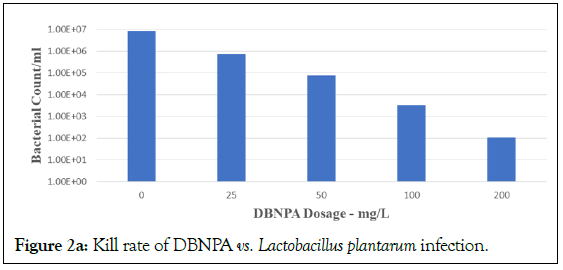
Figure 2a: Kill rate of DBNPA vs. Lactobacillus plantarum infection.
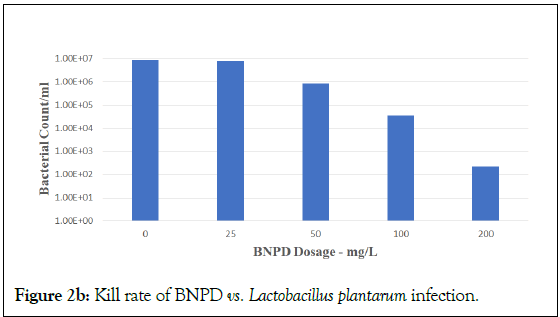
Figure 2b: Kill rate of BNPD vs. Lactobacillus plantarum infection.
DBNPA is seen as effective in a stepwise dose-response from 25 to 200 mg/L, with optimal dose at 200 mg/L. However, BNPD was not efficacious at 25 mg/L. This result is consistent with BNPD applications in laboratory and industrial water field applications that require a typical minimum dose of 30 mg/L in order to start being effective. However, BNPD was efficacious at 100 and 200 mg/L. These results held true for challenges against both Lactobacillus plantarum (Figures 2a and 2b) and Acetobacter cerevisiae (Figures 2c and 2d, respectively). These results using DBNPA and BNPD showed promise for future work in the field.
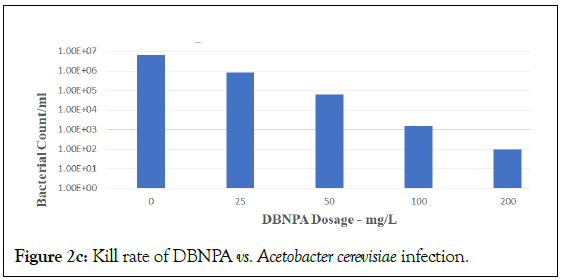
Figure 2c: Kill rate of DBNPA vs. Acetobacter cerevisiae infection.
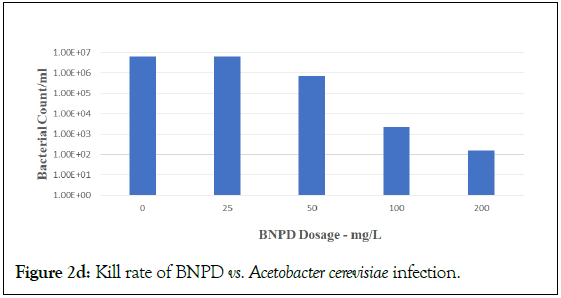
Figure 2d: Kill rate of BNPD vs. Acetobacter cerevisiae infection.
DBNPA and BNPD in field fermentation matrices
The brominated organic biocides were then advanced to a corn- to-ethanol industrial plant for a challenge in their fermentation matrix. The results are given in Table 1. Neither biocide at 50 mg/L provided much of an effect. However, at 100 mg/L BNPD had a kill that approached 2 log10 (1.765) for LAB and total heterotrophic bacteria (1.949). At the same dosage, DBNPA killed 3 log10 LAB and nearly 3 log10 (2.70) total heterotrophic bacteria. Therefore, in this case, the results distinguish DBNPA as a practical dosage for field applications in corn-to-ethanol. It is also markable that kill rates of >2 logs and ≥ 3 logs achieved during field testing are quite desirable, and that the microbiological results from tests conducted on pilot plant fermentation mass (Figure 2) predicted well the results in industrial corn-to-ethanol plants (Table 1 and 2).
Taking field work one step further, both brominated organic biocides were tested in sugar cane syrup. The dose was determined by the cost of treatment that the ethanol plant chemical engineers thought it could absorb. Gram staining indicated that the majority of the bacteria were gram-positive. As in corn-to-ethanol processing, in sugar-cane-to-ethanol processing, competition for carbon compounds is aggressive, not only by LAB but also by other bacteria growing up in the system. Bacterial contamination leads to increases in lactic acid and acetic acid byproducts. LAB and indigenous bacteria can also outcompete yeast from consuming sugar and minerals. At high levels of bacterial infection, foam can form. Additionally, yeast cells can flocculate [29-31], but in both of these field studies, no flocculation was observed during the time period allotted.
While the results collected from the cane sugar syrup bioethanol plant indicate that the brominated biocides at high dosages were effective (Table 2), a stronger performance was expected of the low dose (50 mg/L) because this dose was treating a smaller population of bacteria than in other experiments. The difference may have arisen because the tests were conducted in an industrial plant environment, or could have also resulted from the amount of sulfur in the plant water used. DBNPA reacts with nucleophilic compounds [32]. In addition to DBNPA attacking sulfhydryl groups of bacterial cell proteins, rendering them inactive, it is likely that residual sulfur reacted with and nullified the effects of both DBNPA and BNPD [33]. Because the sulfur measured in the plant water used in Sao Paulo reached 23.3 mg/L (as SO-2). In the same field tests, antibiotics were employed at 3-5 mg/L (Table 1). In both the corn-to-ethanol and cane-sugar-to-ethanol plant field tests, the Antibiotic results did not indicate satisfactory effects against either the LAB or total population of bacteria.
Byproducts of fermentation
The next set of experiments switched gears to byproducts of fermentation. In the laboratory, fermentation was carried out over the long term (See Methods; Table S1 and Figure S1), and the end products were measured. Achieving fermentation was preliminarily indicated by measuring the rates of mass loss vs. time. The only obvious difference between the treatments was the Infected Control (IC), which appeared to have a smaller total mass loss than the other treatments. This was entirely due to one fermentation flask, however, and its influence is shown by the large error bars for the IC treatment relative to the other treatments. Therefore, the difference in final mass loss is not statistically significant. Note that the mass loss after approximately 16 h seems to be slightly lower for the highest DBNPA doses. Although these differences were reproducible and statistically significant, it does not appear to be important because the mass loss for these two treatments was identical to the Infection-Free Control (IFC) after 24 h, which is only approximately half the industry standard incubation time. At 62 h, the curves for all treatments overlapped and indicated 16 g mass losses over 62 h, except the untreated infected control- were statistically the same. Differences in final mass loss were not statistically significant (Figures 3 and 4).
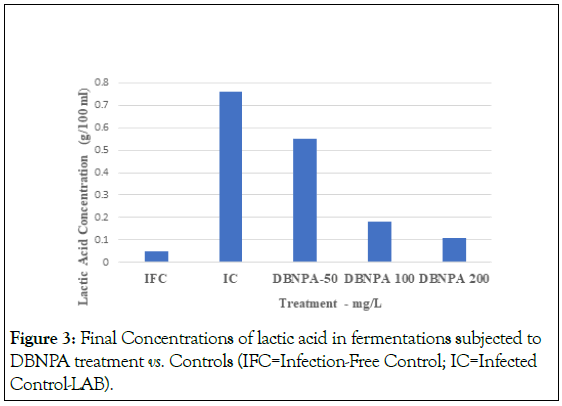
Figure 3: Final Concentrations of lactic acid in fermentations subjected to DBNPA treatment vs. Controls (IFC=Infection-Free Control; IC=Infected Control-LAB).
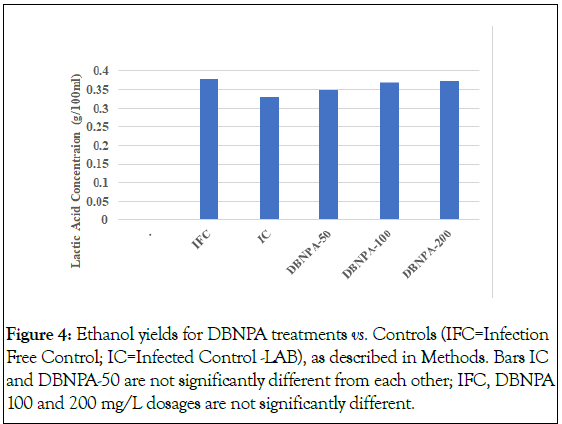
Figure 4: Ethanol yields for DBNPA treatments vs. Controls (IFC=Infection Free Control; IC=Infected Control -LAB), as described in Methods. Bars IC and DBNPA-50 are not significantly different from each other; IFC, DBNPA 100 and 200 mg/L dosages are not significantly different.
The effectiveness of DBNPA applications was also determined by measuring lactic acid and Ethanol yields and comparing those results to those of IFC. Results of these tests are shown in Figures 3 and 4. Analysis of Variance (ANOVA) indicated that significant differences existed among treatments for both endpoints. Tukey’s test for paired comparisons, which compares all possible pairs of treatments, was used to identify the significant differences. The most obvious differences are in the final lactic acid concentrations (Figure 3). As expected, the lowest concentration of lactic acid was observed in the IFC, and the highest occurred in the IC. DBNPA reduced the amount of lactic acid produced at all doses, but there was a clear dose-response relationship. The lowest lactic acid concentration was observed for the 200 mg/L dose, and this concentration was only slightly higher than that observed in the IFC (0.11 ± 0.01 g/100 ml vs. 0.06+0.003 g/100 ml for the IFC). (The difference between the final lactic acid concentrations in the 200 mg/L DBNPA and IFC treatments was statistically significant: P=0.012, where P is the probability that the two concentrations are the same.) The inability of DBNPA to completely eliminate the increase in the final lactic acid concentration even at the highest dose was likely due to the 1-hour incubation period between the inoculating with LAB and introducing the brominated organic biocide.
The differences in ethanol yield were not as dramatic, but statistically significant differences were observed that followed the pattern that is consistent with the trends shown in Figure 3. The ethanol yield of the IC (Figure 4) was significantly lower than the ethanol yield of all other treatments except for the 50 mg/L DBNPA treatment.
Ethanol yields of all DBNPA-treated fermentation mashes were not significantly different from the IFC or from each other. Infection with L. plantarum reduced the yield by approximately 2% relative to the infection-free control, but addition of DBNPA to the concentration of at least 100 mg/L completely eliminated the effect of bacterial infection. Further testing using BNPD was not performed because of the toxicology of the byproducts mentioned on the MSDS. These were namely formaldehyde and nitrosamine. Although these byproducts were yielded nonstoichiometrically and only under high temperatures, the toxic possibility of these chemistries in a plant that handled food were of concern. Hence, the BNPD work was discontinued. DBNPA, which consistently gave the best kill rates throughout the course of this investigation, was apparently the brominated organic biocide of choice. The optimal dosage range was set at 100-200 mg/L. It is wise to note that other brominated biocides (See Methods) and quaternary amines were not attempted because these would attack organics and yeast. Quaternary amines are cationic, cationic biocides react quickly with anionic surfaces, immediately extinguishing potential for complete effect per unit dose.
At this point, future work may include analyzing and quantifying BNPD breakdown in fermentation coproducts vs. time under bioethanol plant conditions. The amount of toxic BNPD byproducts, such as formaldehyde, yielded during fermentation needs to be determined. Test results from field trials using switchgrass and sugar beets as the starting material may also prove to be valuable for the future. Alternative technologies such as taking advantage of synergistic compounds would help lower costs of treatments [34]. However, how and where to apply the compounds are important for success, as proven by other researchers [35]. Applying recombinant DNA bacteriophages is another technique that can be attempted to kill LAB in the field [36]. Regardless of the method used, however, while it is important to focus on killing LAB because of their adaptability to bioethanol systems, and because of the problems they cause [37], it is equally important to control the general bacterial population. If the indigenous population of bacteria is not controlled, the bacteria can set up incipient stages of biofilm. This is actually true of both LAB and heterotrophic bacteria that can tenaciously bind to surfaces [38,39]. Biofilms are known to grow up significantly in 48 h [40]. They can quickly develop on pipes, tanks and various food equipment to protect themselves by producing slime exopolymers [41]. In some cases, they harbor pathogens and cause disease [42-45]. Unfortunately, a narrow-spectrum antimicrobial would not control the biofilm(s), nevertheless, a broad spectrum biocide, such as DBNPA (Table 1), has that capability [46,47].
Conclusion
In this investigation there is compelling evidence to conclude that DBNPA and BNPD are capable of controlling infections of Lactobacillus and Acetobacter species under fermentation conditions that are typical of those used in the fuel-ethanol industry. Based on field trials in corn-to-ethanol and in cane sugar syrup fermentation matrices, both organic bromicides controlled infection by LAB and heterotrophic bacteria in the ethanol fermentation matrices but DBNPA is more effective. Moreover, DBNPA in particular decreases the lactic acid byproduct yield but increases ethanol yield, based on mashes containing 30% (w/w) corn dry solids that were infected with L. plantarum and allowed to ferment. The finalconcentration of lactic acid in untreated fermentation matrices increased by nearly 14-fold (from 0.06% to 0.76% w/v), while the ethanol yield decreased by 2%. Treatment with DBNPA at a concentration of 100 mg/L completely eliminates the LAB effect on ethanol yield and reduces the final lactic acid concentration by 80% or more relative to the infected control. Previous research that quantified the loss of DBNPA during the fermentation process, analytically in the laboratory and during the field trials on the entire corn-to- ethanol process using viable microorganisms, clearly indicate that DBNPA breaks down by first order kinetics and cannot then lead to antimicrobial contamination of DDGS commercial product. Those results also contend that application of DBNPA in corn-to- ethanol fermentation would have no environmental impact, which agrees with previous toxicological and ecological risk assessment studies. Therefore, considering the work conducted using DBNPA, it is totally reasonable and absolutely workable to say that applying DBNPA to bioethanol fermentation can provide a successful alternative to antibiotics for controlling bacterial infections in fuel-ethanol fermentation and obviate the use of antibiotics. Additionally, the difference in bioethanol yield is financially important for ethanol fuel plants. Plant chemical engineers calculate that an increase of only 0.5% in bioethanol yield is approximately a $4 million additional output at a 50 MGY plant.
References
- Harris J. Most U.S. fuel ethanol production capacity at the start of 2022 was in the Midwest. U.S. Energy Information Admin (EIA) independent statistics and analysis. 2023.
- Voegel E. EIA increases 2022, 2023 fuel ethanol production forecasts. 2023.
- Market watch: Global bioethanol market 2023 with upcoming trends, growth rate analysis by top regions, sales volume and industrial updates forecast to 2028. 2023.
- Rosentrater KA. Some physical properties of Distillers Dried Grains with Solubles (DDGS). Appl Eng Agric. 2006;22(4):589-595.
- Skinner KA, Leathers TD. Bacterial contaminants of fuel ethanol production. J Ind Microbiol. Biotechnol. 2004; 31(9):401-408.
[Crossref] [Google Scholar] [PubMed]
- Aquarone E. Penicillin and tetracycline as contamination control agents in alcoholic fermentation of sugar cane molasses. Appl. Microbiol. 1960;8(5):263-268.
[Crossref] [Google Scholar] [PubMed]
- Hynes SH, Kjarsgaard DM, Thomas KC, Ingledew WM. Use of virginiamycin to control the growth of lactic acid bacteria during alcohol fermentation. J Ind Microbiol Biotechnol. 1997;18(4):284-291.
[Crossref] [Google Scholar] [PubMed]
- Stroppa CT, Andrietta MGS, Andrietta SR, Steckelberg C, Serra GE. Use of penicillin and monensis to control bacterial contamination of Brazilian alcohol fermentations. Int Sugar J. 2000; 102(1214);78-82.
- Food and Drug Administration. The judicious use of medically important antimicrobial drugs in food-producing animals. Draft Guidance. 2010;209.
- Food and Drug Administration. Summary report on antimicrobials sold or distributed for use in food-producing animals. Washington DC: Dep Heal Hum Ser. 2011.
- Wiatr CL, Corcoran ML, Mcneel TE, Clark RA, Porto RD, Oppong D, Processes using antibiotic alternatives in bioethanol production. Buckman Laboratories International. 2011.
- Oppong D, Corcoran ML, Porto RD, Mcneel TE, Wiatr CL, Clark RA, Processes using antibiotic alternatives in bioethanol production. Buckman Laboratories International. 2015.
- Oppong D, Corcoran ML, Porto RD, Mcneel TE, Wiatr CL, Clark RA, Processes using antibiotic alternatives in bioethanol production. Buckman Laboratories International. 2018.
- Simpson JV, Wiatr CL. Quantification and Degradation of 2, 2-Dibromo-3-Nitrilopropionamide (DBNPA) in Bioethanol Fermentation Coproducts. World J Microbiol Biotechnol. 2022;38(5):82.
[Crossref] [Google Scholar] [PubMed]
- Hesse BC. On malonnitrile and some its derivatives. [machine translation]. Amer Chem J. 1896;18:723-751.
- Nolan HK, Hechnbleikner I. Seed and plant disinfectants. U.S. Patent. 1947;2:419888.
- Chervenak, MC, Konst, GB, Schwingel, WR. Non-traditional use of the biocide 2, 2-dibromo-3-nitropropionamide (DBNPA) in coatings manufacture. Proc Annu Meet. 2005.
- Kiuru J, Tsitko I, Wathén R. Optimization of biocide strategies on fine paper machines. Bio Res. 2010.
- Wolf PA, Sterner PW. 2, 2-Dibromo-3-nitrilopropionamide, a compound with slimicidal activity. Appl. Microbiol. 1972; 24(4):581-584.
[Crossref] [Google Scholar] [PubMed]
- Exner JH, Burk GA, Kyriacou D. Rates and products of decomposition of 2, 2-dibromo-3-nitrilopropionamide. J. Agric Food Chem. 1973;21(5):838-842.
- Zheng J, Wittouck S, Salvetti E, Franz CM, Harris HM, Mattarelli P, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782-2858.
[Crossref] [Google Scholar] [PubMed]
- Bischoff KM, Skinner-Nemec KA, Leathers TD. Antimicrobial susceptibility of lactobacillus species isolated from commercial ethanol plants. J. Ind. Microbiol. Biotechnol. 2007;34(11):739-744.
[Crossref] [Google Scholar] [PubMed]
- Lushia W, Heist P. Antibiotic resistant bacteria in fuel ethanol fermentations. Ethanol Prod Mag. 2005;11: 80-81.
- Alexander M. Microbial Ecology.1971
- Krantz M, Nordlander B, Valadi H, Johansson M, Gustafsson L, Hohmann S. Anaerobicity prepares saccharomyces cerevisiae cells for faster adaptation to osmotic shock. Eukaryotic cell. 2004;3(6):1381-1390.
[Crossref] [Google Scholar] [PubMed]
- Wiatr CL, Corcoran ML, McNeel TE, Porto B DeCassia R, Oppong D. Processes usipng in situ generated antibiotic alternatives in bioethanol fermentation. 2018.
- Barros, AC, Melo, LF, Pereira A. A multi-purpose approach to the mechanisms of action of two biocides (benzalkonium chloride and dibromonitrilopropionamide): discussion of Pseudomonas fluorescens’ viability and death. Front Microbiol. 2022;13(842414): 1-13.
- Frayne C. The selection and application of nonoxidizing biocides for cooling water systems. Analyst. 2001;8(2):9-16.
- Laluce C, Leite GR, Zavitoski BZ, Zamai TT, Ventura R. Fermentation of sugarcane juice and molasses for ethanol production. Sugarcane based biofuels and bioproducts. 2016;2(8):53-86.
- Basso LC, Basso TO, Rocha SN. Ethanol production in Brazil: The industrial process and its impact on yeast fermentation. Biofuel production-recent developments and prospects. 2011;1530:85-100.
- Skinner KA, Leathers TD. Bacterial contaminants of fuel ethanol production. J Ind Microbiol Biotechnol. 2004;31(9):401-408.
[Crossref] [Google Scholar] [PubMed]
- Chapman JS. Biocide resistance mechanisms. Int Biodeterior Biodegradation. 2003;51(2):133-138.
- Williams TM, McGinley HR. Deactivation of industrial water treatment biocides. 2010.
- Wiatr CL, Corcoran ML, McNeel TE, Clark RA, Porto RD, Oppong D, Synergistic combination of DBNPA and polycyclic antibacterial peptide as biocide in bioethanol production. inventors; Buckman Laboratories International, assignee.2015.
- Zhang L, Holle MJ, Kim JS, Daum MA, Miller MJ. Nisin incorporation enhances the inactivation of lactic acid bacteria during the acid wash step of bioethanol production from sugarcane juice. Lett Appl Microbiol. 2019;69(1):50-56.
[Crossref] [Google Scholar] [PubMed]
- Lu SY, Bischoff KM, Rich JO, Liu S, Skory CD. Recombinant bacteriophage LysKB317 endolysin mitigates Lactobacillus infection of corn mash fermentations. Biotechnol Biofuels. 2020;13(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Beckner M, Ivey ML, Phister TG. Microbial contamination of fuel ethanol fermentations. Lett Appl Microbiol. 2011;53(4):387-394.
[Crossref] [Google Scholar] [PubMed]
- Ramírez MD, Smid EJ, Abee T, Groot MN. Characterisation of biofilms formed by Lactobacillus plantarum WCFS1 and food spoilage isolates. Int J Food Microbiol. 2015;207:23-29.
[Crossref] [Google Scholar] [PubMed]
- Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277.
[Crossref] [Google Scholar] [PubMed]
- Caro-Astorga J, Frenzel E, Perkins JR, Álvarez-Mena A, De Vicente A, Ranea JA, et al. Biofilm formation displays intrinsic offensive and defensive features of Bacillus cereus. NPJ Biofilms Microbiomes. 2020;6(1):3.
[Crossref] [Google Scholar] [PubMed]
- Wiatr CL. Bacterial resistance to antimicrobials in a cooling water system, Part2. Analyst. 2016;23(2):10-16.
- Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399(10325):629-655.
[Crossref] [Google Scholar] [PubMed]
- de Kraker ME, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):1002184.
[Crossref] [Google Scholar] [PubMed]
- Van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin. 2016;30(2):377-390.
[Crossref] [Google Scholar] [PubMed]
- Da-Silva-Correa LH, Smith H, Thibodeau MC, Welsh B, Buckley HL. The application of non-oxidizing biocides to prevent biofouling in reverse osmosis polyamide membrane systems: A review. J WATER SUPPLY RES T.2022;71(2):261-292.
- Klaine SJ, Cobb GP, Dickerson RL, Dixon KR, Kendall RJ, Smith EE, et al. An ecological risk assessment for the use of the biocide, dibromonitrilopropionamide (DBNPA), in industrial cooling systems. Environ Toxicol Chem. 1996;15(1):21-30.
Citation: Wiatr CL, Bazzell J, Porto RdB (2023) Brominated Organic Biocides Control Lactic Acid-Producing Bacteria in Bioethanol Fermentation Matrices. J Microb Biochem Technol. 15:549.
Copyright: © 2023 Wiatr CL, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

