Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2016) Volume 1, Issue 1
Body Weight Reduction and QTc Interval in Obesity
Abstract
Introduction: Obesity is associated with wide variety of electrocardiographic (ECG) abnormalities. Prolonged QTc interval is common in obesity and it represents a delayed ventricular repolarisation which is associated with ventricular arrhythmias, syncope and sudden cardiac death. The aim of our study is to evaluate the effects of weight reduction on QTc interval in obese subjects. Materials and Methods: The study included 74 obese subjects aged 41.78 ± 10.70 years, treated with a lowcalorie diet (800 kcal/day) for two weeks. All patients underwent a standard resting 12-lead surface ECG before and after the treatment. QTc intervals were corrected by Bazett’s formula. Results: The average loss in body weight was 10.26 ± 3.63%, and the average reduction in body mass index was 9.39 ± 4.23%. There were statistically significant differences for all electrocardiographic parameters (p<0.01) except for the QTc dispersion (p>0.05) before and after the treatment with low-calorie diet. Heart rate was also statistically significant reduced after the low-calorie diet treatment. Prevalence of prolonged QTc interval was 24.32% before and 9.21% after the treatment. Conclusion: The results show that obesity causes prolongation of QTc interval. Significant weight reduction of about 10% leads to favorable electrocardiographic changes and significant shortening of the QTc interval.
Keywords: Obesity; Electrocardiography; Arrhythmia; Weight loss; Sudden cardiac death
Introduction
Obesity is defined as an excessive accumulation of body fat to the extent that causes serious health problems and complications in many different organs and organ systems [1]. Obesity follows an increased risk of developing chronic diseases such as cardiovascular diseases, hypertension, coronary heart disease, cerebrovascular disease and peripheral arterial disease [2]. Obesity is associated with a higher incidence of ventricular arrhythmias that can cause sudden cardiac death [3] even in those patients who had no previously known heart disease [4,5]. Cardiovascular complications in obesity are associated with various electrocardiographic (ECG) abnormalities. One of them is prolonged QTc interval, which hallmarks delayed ventricular repolarization, and it represents a risk factor for the development of ventricular tachyarrhythmias, syncope and sudden cardiac death [6,7]. Prolonged QTc interval in obesity was first described twenty years ago [8]. Many studies that followed showed that obesity can cause prolonged QTc interval [9-11]. Much of comorbidities associated with obesity can be improved or prevented with body weight (BW) reduction [12]. Effects of changes in body mass index (BMI) on cardiovascular risk are verified in the Framingham study. It was noticed that 20% of BW loss reduces the risk of cardiovascular diseases by 40% [13]. Many studies observed the effects of weight reduction on the QTc interval in obese patients, but the results are conflicting. The most of the studies observed that QTc interval is shortening during the weight reduction [14-17], but some studies haven’t shown significant changes in the QTc interval during weight reduction [12,18]. It is surely important to monitor the QTc interval during the treatment of obesity in order to prevent adverse cardiovascular events. The aim of our study is to evaluate the effects of weight reduction on QTc interval in obese patients.
Materials and Methods
Obese men and women were recruited from the Clinic for Endocrinology, Diabetes and Metabolic Disorders, Clinical Center of Vojvodina, Novi Sad. Subjects were included in weight loss program. Inclusion criteria were age 18 to 65 and BMI ≥ 30 kg/m². We excluded those with a previous history of diabetes mellitus, hypertension, heart, hepatic, kidney, psychiatric, malignant, infectious disorders and those taking drugs that can influence QT interval. Levels of potassium, sodium and calcium were measured before and during the investigation and we have excluded 3 patients because of existence of electrolyte imbalances. The study was performed according to the Declaration of Helsinki, and informed consent was obtained from all participants. The study has local ethics committee approval.
With the subjects wearing light indoor clothes and no shoes BW and body height (BH) were measured using calibrated beam-type balance to the nearest 0.1 kg and Harpenden anthropometer to the nearest 0.1 cm respectively, and BMI was calculated (BMI=BW/BH2 (kg/m2)).
The obese subjects were treated with conventional diet consisting of a low-calorie diet (LCD) of 800 kcal/day. During the active weight loss programme which lasted two weeks all subjects were required to eat the same low calorie diet of nutritionally balanced prepared foods. The prescribed diet contained approximately 30% of calories from fats, 50% from carbohydrates and 20% from proteins. The subjects were required to take three to four liters of sparkling mineral water per day, and a multivitamin tablets (Oligovit), which contains: Vitamin A 5000 IU; Vitamin B1 5 mg; Vitamin B2 5 mg; Vitamin B6 2.5 mg; Vitamin B12 2.5 mg; 50 mg of nicotine amide; vitamin C 100 mg; vitamin D3 500 IU; Vitamin E 12.5 mg; Calcium pantothenate 10 mg; CaHPO4 200 mg; fluoride 0.5 mg; iron 10 mg; copper 0.5 mg; manganese 0.5 mg; cobalt 0.05 mg; magnesium 3 mg; zinc 0.75 mg; molybdenum 0.1 mg; potassium 2.5 mg; methyl hydroxybenzoate 0.06 mg; the energy value of this product was 6.68 kJ (1.59 kcal).
ECG records were done twice at approximately the same time each morning in fasting subjects on day 0 (at the beginning of weight loss program) and day fourteen, at the end of intervention. All of the subjects in the study group underwent a standard resting 12-lead surface ECG recorded at a paper speed of 25 mm/s and gain of 10 mm/mV. The ECGs were analyzed by one reader who was unaware of the characteristics of the subject. Reader was trained to obtain the minimum of intravariability of measurements. The ECG parameters were measured manually by using graduated lens. QT intervals were measured from the beginning of the QRS complex to the visual return of the T-wave to the isoelectric line. QT intervals and R-R intervals were measured in all of 12 derivations in three consecutive cardiac cycles and then averaged. The longest QT was accepted as the QTmax interval, and the shortest QT was accepted as the QTmin interval. The difference between the QTcmax and QTcmin was defined as QTc dispersion. All the intervals were corrected by the Bazett formula=QT Interval/√ (RR interval). Statistical analyses were performed using the SPSS (version 11.0) software. All variables were expressed as mean ± standard deviation or percentage (%). We used the Mann‑Whitney test for nonparametric unpaired variables when comparing the groups before and after weight reduction and the Student’s t test for parametric variables, p value of 0.05 or less was considered to indicate statistical significance.
Results
The study included 74 obese subjects, 41.78 ± 10.70 years old treated with LCD. The average loss in BW was 10.26 ± 3.63%, and the average reduction in BMI was 9.39 ± 4.23%. The characteristics of the study population are listed in Table 1. There were statistically significant differences in BW and BMI before and after the treatment (p<0.01). Prevalence of prolonged QTc interval (over 440 msec) was 24.32% in obese subjects before treatment. After weight reduction prevalence of prolonged QTc interval was reduced to 9.21%. There were statistically significant differences for all electrocardiographic parameters (p<0.01) except for the QTc dispersion (p>0.05) before and after the treatment with low-calorie diet. Decline in heart rate (HR) after the treatment was also statistically significant (Table 1). There was statistically significant difference in systolic blood pressure before and after weight reduction program (131.00 ± 21.92 mmHg vs. 113.33 ± 8.84 mmHg, p<0.01) while the change in diastolic blood pressure was not significant (84.90 ± 13.26 mmHg vs. 78.50 ± 4.76 mmHg, p>0.05). Changes in blood pressure were not related to QTc interval changes. Considering the lipid profile, there was significant decrease in triglyceride levels (1.69 ± 1.49 mmol/l vs. 1.02 ± 0.58 mmol/l, p<0.01), while the changes in total cholesterol (5.34 ± 1.20 mmol/l vs. 5.04 ± 0.92 mmol/l, p>0.05), LDL cholesterol (3.56 ± 1.01 mmol/l vs. 3.14 ± 0.80 mmol/l, p>0.05) and HDL cholesterol (1.06 ± 0.23 mmol/l vs. 1.14 ± 0.31 mmol/l, p>0.05) levels before and after weight reduction were not significant. Also, there were no significant correlations between changes in lipid parameters and QTc interval changes.
| Parameter | Before | After | t-value | p-value |
|---|---|---|---|---|
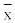 ± SD ± SD |
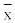 ± SD ± SD |
|||
| BW (kg) | 140.78 ± 31.74 | 125.45 ± 26.53 | 3.19 | p<0.01 |
| BMI (kg/m2) | 49.52 ± 9.67 | 44.36 ± 8.16 | 3.51 | p<0.01 |
| QTc (msec) | 422.82 ± 24.21 | 401.88 ± 27.32 | 4.93 | p<0.01 |
| QTcmax (msec) | 446.07 ± 30.26 | 426.86 ± 31.03 | 4.91 | p<0.01 |
| QTcmin (msec) | 396.89 ± 25.90 | 379.27± 31.05 | 4.79 | p<0.01 |
| QTcdisp (msec) | 49.18 ± 21.80 | 47.58±16.90 | 0.53 | p>0.05 |
| HR (bpm) | 70.61 ± 10.47 | 65.15 ± 10.10 | 4.08 | p<0.01 |
Table 1: Characteristics of subjects before and after weight reduction treatment.
Discussion
In our study, obese subjects, after the treatment with LCD, showed a significant reduction in BW and BMI. Obesity is associated with a numerous of electrocardiographic abnormalities. Some of them are harmless but some may represent changes in cardiac morphology which are associated with obesity or its comorbidities [19]. Ventricular repolarization disturbances caused by left ventricle enlargement and eccentric hypertrophy of the left and right ventricular wall in obesity may lead to malignant cardiac arrhythmias and sudden cardiac death. Many of these changes are reversible [20,21]. Alpert et al. and Alexander et al. showed that significant weight loss in extremely obese patients leads to favorable cardiac structural, hemodynamic and electrocardiographic changes [22-24]. Al-Salameh et al. concluded that weight reduction after sleeve gastrectomy in morbidly obese patients was associated with a significantly lower QTc interval [25].
In our research 24.3% obese patients had prolonged QTc interval before the treatment and after weight reduction percentage of prolonged QTc interval was significantly reduced to 9.21%. BW loss of 10.26 ± 3.63% is associated with significant shortening of QTc, QTcmax, QTcmin intervals and HR reduction. QT dispersion was not significantly shortened. Shortening of the QTc interval and HR reduction decreases the risk of potentially fatal arrhythmias that lead to a reduction in cardiovascular mortality [12]. In clinical studies dealing with this problem, the conclusions are still controversial. Mshui et al. showed that QTcmax and QTcmin intervals decreased after weight reduction, with no significant change in QTc dispersion [26]. Similar results we have obtained in our study. In contrast, Grupta et al. reveled that decrease in QT dispersion was due to increase in the QTcmin interval in most patients treated with liquid protein diet for weight loss [27]. In our research, we concluded that a significant loss in BW of about 10% leads to favorable electrocardiographic changes and the significant shortening of the QTc interval.
Electrolyte imbalances that occur during obesity treatment may aggravate or prolong QTc. The risk of sudden cardiac death in the obese patients is influenced by structural, functional and metabolic factors [28]. Therefore, it is necessary to monitoring these patients electrocardiographically to prevent potentially fatal arrhythmias and sudden cardiac death.
Acknowledgements
We would like to thank to all our colleagues who helped us during the work on this study, and to the participants without whom this study could not be performed.
References
- Ito H, Nakasuga K, Ohshima A, Sakai Y, Maruyama T, et al. (2004) Excess accumulation of body fat is related to dyslipidemia in normal-weight subjects. Int J Obes 28: 242-247.
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, et al. (2001) Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161: 1581-1586.
- Drenick EJ, Bale GS, Seltzer F, Johnson DG (1980) Excessive mortality and causes of death in morbidly obese men. JAMA 243: 443-445.
- Kannel WB, Plehn JF, Cupples LA (1998) Cardiac failure and sudden death in the Framingham Study. Am Heart J 115: 869-875.
- Messerli FH, Nunez BD, Ventura HO, Synder DW (1987) Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med 147: 1725-1728.
- Vlay SC, Mallis GI, Brown EJ, Cohn PF (1984) Documented sudden cardiac death in prolonged QT syndrome. Arch Intern Med 144: 833-835.
- Moss AJ (1993) Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol 72: 23B-25B.
- Frank S, Colliver JA, Frank A (1986) The electrocardiogram in obesity: statistical analysis of 1,029 patients. J Am Coll Cardiol 7: 295-299.
- Esposito K, Marfella R, Gualdiero P, Carusone C, Pontillo A, et al. (2003) Sympathovagal balance, nighttime blood pressure, and QT intervals in normotensive obese women. Obes Res 11: 653-659.
- Kim JA, Park YG, Cho KH, Hong MH, Han HC, et al. (2005) Heart rate variability and obesity indices: emphasis on the response to noise and standing. J Am Board Fam Pract 18: 97-103.
- Giraldi FP, Manzoni G, Michailidis J, Scacchi M, Stramba-Badiale M, et al. (2011) High prevalence of prolonged QT interval in obese hypogonadal males. Obesity 19: 2015-2018.
- Gondoni LA, Titon AM, Montano M, Caetani G, Nibbio F, et al. (2011) The myth of QT shortening by weight loss and physical training in obese subjects with coronary heart disease. Obesity 19: 200-203.
- Hubert HB, Feinleib MPH, McNamara PM, Castelli WP (1983) Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67: 968-977.
- Seyfeli E, Duru M, Kuvandik G, Kaya H, Yalcin F (2006) Effect of weight loss on QTc dispersion in obese subjects. Anadolu Kardiyol Derg 6: 126-129.
- Papaioannou A, Michaloudis D, Fraidakis O, Petrou A, Chaniotaki F, et al. (2003) Effects of weight loss on QT interval in morbidly obese patients. Obes Surg 13: 869-873.
- Corbi GM, Carbone S, Ziccardi P, Giugliano G, Marfella R, et al. (2002) FFAs and QT intervals in obese women with visceral adiposity: effects of sustained weight loss over 1 year. J Clin Endocrinol Metab 87: 2080-2083.
- Carella MJ, Mantz SL, Rovner DR, Willis PW, Gossain VV, et al. (1996) Obesity, adiposity, and lengthening of the QT interval: improvement after weight loss. Int J Obes 20: 938-942.
- Pringle TH, Scobie IN, Murray RG, Kesson CM, Maccuish AC (1983) Prolongation of the QT interval during therapeutic starvation: a substrate for malignant arrhythmias. Int J Obes 7: 253-261.
- Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA (2005) Obesity and the electrocardiogram. Obes Rev 6: 275-281.
- Amad RH, Brennan JC, Alexander JK (1965) The cardiac pathology of chronic exogenous obesity. Circulation 32: 740-745.
- Warnes CA, Roberts WC (1984) The heart in massive (more than 300 pounds or 136 kilograms) obesity: analysis of 12 patients studied at necropsy. Am J Cardiol 54: 1087-1091.
- Alpert MA, Terry BE, Hamm CR, Fan TM, Cohen MV, et al. (2001) Effect of weight loss on the ECG of normotensive morbidly obese patients. Chest 119: 507-510.
- Alpert MA, Alexander JK (1998) Cardiac morphology and obesity in man: The Heart and Lung in Obesity, Armonk, NY, Futura Publishing.
- Alexander JK, Alpert MA (1998) Hemodynamic alterations with obesity in man: The Heart and Lung in Obesity, Armonk, NY, Futura Publishing.
- Al-Salameh A, Allain J, Jacques A, Verhaeghe P, Desailloud R (2014) Shortening of the QT interval is observed soon after sleeve gastrectomy in morbidly obese patients. Obes Surg 24: 167-170.
- Mshui ME, Saikawa T, Ito K, Hara M, Sakata T (1999) QT interval and QT dispersion before and after diet therapy in patients with simple obesity. Proc Soc Exp Biol Med 220: 133-138.
- Gupta AK, Xie B, Thakur RK, Maheshwari A, Lokhandwala Y, et al. (2002) Effect of weight loss in QT dispersion in obesity. Indian Heart J 54: 399-403.
- Plourde B, Sarrazin JF, Nault I, Poirier P (2014) Sudden cardiac death and obesity. Expert Rev Cardiovasc Ther 12: 1099-1110.
Copyright: © 2016 Milovancev A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.