Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 14, Issue 1
Bioequivalence Study of Entecavir 0.5 mg Tablets in Healthy Thai Volunteers Under Fasting Conditions
Vipada Khaowroongrueng1*, Charinthon Seeduang1, Suchada Rakphung1, Mariam Duereh1, Lalinthip Saeaue1, Busarat Karachot1, Isariya Techatanawat1, Porranee Puranajoti2 and Praphassorn Surawattanawan12International Bio Service Co.,Ltd., 888 Golden Jubilee Medical Center, Mahidol University, Nakhon Pathom 73170, Thailand
Received: 03-Jan-2022, Manuscript No. JBB-22-15354; Editor assigned: 05-Jan-2022, Pre QC No. JBB-22-15354 (PQ); Reviewed: 19-Jan-2022, QC No. JBB-22-15354; Revised: 21-Jan-2022, Manuscript No. JBB-22-15354 (R); Published: 28-Jan-2022, DOI: 10.35248/0975-0851.22.14.448
Abstract
Entecavir is a nucleoside polymerase inhibitor indicated for chronic hepatitis B infection in order to minimize the development of serious consequences. The Government Pharmaceutical Organization (GPO), Thailand has developed HEPA-EN®, entecavir 0.5 mg tablets as a generic substitute for the corresponding innovator product, Baraclude® (Bristol-Myers Squibb Company, USA) to enhance patient adherence to continuous treatment. The bioequivalence study was conducted under fasting conditions using a randomized-sequence, open-label, 2-period crossover design. The plasma samples were collected for 72 hours in both study periods and analyzed using a validated liquid chromatography tandem mass spectrometry method. The 90% CIs of the geometric least squares mean ratio between the formulations of log-transformed AUC0-72h and Cmax were 95.82-107.00% and 95.40-122.32%, respectively which were within the acceptance range for bioequivalence of 80.00-125.00%. The analysis of variance did not show any significant difference between the two formulations. Wilcoxon signed-rank test showed no significant difference in median tmax between two formulations. It was concluded that two entecavir 0.5 mg tablet formulations were bioequivalent based on insignificant difference in terms of rate and extent of absorption describing by peak drug concentration (Cmax) and area under concentration-time curve (AUC0-72h ).
Keywords
Entecavir; Bioequivalence; Pharmacokinetics; Hepatitis B virus
Introduction
Chronic hepatitis B infection is a major cause of cirrhosis and hepatocellular carcinoma. The estimated prevalence of chronic hepatitis B infection in Thailand was 5.1% [1]. Moreover, the prevalence of hepatocellular carcinoma in chronic hepatitis B infection in Thailand was 23.2% which was strongly associated with the evidence of decompensate liver disease [2]. It is recommended that patients with HBeAg-positive (marker of infectivity and active replication), moderate or severe hepatitis and elevated liver enzyme should receive antiviral therapy to suppress viral replication as well as to minimize the development of serious consequences [3].
Entecavir is a guanosine nucleoside antiviral that must be phosphorylated to the active triphosphate form to exert the activity against Hepatitis B Virus (HBV) polymerase, thereby inhibiting HBV DNA synthesis [4]. It is indicated for the treatment of chronic HBV infection with compensated and decompensated liver disease. The recommended dose of entecavir in adults is 0.5 mg once daily but it can be increased to 1 mg daily for lamivudine-refractory patients and patients with decompensated liver disease [5,6].
Following oral administration, entecavir is rapidly absorbed with the peak steady state plasma concentration of 4-7 ng/mL after a 0.5 mg dose and 8-12 ng/mL after a 1.0 mg dose which is achieved within 1 hour [7]. Entecavir is not a substrate of cytochrome P450 enzymes (CYP450), thus drug interaction upon coadministration with potent CYP450 inducers or inhibitors is insignificant [8]. The terminal elimination half-life is approximately 128-149 hours after multiple dosing at 0.5 and 1.0 mg. Entecavir is predominantly cleared by kidney as unchanged form accounted for around 70% of the administered dose. Therefore, dose adjustment is required in patients with renal impairment [7,9]. The pharmacokinetics of entecavir is linear in the therapeutic range. Significant accumulation is observed after multiple dosing with the steady state achieved in 10 days [9].
Patients may continue the treatment unless there is evidence of HBsAg seroconversion or drug resistance [6,10]. The Government Pharmaceutical Organization (GPO), Thailand has developed HEPA-EN®, entecavir 0.5 mg tablets as a generic substitute for the corresponding innovator product, Baraclude® to enhance patient adherence to continuous treatment. The bioequivalence study was conducted to compare pharmacokinetic parameters describing the rate and extent of absorption of the test and reference formulations, and to evaluate the tolerability of the formulations in healthy Thai subjects for generic drug registration in Thailand.
Material and Methods
Study products
HEPA-EN®, entecavir 0.5 mg tablets (Lot No. S630039) manufactured by GPO, Thailand was used as the test product. Baraclude®, entecavir 0.5 mg tablets (Lot No. ABQ9977) manufactured by AstraZeneca Pharmaceuticals LP for Bristol- Myers Squibb Company, USA was used as the reference product.
Study subjects
Sample size calculation was based on probability of greater than 95% for concluding bioequivalence within the acceptance bioequivalence limits of 80.00-125.00% at a significant level of 5% [11]. The maximum intra-subject variability for the primary pharmacokinetic parameter, Cmax of entacavir was around 11% and the expected T/R ratio was 90% which yielded a sample size of 20 subjects [12,13]. However, 28 healthy Thai subjects were enrolled considering 30% dropout and withdrawal rate.
The age and body mass index of subjects were within the range of 18-55 years and 18-30 kg/m2,respectively. All subjects had acceptable medical history, physical examination results and clinical laboratory measurements prior to study initiation. Female subjects were not pregnant or breastfeeding throughout the study. The subjects had no history of hypersensitivity to entecavir or any excipients, allergy to other medications, alcohol dependence, drug abuse, recent blood donation, and recent clinical drug research participation. They were instructed to abstain from smoking and taking any medications prior to dosing and during the entire study. Consumption of any grapefruit, pomelo or orange-based products, and xanthine containing products were restricted at least 24-48 hours prior to dosing and throughout the study. All subjects provided the written informed consent before study participation at International Bio Service Co., Ltd., Golden Jubilee Medical Center, Mahidol University, Thailand.
Study design
A randomized-sequence, open-label, 2-period crossover design was used. All enrolled subjects were admitted to the clinical facility one day prior to study initiation. Twenty-eight subjects were enrolled and randomly divided into two groups, Test-Reference (TR) and Reference-Test (RT). The investigational product, either test or reference was administered after at least 10-hour fasting in each period as per the randomization schedule. The activities of each subject were standardized in both periods including administration of drug with 240-mL water in sitting posture, food restriction for 4 hours post-dose, and water intake restriction for an hour pre and post-dose. The washout period between the study periods was 63 days. Physical and clinical laboratory examinations were performed periodically to evaluate tolerability and to ensure welfare of the study subjects. The subjects were monitored for any adverse events or complaints throughout the study. The bioequivalence studies were conducted as per the protocol, ICH ‘Guidance on Good Clinical Practice’, Declaration of Helsinki, and the Standard Operation Procedures (SOPs) of International Bio Service Co., Ltd., Golden Jubilee Medical Center, Mahidol University, Thailand. The clinical study protocol was approved by the Institute for the Development of Human Research Protection (IHRP), Department of Medical Sciences, Ministry of Public Health, Thailand.
Blood sampling
Total 23 blood samples were drawn at 0 (pre-dose sample) and 0.08, 0.17, 0.25, 0.33, 0.5, 0.67, 0.83, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 3, 4, 6, 12, 24, 36, 48 and 72 hours post-dose through an indwelling intravenous cannula placed in the forearm vein of the subjects and transferred into dipotassium ethylenediaminetetraacetate (K2EDTA) vacutainer. Blood samples were centrifuged at 3,000 ± 100 relative centrifugal force (rcf) for 5 minutes at below 10°C to obtain plasma for entecavir assay. Each plasma sample was separated into two aliquots, and subsequently stored upright in a freezer maintained below -65°C until analysis.
Study sample analysis
The plasma samples were analyzed at GPO, Thailand as per in- house SOPs complying with the Principles of Good Laboratory Practice (GLP) and the international guidelines [14-16]. The samples from the same subject were analyzed in the same analytical run along with 8 calibration standards ranging from 50.223 to 10046.333 pg/mL. The quality control samples at 4 different levels were also included in each analytical batch. Entecavir and the internal standard, entecavir-d2 were extracted from 300 μL of plasma using solid phase extraction technique. Briefly, 750 μL of water was added to each tube and vortexed. The samples were centrifuged at 4500 ± 100 rcf for 5 minutes at 10°C. The StrataTM -X 33 um, 30 mg/l mL cartridges were conditioned using methanol followed by water. Thereafter, each centrifuged sample was loaded into conditioned cartridges. The cartridges were then washed by water and subsequently eluted using methanol. The eluent was evaporated at 50°C to dryness and reconstituted with 200 μL of methanol:water (20:80) solution.
The processed samples were analyzed using a validated Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) method: NexeraTM (Shimadzu Corporation, Japan) coupled with TSQ Quantum Ultra (Thermo Fisher Scientific, USA). Each sample was injected onto Kromasil 100-5C8 150 × 4.6 mm, 5 um column. The isocratic mobile phase consisting of 10 mM ammonium hydrogen carbonate buffer pH 9.5 and methanol (55:45, v/v) was pumped at a flow rate of 0.7 mL/min. The autosampler and column oven temperatures were set at 4°C and 40°C, respectively. The transition of precursor to product ion was monitored in positive mode at m/z 278.100 to 152.000 for entecavir, and m/z 280.100 to 83.100 for entecavir-d2. Data acquisition and evaluation of chromatographic data were performed using XcaliburTM version 4.0.27.42 and LCquanTM version 3.0.26.0 (Thermo Fisher Scientific Inc., USA).
The study samples having concentrations close to maximum concentration and in the elimination phase of each subject in each period were chosen for Incurred Sample Reanalysis (ISR) according to EMA guideline on bioanalytical method validation [15]. However, the concentrations from ISR were not used for pharmacokinetic calculation.
Pharmacokinetic and statistical analysis
The pharmacokinetic parameters were calculated by non- compartmental analysis using Phoenix WinNonlin Software Version 6.4 (Pharsight Corporation, USA). The maximum concentration (Cmax) and time at maximum concentration (tmax) of entecavir were directly obtained from the pharmacokinetic profiles. The truncated area under the curve from time zero to 72 hours (AUC0-72h) of pharmacokinetic profiles was calculated using the trapezoidal rule. The AUC0-72h and Cmax were reported as primary pharmacokinetic parameters, whereas the tmax was reported as a secondary pharmacokinetic parameter.
The statistical analysis was carried out using PROC GLM (SAS® Version 9.4, SAS Institute Inc., USA). Analysis of Variance (ANOVA) was performed for log-transformed primary pharmacokinetic parameters: AUC0-72h and C max. Effects of period, treatment, and sequence on primary pharmacokinetic parameters were included in ANOVA mixed-effect model. The significance of these effects was determined using F-test. The 90% Confidence Intervals (CIs) for the ratio of geometric least squares mean (test/reference) were calculated for the log-transformed primary pharmacokinetic parameters. Bioequivalence was to be concluded when the 90% CIs were within the acceptable range of 80.00-125.00%. Wilcoxon signed-rank test was performed to compare tmax of the test and reference products. All statistical calculations were performed at a significance level of 5% (α=0.05).
Result
Demographic characteristics of subjects
In period I, 28 subjects were enrolled and there were 5 subjects dropped out from the study before rescreening due to personal reason. Two subjects were withdrawn by the principal investigator due to fever and two additional subjects dropped out before check- in of period II. Therefore, 19 subjects completed the study, and their pharmacokinetic data were used for statistical comparison. The demographic characteristics of enrolled and completed subjects are summarized in Table 1.
| Demographic characteristics | Enroll subjects (N=28) | Completed subjects (N=19) |
|---|---|---|
| Age (year) | 31.36 ± 8.22 | 32.68 ± 8.54 |
| Weight (kg) | 62.23 ± 10.87 | 62.08 ± 11.07 |
| Height (m) | 1.64 ± 0.09 | 1.63 ± 0.09 |
| BMI (kg/m2) | 22.98 ± 3.12 | 23.31 ± 3.10 |
Table 1: Demographic characteristics of enrolled and completed subjects (Mean ± SD).
Sample analysis
A total of 1,081 collected samples including samples from drop-out and withdrawn subjects were successfully analyzed. There were 29 samples accounted for 2.7% of total samples were reanalyzed. The correlation coefficient calculated from 8 calibration standards was more than 0.99 for all analytical runs. The between-run precision and accuracy of the calibration standards ranged from 1.4-3.0% CV and 98.8-100.8% of the nominal concentrations, respectively. The quality control samples included in the analytical batch had the precision less than 7.0% CV and the accuracy within ±5% of the nominal concentrations. ISR were carried out in two separate analytical runs for 116 selected samples. The difference between original and ISR concentrations of 113 incurred samples was less than 20% accounted for 97.4% of selected samples. The ISR results met the acceptance criteria as per EMA guideline on bioanalytical method validation [15]. The reanalysis using incurred samples confirmed reproducibility and reliability of the concentration data used for pharmacokinetic analysis.
Pharmacokinetic and statistical analysis
According to the data, entecavir was rapidly absorbed with the mean Cmax around 4-5 ng/mL which was attained at median tmax of 40 minutes after oral administration. The mean AUC0-72h of approximately 13 ng.hr/mL was comparable between the test and reference products. The mean plasma concentration-time profiles of entecavir after administration of the test and reference products under fasting conditions are illustrated in Figure 1. Pharmacokinetic parameters of entecavir for the test and reference products are summarized in Table 2.
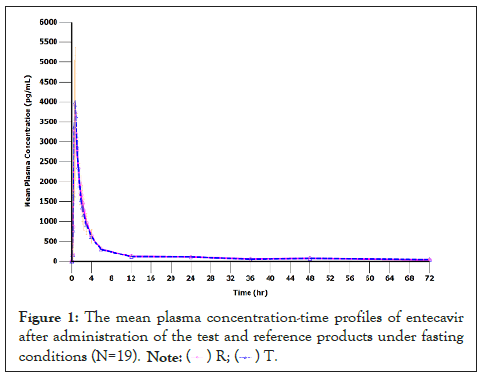
Figure 1:The mean plasma concentration-time profiles of entecavir after administration of the test and reference products under fasting conditions (N=19). ( ) R; (
) R; ( ) T.
) T.
| Parameter (Unit) | Un-transformed data (Mean ± SD, N=19) | |
|---|---|---|
| Test | Reference | |
| AUC0-72h (ng.hr/mL) | 13.2 ± 2.52 | 13.1 ± 2.77 |
| Cmax (ng/mL) | 4.61 ± 1.21 | 4.37 ± 1.59 |
| tmax (hr, in median (min,max)) | 0.67 | 0.67 |
| (0.5, 1) | (0.33, 2.25) | |
Table 2: Pharmacokinetic parameters of entecavir for the test and reference products.
On the ANOVA for log-transformed AUC0-72h and Cmax, no significant effects of sequence, formulation or period were observed (Table 3, p-value > 0.05). The 90% CIs of the geometric least squares mean ratio between the formulations of log- transformed AUC0-72h and C max were within the acceptance range for bioequivalence. Wilcoxon signed-rank test did not detect the significant difference in the median tmax between the test and reference products given under fasting conditions (p-value>0.05).
| Parameter | Geometric least squares mean ratio (90% CI) | Power | Intra subject CV (%) | ANOVA (p-value) | ||
|---|---|---|---|---|---|---|
| Sequence | Formulation | Period | ||||
| ln (AUC0-72h) | 101.3 (95.82-107.00) | 100 | 9.8 | 0.7562 | 0.6992 | 0.2787 |
| ln (Cmax) | 108.0 (95.40-122.32) | 90.8 | 22.3 | 0.7236 | 0.295 | 0.3099 |
Table 3: Statistical comparison of primary pharmacokinetic parameters between the test and reference products (N=19).
Tolerability
Both test and reference products were well tolerated by the study subjects. Twelve post-dose adverse events were reported in 9 subjects who received the test product whereas ten post- dose adverse events were reported in 8 subjects who received the reference product (Table 4). The most frequently reported adverse event in this study was related to hematologic changes including increased alanine aminotransferase, decreased basophil, decreased eosinophil, decreased monocyte, increased neutrophil, increased platelet count, decreased red blood cell count, and increased white blood cell count. The adverse events were mild in the intensity,except that the severity of fever was determined to be moderate but could be resolved with the antipyretic.
| Adverse event | Incidence (N) | |
|---|---|---|
| Test | Reference | |
| Abdominal pain | 1 | 0 |
| Fever | 1 | 1 |
| Dizziness | 1 | 1 |
| Headache | 1 | 0 |
| Increased ALT | 1 | 0 |
| Hematologic changes | 7 | 8 |
| Total | 12 | 10 |
Table 4: List of adverse events.
Discussion
In the present study, entecavir 0.5 mg tablets were administered under fasting conditions. Although the medicine can be taken with or without food, demonstration of bioequivalence under fasting conditions is more sensitive to detect the differences between the test and reference formulations [12,14]. The truncated AUC at 72 hours was used for bioequivalence assessment since it should cover the absorption phase of long half-life drug in the immediate release dosage form, thereby adequately describing and differentiating the biopharmaceutical performance between the formulations [14]. The washout period between the administrations of two formulations was 63 days to ensure complete drug elimination given that at least 5 half-lives are required [17]. No significant amounts of drug were found in any pre-dose samples indicating sufficient washout of drug between study periods. Even though entecavir is a prodrug and must be converted to its active form to exert the activity, the conversion occurs intracellular. Thus the rate and extent of absorption derived from entecavir well represent drug release from the formulation for bioequivalence evaluation [14].
The bioequivalence of entecavir 0.5 mg tablets was successfully demonstrated using the data from 19 healthy Thai subjects with the power greater than 90% for both AUC0-72h and Cmax. The 90% CI of the geometric least squares mean ratio between the formulations of log-transformed AUC0-72h and Cmax met the standard bioequivalence criteria. The ANOVA did not show any significant effects of period, sequence and treatment (formulation) on the primary pharmacokinetic parameters. Wilcoxon signed-rank test showed no significant difference in median tmax between two formulations. The results in this study indicated bioequivalence in the terms of rate and extent of absorption between the test and reference formulations. The intra-subject variability of Cmax observed in the present study was higher than the previously reported value [12,13]. However, the referred study was conducted in Chinese volunteers for bioequivalence evaluation of 1 mg entecavir tablets. Another bioequivalence study conducted in healthy male Chinese volunteers demonstrated the mean Cmax at approximately 5 ng/mL with the mean tmax at 40 min following administration of entecavir 0.5 mg tablets which were similar to those observed in healthy Thai volunteers. However, the study in Chinese volunteers was designed to collect the sample for 96 hours allowing the determination of terminal half-life which was around 127 hours for the reference formulation [18]. The adverse events observed in this study were in agreement with the literature data [19]. No serious adverse events occurred during conducting the study indicating good tolerability of the formulations.
Conclusion
The statistical comparison of AUC0-72h, and Cmax of the test and reference formulations indicated that there was no significant difference between two formulations in terms of rate and extent of absorption. The study successfully established bioequivalence between HEPA-EN® and Baraclude®. The test and reference formulations were well tolerated and no subjects developed serious adverse events.
Acknowledgement
This study was supported by the Government Pharmaceutical Organization (GPO), Thailand.
REFERENCES
- Leroi C, Adam P, Khamduang W, Kawilapat S, Ngo-Giang-Huong N, Ongwandee S, et al. Prevalence of chronic hepatitis B virus infection in Thailand: A systematic review and meta-analysis. Int J Infect Dis. 2016; 51: 36-43.
[Crossref], [Google Scholar], [PubMed]
- Wanich N, Wanich N, Vilaichone RK, Chotivitayatarakorn P, Siramolpiwat S. High prevalence of hepatocellular carcinoma in patients with chronic hepatitis B infection in Thailand. Asian Pacific J Cancer Prev. 2016; 17: 2857-2860.
[Google Scholar], [PubMed]
- D’Souza R, Foster GR. Diagnosis and treatment of chronic hepatitis B. J R Soc Med. 2004; 97(): 318-321.
[Google Scholar], [PubMed]
- Langley RD, Walsh AW, Baldick CJ, Eggers BJ, Rose RE, Levine SM, et al. Inhibition of hepatitis B virus polymerase by entecavir. J Virol. 2007; 81: 3992-4001.
[Crossref], [Google Scholar], [PubMed]
- Palumbo E. Pharmacotherapy of chronic hepatitis B with Entecavir. Clin Med Ther. 2009; 1: 2172.
[Crossref], [Google Scholar]
- Keating GM. Entecavir. Drugs. 2011; 71: 2511-2529.
- Robinson DM, Scott LJ, Plosker GL. Entecavir: A review of its use in chronic hepatitis B. Drugs. 2006; 66: 1605-1622.
[Crossref], [Google Scholar], [PubMed]
- Matthews SJ. Entecavir for the treatment of chronic hepatitis B virus infection. Clin Ther. 2006; 28(2): 184-203.
[Crossref], [Google Scholar], [PubMed]
- Yan JH, Bifano M, Olsen S, Smith RA, Zhang D, Grasela DM, et al. Entecavir pharmacokinetics, safety, and tolerability after multiple ascending doses in healthy subjects. J Clin Pharmacol. 2006; 46(11): 1250-1258.
[Crossref], [Google Scholar], [PubMed]
- Gish RG, Lok AS, Chang T, de Man RA, Gadano A, Sollano J, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007; 133(5): 1437-1444.
[Crossref], [Google Scholar], [PubMed]
- Zhang P. A simple formula for sample size calculation in equivalence studies. J Biopharm Stat. 2003; 13(3): 529-538.
[Crossref], [Google Scholar], [PubMed]
- World Health Organization. Notes on the design of bioequivalence study: Entecavir. 2021; 1-2.
- Jin J, Liu J, Chen J, Zhao L, Ma Z, Chen X, et al. Bioequivalence evaluation of 2 tablet formulations of Entecavir in healthy Chinese volunteers: A single-dose, randomized-sequence, open-label crossover study. Arzneimittelforschung. 2012; 62: 113-116.
[Crossref], [Google Scholar], [PubMed]
- European medicines agency. Guideline on the investigation of bioequivalence. Committee for Medicinal Products for human use (CHMP), London. 2010.
- European Medicines Agency. Guideline on bioanalytical method validation. Committee for Medicinal Products for human use (CHMP), London. 2011.
- U.S. Food and Drug Administration. Guidance for industry: Bioanalytical method validation. Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM), Silver Spring. 2018.
- U.S. Food and Drug Administration. Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. Center for Drug Evaluation and Research (CDER), Silver Spring. 2013.
[PubMed]
- Ding Y, Song M, Shi XL, Gu X, Wen AD, Yang L, et al. Relative bioavailability and bioequivalence of entecavir dispersible tablets in healthy Chinese male volunteers. Chinese J New Drugs. 2010; 19: 590-594.
- Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017; 9(5): 227-241.
[Crossref], [Google Scholar], [PubMed]
Citation: Khaowroongrueng V, Seeduang C, Rakphung S, Duereh M, Saeaue L, Karachot B, et al. (2022) Bioequivalence Study of Entecavir 0.5 mg Tablets in Healthy Thai Volunteers Under Fasting Conditions. J Bioequiv Availab. 14:448.
Copyright: © 2022 Khaowroongrueng V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

