Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2023) Volume 12, Issue 3
Assessment of Blighia sapida on Cholinergic and Antioxidant Enzymes; Possible use of the Plant Stem-Bark Extract as a Biological Pest Controlling Agent
Adekola Mukaila Babatunde1*, Taiwo Adewale Mathew1, Oriyomi Vincent Olumayowa2, Ogunleye Olalekan Seyi1, Oyebamiji Adeola Tope1, Raheem Abdul Rasheed Omotola1, Kasali Sodiq Oluwafemi1 and Bello Iyabo Raliat12Department of Biochemistry and Forensic Science, First Technical University, Ibadan, Nigeria
Received: 05-Jun-2023, Manuscript No. BABCR-23-21715; Editor assigned: 07-Jun-2023, Pre QC No. BABCR-23-21715 (PQ); Reviewed: 22-Jun-2023, QC No. BABCR-23-21715; Revised: 28-Jun-2023, Manuscript No. BABCR-23-21715 (R); Published: 06-Jul-2023, DOI: 10.35248/2161-1009.23.12.491
Abstract
Reduction of cholinergic and metabolizing enzymes by natural products are safer pest-controlling alternatives. The study investigated the ability of Blighia sapida stem-bark extract relative to Rambo, a synthetic pesticide, to interfere with Acetylcholinesterase (AChE), Glutathione S-Transferase (GST), and biomarkers in the brain, liver, and blood of Wistar rat. Rat brain and liver were excised and blood was collected into heparinized tubes at the end of a 28- day experiment for biochemical investigations. The activities of AChE and GST decreased at a dose-dependent rate (P<0.05). A non-significant difference in Alkaline Phosphatase (ALP) for all the treated groups and a dose-dependent increase in total protein concentration was detected. The extract did not significantly alter Alanine Transaminase (ALT), Aspartate Transaminase (AST), and ALP, particularly at repeated doses of 50 mg/kg and 100 mg/kg. The extract of Blighia sapida reduced AChE and GST activity; a property that could be exploited in the formulation of pest control agents.
Keywords
Hydro-alcohol extract; Acetylcholinesterase; Glutathione S-transferase; Rats; Biomarkers
Introduction
Since ancient times, man has engaged in agriculture to meet his food needs and used pesticides to prevent pre-and post-harvest losses of the crop from pests [1]. Pest attack on crops is a threat to global food security and its control with synthetic pesticides is associated with health and environmental problems [2]. Pesticides are chemicals formulated to destroy, control, or prevent pests but because their mode of action is not specific, they also affect non-target organisms including humans [3,4]. Synthetic pesticides exhibit harmful effects such as neurological, behavioral dysfunctions, hormonal imbalances, kidney and liver disorders, genotoxicity, and others due to their persistence in the environment and absorption by humans/animals thereby constituting major causes of environmental and health problems [5,6]. The effects of synthetic pesticides on the environment and organisms have led to the search for safer alternatives from natural sources [7].
According to Yang, et al. [8], plants exhibit pesticide activity by interfering with cholinergic and antioxidant enzymes such as acetylcholinesterase and glutathione S-transferase. Acetylcholinesterase (AChE) terminates nerve impulses by catalyzing the hydrolysis of a neurotransmitter, acetylcholine into acetic acid and choline in the nervous system of various organisms. Also, Glutathione S-Transferases (GST) play an important role in pesticide resistance and detoxification [9]. GSTs are the main cytosolic enzymes that catalyze the conjugation of electrophile molecules with reduced Glutathione (GSH), making potentially toxic substances more water-soluble and less toxic [10]. Inhibitors of AChE and GST are good pesticidal agents [11]. Culturally, Blighia sapida stem-bark powder is usually mixed with seeds of African finger (Abelmoschus esculentus) and pearl millet (Pennisetum glaucum) before sowing to avoid insect attacks. Presently, there is no scientific support for this practice, hence this study.
Materials and Methods
Sample collection
Fresh stem-barks of Blighia sapida were obtained from a farm, at the Federal University of Agriculture Abeokuta, Ogun State Nigeria. The plant had been earlier identified and authenticated at IFE Herbarium, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria. The specimen copy was deposited at the Herbarium and specimen voucher number 17623 was given.
Preparation of hydro-alcohol extract of plant materials
Blighia sapida stem bark was air-dried for two weeks at 25 ± 2°C and ground into powder by an electrical Grinding Machine (SR-14733, Marlex, Daman). The powder material (1066.71 g) was macerated in 70% (v/v) ethanol/water for 72 hours at room temperature using the method of Handa, et al. The resulting suspension was filtered and strained with a muslin cloth. The filtrates were pooled together and concentrated with a rotary evaporator at 40°C to yield a residue termed Ethanol Extract (EE). The resulting extract was weighed, labelled, and stored in the desiccator until required for further analysis.
Experimental animals
Thirty Wistar rats, weighing between 150 g and 220 g were obtained from the Department of Anatomy, University of Ibadan, Ibadan, Nigeria. The rats were acclimatized to the laboratory conditions for two weeks in the Animal House. Rats were fed with standard pellets obtained from Ladokun Feeds, Ibadan, Oyo State, Nigeria, and were allowed free access to clean water. The principle of laboratory animal care (NIH publication No. 85-23) guidelines and procedures were followed for the study (NIH Publication Revised, 1996). Animal handling and care complied with international laboratory animal use and care guidelines. The approval of the departmental animal ethical committee, the Federal University of Agriculture Abeokuta Environmental Management and Toxicology (FUNAAB/EMT/20142094) was obtained prior to the experiment.
Determination of LD50 of the Blighia sapida extract
The lethal dose (LD50) of the ethanol extract was designed in two phases [12]. Nine (9) Wistar rats were divided into three groups (3 Wistar rats per group) in phase one. The ethanol extract was given at 10 mg/kg, 100 mg/kg, and 1000 mg/kg body weight orally to all the groups (1, 2, and 3) respectively once before feeding. The Wistar rats were checked regularly for signs of toxicity, like withdrawal to a corner of the cage, rough fur, and salivation during the first 4 hours. Then, they were observed for the next 24 hours and every other day for 14 days, for effects of toxicity. In phase two, 3 Wistar rats were divided into 3 groups (1 rat per group), and 1600 mg/ kg, 2900 mg/kg, and 5000 mg/kg body weight of the extract were administered orally, this followed the procedure of the first phase.
Experimental Design
Thirty (30) rats were randomly divided into five groups, and each group contained six rats [13];
• Group 1–received 1.0 ml of distilled water
• Group 2–received Rambo pesticide 10% (w/v)
• Group 3–received 50 mg/kg EE
• Group 4–received 100 mg/kg EE
• Group 5–received 150 mg/kg EE
Based on the result of the acute test (LD50) conducted, three doses (50 mg/kg, 100 mg/kg, and 150 mg/kg) of B. sapida ethanol extract were chosen for the study. The rats were treated by gavage administration of the extract and locally produced insecticide ‘Rambo’ containing 0.6% permethrin (synthetic pesticide) every other day for 28 days. On day 28, the animals fasted overnight before being sacrificed under light chloroform anaesthesia, their blood was obtained through the cardiac puncture, and organs of interest were excised. Collected blood samples were kept in separate heparinized tubes while the organs were stored in plain sample bottles. The samples were labelled and used for the estimation of biochemical parameters.
Preparation of blood plasma
Blood plasma was prepared according to the standard procedure [14]. Typically, blood samples collected in heparinized tubes were centrifuged at 3000 rpm for 10 min in a Table Centrifuge (Model 90-2) at 25°C. The plasma was collected into sterile tubes with sterile Pasteur pipettes and used for biochemical analyses.
Preparation of liver and brain homogenates
Liver and brain homogenates were prepared as described by Babalola, et al. [15]. One gram (1 g) of tissue was homogenized with 100 mM of phosphate buffer, pH 7.2, to produce 10% (w/v) homogenates with pestle and mortar. The homogenates were carefully transferred into centrifuge tubes and volumes were adjusted to 10 ml. This was centrifuged at 4000 rpm for 30 min in a Table Centrifuge (Model 90-2) at 25 °C. The supernatants were collected into clean sample bottles, labelled, and kept in the freezer for the assay of biomarker enzymes.
Determination of biochemical parameters
The biochemical parameters were assayed according to the methods described by Klein and Kaufman [16], for ALP, total protein by Lowry, et al. [17], Habig et al. [18], for GST, and Voss and Sachsse [19], for AChE.
Histopathological examination
Liver samples were cleared from adhering tissues, fixed in 10% formal saline, dehydrated through ascending grades of alcohol (50%, 70%, 80%, 90%, and 100%) and cleared in xylene. The wax-infiltrated tissues were embedded in paraffin wax and 5 μm thick sections were prepared from the tissues using a LEICA rotary microtome. These sections were floated in a water bath (45°C) to allow spreading of the folded sections and mounted on glass slides Dewaxed slide sections were then rehydrated in descending grades of alcohol (100%, 90%, 80%,70%, and 50%) and stained with hematoxylin and eosin. Sections were differentiated in a mixture of (1% hydrochloric acid in 70% alcohol) to remove excess dye from the tissues. The stained tissues were observed under the microscope (LEICA DM750) interfaced with a LEICA (ICC50) camera.
Statistical analysis
The data obtained were analyzed using one-way Analysis of Variance (ANOVA) followed by Tukey-Kramer multiple comparisons test using the software Graph pad Prism 5. The statistical significance was set at p<0.05. Values were expressed as mean ± Standard Error of the Mean (SEM).
Results
The acute toxicity test showed that the extract is nontoxic at the highest dose (5000 mg/kg) tested. No toxic symptoms or mortalities were observed in the two phases of the acute toxicity test (LD50>5000 mg/kg).
Biochemical parameters
The biochemical parameters of plasma, liver, and brain homogenates of Wistar rats administered with different concentrations of B. Sapida extract and Rambo are shown in Table 1. A significant decrease in AChE activity in the experimental animal that received a high dose (150 mg/kg) of the extract was detected. Also, there was a significant decrease in plasma GST activity at 100 mg/kg and 150 mg/kg extract. The decrease in these enzymes is similar to Rambo which was significant when compared to the control group. There was no significant difference in the activity of alkaline phosphatase in the test groups as compared to the control group. A dose-dependent increase in protein concentration was observed.
Histopathological examination
Histopathological investigation of liver sections in control rats showed normal cellular integrity and normal lobular architecture with central veins and radiating cords of hepatocytes, separated by blood sinusoids of the liver. However, no marked lesion or congestion was observed in the groups treated with 50 mg/kg, 100 mg/kg, and 150 mg/kg of B. sapida extract. A mild lesion and portal congestion were observed in the group that was administered 10% (w/v) Rambo pesticide (Figures 1-5).
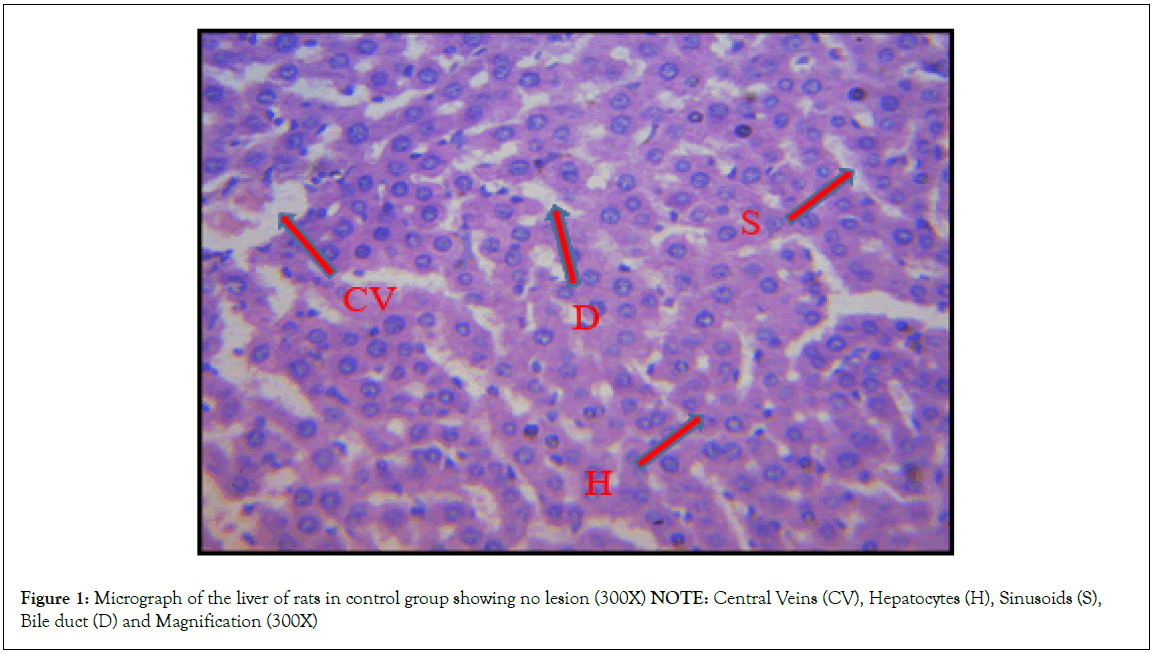
Figure 1: Micrograph of the liver of rats in control group showing no lesion (300X)
NOTE: Central Veins (CV), Hepatocytes (H), Sinusoids (S),
Bile duct (D) and Magnification (300X)
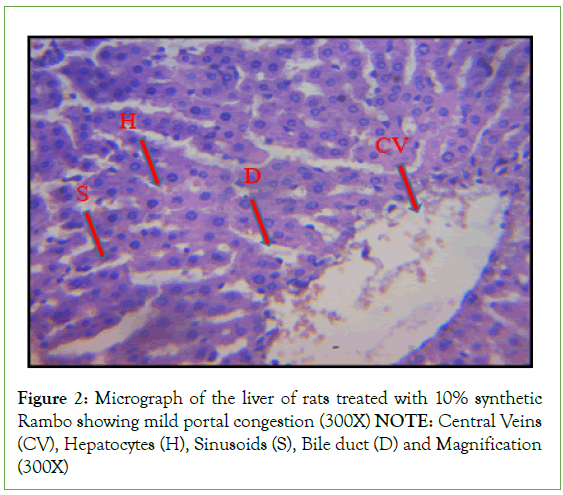
Figure 2: Micrograph of the liver of rats treated with 10% synthetic
Rambo showing mild portal congestion (300X)
NOTE: Central Veins
(CV), Hepatocytes (H), Sinusoids (S), Bile duct (D) and Magnification
(300X)
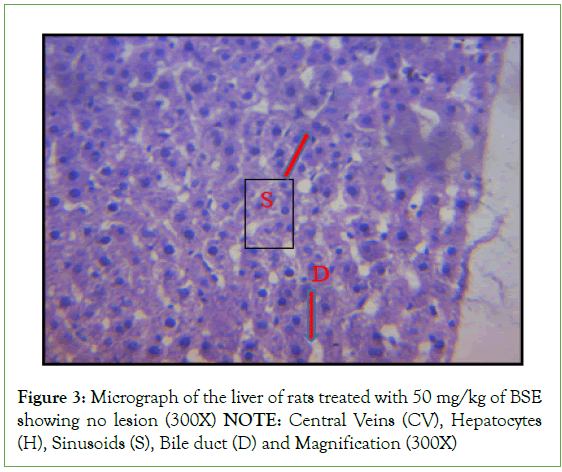
Figure 3: Micrograph of the liver of rats treated with 50 mg/kg of BSE
showing no lesion (300X)
NOTE: Central Veins (CV), Hepatocytes
(H), Sinusoids (S), Bile duct (D) and Magnification (300X)
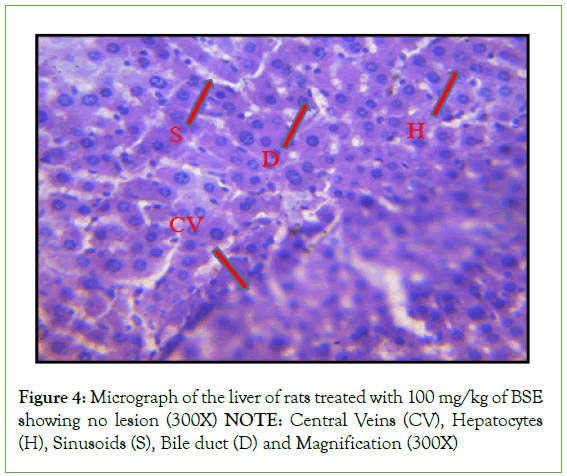
Figure 4: Micrograph of the liver of rats treated with 100 mg/kg of BSE
showing no lesion (300X)
NOTE: Central Veins (CV), Hepatocytes
(H), Sinusoids (S), Bile duct (D) and Magnification (300X)
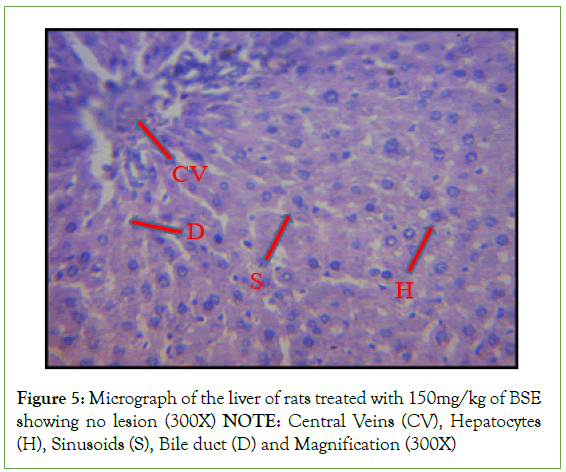
Figure 5: Micrograph of the liver of rats treated with 150mg/kg of BSE
showing no lesion (300X)
NOTE: Central Veins (CV), Hepatocytes
(H), Sinusoids (S), Bile duct (D) and Magnification (300X)
Discussion
In this study, no death was recorded during the acute and sub- acute toxicity tests with graded doses of ethanol extract of B. Sapida. No symptom of toxicity was noticed and no significant changes in appearances were observed when the treated groups were compared with the control group. The results of this study showed that B. sapida decreased the activity of cholinergic and antioxidant enzymes in Wistar rats administered with high doses of the extract in a similar pattern as a synthetic pesticide (Rambo). According to Malhat, et al. [20], this effect is typical of botanicals that interrupt the proper functioning of the insect nervous system, causing uncontrollable excitation, tremor, and, death as a result of an accumulation of acetylcholine. Accumulation of acetylcholine at neuron synapses and neuromuscular junctions has been reported to cause effects such as paralysis and muscle weakness [21]. Glutathione S-Transferases (GST) is the main enzyme that catalyzes the conversion of foreign chemicals to hydrophilic, less toxic, and easily excreted materials from the system [22,23]. A low dose (50 mg/kg) of the extract exhibited no significant reduction in GST activities, however, higher doses of 100 mg/kg and 150 mg/kg caused a significant reduction in the activity of GST. The findings of this study are similar to that of Oni et al. in which the administration of Acalypha wilkesiana extract which reduced GST killed Callosobruchus maculatus [24]. Similarly, studies by Ebadollahi et al. and Tarigan et al. have also reported the pesticidal activity of different botanical extracts that reduced GST activity. The observed effects of the B. Sapida extract on the activities of AChE and GST indicate that the metabolites in the extract may serve as an effective botanical pesticide [25,26].
Alkaline phosphatase is a membrane-bound enzyme and it plays a critical role in the transportation of metabolites across the cell membrane, conditions such as liver damage and bone disease lead to its alteration which affects the transport of metabolites and membrane permeability [27]. Also, alkaline phosphatase has been referred to as a family of zinc metalloenzymes, and an increase in its activity is an indication of biliary process obstruction [28]. The ALP activity of the rats treated with different doses of ethanol extract of B. sapida exhibited no significant difference at (p>0.05) when compared to the control rats which indicates that the extract may not cause cellular leakage of the enzyme and obstruction of normal biliary process. The results of ALP in this study implied that phytoconstituents in the plant extract were neither toxic nor caused damage to the membrane at the doses considered, hence transportation of metabolite remains intact. This result was also confirmed by histopathological examination of the liver (Figure 1), in which the micrograph of the liver of rats treated with different doses of the plant extract revealed no lesion. Plasma alkaline phosphatase is one of the liver biomarker enzymes, whose evaluation provides details of pathological damage to the tissue [29]. A high level of plasma ALP activity has been linked with hepatobiliary dysfunction which is likely a result of hepatobiliary injury and cholestasis [30]. The nonsignificant value of ALP activities observed in this study implied that the doses of the extract considered may not affect the hepatobiliary function of the liver.
The assessment of the activities of enzymes such as ALT and AST provides information on liver function. The ethanol extract of B. sapida stem-bark displayed a dose-dependent increase in the activities of ALT as well as AST in the rats. The results of this study indicated that administration of the plant extract as bio pesticide may not alter the functions of the liver as displayed by liver marker enzymes. Higher levels of these liver enzymes indicating hepatotoxicity have been reported by different authors [31].
The liver is mainly involved in the synthesis of plasma protein, damage to which causes a reduction in the protein concentration [32]. A dose-dependent increase in protein concentration was observed in the groups that were administered with different doses of ethanol extract of B. sapida which implies that the extract is not toxic at the doses considered because a decrease in the level of total protein has been associated with damage to the liver function caused by toxicity [33]. The results of this study imply that bioactive compounds present in the study plant may cause a reduction in the activities of enzymes involved in the metabolism of foreign substances (pesticides) thereby leading to consequences such as tremor, reduction in the rate of survival, and death. The bioactive compounds in the plant may also serve as growth retardants, cause a reduction in the rate of reproduction, and loss of weight in the larva, pupa, and adult [34].
The toxic effects of natural products in animals and humans can be evaluated by using physiological parameters such as but not limited to histological examination. The histopathological examination of the liver in control rats showed normal cellular integrity and normal lobular architecture with Central Veins (CV) and radiating cords of Hepatocytes (H), separated by blood Sinusoids (S) of the liver. However, no marked lesion or congestion was observed in the groups treated with 50 mg/kg, 100 mg/kg, and 150 mg/kg of B. Sapida extract. A mild lesion and portal congestion was observed in the group that was administered with 10% (w/v) Rambo pesticide. However, Adekola et al. reported distinct hepatocytes, clear central vein, and mildly congested sinusoids in rats administered with 250 mg/kg ethanol extract of B. sapida.
Conclusion
Natural products that can reduce cholinergic and metabolizing enzymes serve as safer alternatives for pest control. The hydro- ethanol extract of Blighia sapida significantly reduced the activity of acetylcholinesterase and glutathione-s-transferases which are involved in neurotransmission and detoxification, respectively. This implies that the potent extract interfered with the detoxification process to make the insect pest more vulnerable to the toxic effects of potent extract. This is suggestive of B. sapida as having neurodegenerative ability peculiar to pesticide agents. Hence, these properties of Blighia sapida could be harnessed in the development of pest control agents as an alternative agent for the mitigation of pests in agriculture.
Acknowledgment
The assistance rendered by Prof. O. O. Babalola and Dr. J. I. Olawuni, Department of Biochemistry and Molecular Biology, Obafemi Awolowo University, Ile-Ife, Nigeria during this research is highly appreciated.
Authors Contribution
AMB, TAM: Conceptualization. AMB, OVO, OOS, OAT, RAO, KSO, BIR: Investigation; Methodology; Project administration; Data curation; Formal analysis. AMB: Supervision; Validation; Original draft preparation. AMB, OVO: Writing- Reviewing and Editing. All the authors have read and approved the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author, (AMB), upon reasonable request.
Ethics Approval
The principle of laboratory animal care (NIH publication No. 85- 23) guidelines and procedures were followed for the study (NIH publication revised, 1985). The approval of the departmental animal ethical committee, Federal University of Agriculture Abeokuta/ Environmental Management and Toxicology (FUNAAB/ EMT/20142094) obtained prior to the experiment.
References
- Adekola MB, Areola JO, Omisore NO, Asaolu FT, Ogunleye SG, Apalowo OE, et al. Sub-chronic toxicity study of ethanol stem-bark extract of Blighia sapida (Sapindaceae) in wistar rats. Heliyon. 2020;6(2):e02801.
[Crossref] [Google Scholar] [PubMed]
- Adeoti OT, Belonwu DC, Wegwu MO, Osuoha JO. Implication of acute, subchronic and chronic exposure to different pesticides via inhalation on male wistar rats. J Biosci Bioeng.2017;5(4):74-85.
- Babalola OO, Areola JO. Interactive roles of terpenoid extract from the leaves of neem plant (Azadirachta indica, A. Juss) on lead induced toxicity in pregnant rabbits. J Med Plant Res. 2010;4(12):1102-1107.
- Chawla R. Biochemical tests for total proteins, albumin and pyruvate.1999.
- Chowdhary K, Kumar A, Sharma S, Pathak R, Jangir M. Ocimum sp.: Source of biorational pesticides. Ind Crops Prod. 2018;122:686-701.
- Dobritzsch D, Grancharov K, Hermsen C, Krauss GJ, Schaumlöffel D. Inhibitory effect of metals on animal and plant glutathione transferases. J Trace Elem Med Bio. 2020;57:48-56.
[Crossref] [Google Scholar] [PubMed]
- Ebadollahi A, Khosravi R, Sendi JJ, Honarmand P, Amini RM. Toxicity and physiological effects of essential oil from Agastache foeniculum (Pursh) Kuntze against Tribolium castaneum herbst (Coleoptera: Tenebrionidae) larvae. Annu Res Rev Biol. 2013:649-658.
- Sub-chronic toxicity study of ethanol stem-bark extract of Blighia sapida (Sapindaceae) in wistar rats
- Gullner G, Komives T, Király L, Schröder P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front Plant Sci. 2018;9:1836.
[Crossref] [Google Scholar] [PubMed]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem.1974;249(22):7130-7139.
[Google Scholar] [PubMed]
- Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction technologies for medicinal and aromatic plants, international centre for science and high technology. Trieste.2008;2:21-25.
- Kasthuri OR, Ramesh B. Toxicity studies on leaf extracts of Alternanthera brasiliana (L.) Kuntze and Alternanthera bettzickiana (Regel) Voss. J Appl Pharm Sci.2018;8(10):82-89.
- Kaur M, Kumar R, Upendrabhai DP, Singh IP, Kaur S. Impact of sesquiterpenes from Inula racemosa (Asteraceae) on growth, development and nutrition of Spodoptera litura (Lepidoptera: Noctuidae). Pest Management Science. 2017;73(5):1031-1038.
[Crossref] [Google Scholar] [PubMed]
- Kaur R, Mavi GK, Raghav S, Khan I. Pesticides classification and its impact on environment. Int J Curr Microbiol Appl Sci. 2019;8(3):1889-1897.
- Klein B, Kaufman JH. Automated alkaline phosphatase determination: III. Evaluation of phenolphthalein monophosphate. Clin Chem.1967;13(4):290-298.
[Google Scholar] [PubMed]
- Lee SK, Lee DR, Choi BK, Palaniyandi SA, Yang SH, Suh JW, et al. Glutathione S-transferase pi (GST-pi) inhibition and anti-inflammation activity of the ethyl acetate extract of Streptomyces sp. strain MJM 8637. Saudi J Biol Sci.2015;22(6):744-751.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275.
[Google Scholar] [PubMed]
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275-287.
[Crossref] [Google Scholar] [PubMed]
- Malhat FM, Loutfy NM, Greish SS, Ahmed MT. A review of environmental contamination by organochlorine and organophosphorus pesticides in Egypt. J Toxicol Risk Assess. 2018;4:13.
- Mu’azu AB, Baba YB, Matinja AI. Biochemical and histopathological effect of Detarium microcarpum stem bark extract in wistar albino rats. Asian J Biochem. 2020;20:1-9.
- Nartop D, Yetim NK, Özkan EH, Sarı N. Enzyme immobilization on polymeric microspheres containing Schiff base for detection of organophosphate and carbamate insecticides. J Mol Struct. 2020;1200:127039.
- Ogunleye GS, Fagbohun OF, Babalola OO. Chenopodium ambrosioides var. ambrosioides leaf extracts possess regenerative and ameliorative effects against mercury-induced hepatotoxicity and nephrotoxicity. Ind Crops Prod. 2020;154:112723.
- Oni MO, Ogungbite OC, Oguntuase SO, Bamidele OS, Ofuya TI. Inhibitory effects of oil extract of green Acalypha (Acalypha wilkesiana) on antioxidant and neurotransmitter enzymes in Callosobruchus maculatus. j basic appl zool. 2019;80(1):1-3.
- Ore A, Olayinka ET. Influence of moxifloxacin on hepatic redox status and plasma biomarkers of hepatotoxicity and nephrotoxicity in rat. Biochem Res Int. 2015;2015:192724.
[Crossref] [Google Scholar] [PubMed]
- Jayaraj R, Megha P, Sreedev P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol. 2016;9(3-4):90-100.
[Crossref] [Google Scholar] [PubMed]
- Saroj A, Oriyomi OV, Nayak AK, Haider SZ. Natural remedies for pest, disease and weed control. 2020.
- Shirode DS, Kulkarni AV, Jain BB. Effect of blumea lacera on tissue gsh, lipid peroxidation and hepatic cells in ethanol induced hepatotoxicity in rats. Int J Pharm Pharm Sci. 2019.
- Simon JP, Parthasarathy M, Nithyanandham S, Katturaja R, Namachivayam A, Prince SE, et al. Protective effect of the ethanolic and methanolic leaf extracts of Madhuca longifolia against diclofenac-induced toxicity in female Wistar albino rats. Pharmacol Rep. 2019;71(6):983-993.
[Crossref] [Google Scholar] [PubMed]
- Tarigan SI, Harahap IS. Toxicological and physiological effects of essential oils against Tribolium castaneum (Coleoptera: Tenebrionidae) and Callosobruchus maculatus (Coleoptera: Bruchidae). J Biopest. 2016;9(2):135.
- Umar A, Mgutu AJ, Piero MN, Ann NW, Maina GS. In Vitro anti-acetylcholinesterase activity of crude fruits sap extract of Solanum incanum in green peach aphids. J Develop Drugs. 2015;4(142):2.
- Voss G, Sachsse K. Red cell and plasma cholinesterase activities in microsamples of human and animal blood determined simultaneously by a modified acetylthiocholine/DTNB procedure. Toxicol Appl Pharmacol. 1970;16(3):764-772.
[Crossref] [Google Scholar] [PubMed]
- Wilson CL, Huber DM. Synthetic pesticide use in Africa: Impact on people, animals, and the environment. 2021.
- Yang WD, Liu YR, Liu JS, Liu Z. Inhibitory effects and chemical basis of eucalyptus orelliana wood meals on the growth of Alexandrium tamarense. 2008;29(8):2296-2301.
[Google Scholar] [PubMed]
- Yılmaz M, Sebe A, Ay M, Gürger M. Organophosphate poisoning and intermediate syndrome. Annu Rev Resour. 2016;25(1):70-83.
Citation: Babatunde AM, Mathew TA, Olumayowa OV, Seyi OO, Tope OA, Omotola RAR, et al (2023) Assessment of Blighia sapida on Cholinergic and Antioxidant Enzymes; Possible use of the Plant Stem-Bark Extract as a Biological Pest Controlling Agent. Biochem Anal Biochem. 12:491.
Copyright: © 2023 Babatunde AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

