Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 1
Antiestrogen Treatment for Androgen-Insensitive or Castration-Resistant Prostate Cancer, Identification of Estrogen Stem Cells, and Estrogen Receptors
Akhouri A Sinha*Received: 16-Jan-2023, Manuscript No. JCM-22-19753; Editor assigned: 18-Jan-2023, Pre QC No. JCM-22-19753 (PQ); Reviewed: 01-Feb-2023, QC No. JCM-22-19753; Revised: 08-Feb-2023, Manuscript No. JCM-22-19753 (R); Published: 17-Feb-2023, DOI: 10.35248/2157-2518.23.14.409
Abstract
Objective: A variety of treatments (such as bilateral castration, chemical castration, adrenalectomy and hypophysectomy) have not cured adenocarcinoma of Prostate Cancer (PC). We hypothesize that sub type (s) can exist resulting in the failures of the above treatments.After Androgen Deprivation Therapy (ADT), PC emerges as androgen-insensitive or Castration-Resistant Prostate Cancer (CRPC).Our study is designed to identify a sub type of PC.
Materials and Methods: DES (diethylstilbestrol) treated and untreated prostate biopsy specimens were utilized to identify two sub-types, androgen-sensitive and androgen-insensitive, tumours. Additional studies, such as localization of isotopic estrogen and estrogen antibodies, were designed to further define the sub types of PC.
Results: Localization of isotopic estrogen showed silver grains in cancer cells and not in the controls by electron microscopic autoradiography. Estrogen antibody IgG localized in the nucleus by electron microscopy. After the removal of androgen by the ADT, estrogen-dependent stem cells and estrogen receptors were identified.
Conclusion: ADT alone does not treat the estrogen-dependent PC. PC/CRPC has caused over 375,000 deaths in the world in 2021. Both androgen and estrogen dependent PC need to be treated concurrently. Antiestrogen treatment (e.g., Tamoxifen, etc.) is the appropriate for the sub type of estrogen dependent PC.Clinical trials are expected to determine the sequence of ADT (including inhibition of Hypothalamic Luteinizing Hormone-Releasing Hormone and antiestrogen treatments, dosages, timing, and duration of treatments. Antiestrogen treatment combined with ADT, will hopefully begin an era of curing PC just as the antibiotics have cured bacterial infections.
Keywords
Androgen-insensitive, Castration-resistant prostate cancer, Estrogen-dependent cancer, Estrogen- dependent stem cells, Estrogen receptors, Antiestrogen treatment for increased survival
INTRODUCTION
The author joined the Minneapolis Veterans Affairs Medical Center (VAMC) as a scientist and electron microscopist for the ongoing study of VA Cooperative Urological Group (VACRG). Most of the VACURG pathology was based on hematoxylin and eosin-stained sections using light microscopy [1]. This allowed the author to study prostate specimens using electron microscopy and a variety of other techniques.His study indicated that PC did not develop in the womb and after birth until after the age of about 30 [1, 2].The average age for PC at the VAMC patients is about 68 years. All these individuals were undoubtedly, exposed to the circulating carcinogens, but only older men developed PC. The reasons for these differences are unknown.Stem cell genes were exposed to circulating carcinogens and gene mutation occurs in some organs, such as in prostate, but not in lungs and other solid organs.Our earlier study provides partial explanation [1]. We had suggested that after organogenesis, organ organ-specific-genes are retained, and unrelated genes were either deleted or inactivated. This undoubtedly prevents development of cancers in several organs concurrently, such as prostate and lungs.Dual primary cancer is relatively rare [3, 4]. This area requires additional studies.
Benign prostate and its cancer and benign breast and its cancer contain testosterone, estrogen, and progesterone [5, 6]. Ratios of steroid hormones differ in males and females. We had published a brief report on the pathway of steroid hormones as have others [7]. Earlier, White treated removed androgen in PC by bilateral castration [8]. Subsequently, chemical castration treatment DES (Diethylstilbestrol) was used by Huggins and Associates in the 1940s, and that was followed by adrenalectomy and hypophysectomy [9-12]. These treatments did not cure PC.Our analysis of DES-treated and untreated biopsies identified two sub- types of PC, namely, androgen-insensitive, and androgen-sensitive in resin (Epon)-embedded and methylene blue or toluidine blue- stained sections by light and electron microscopy [13]. Others have suggested the role of estrogen in prostate carcinogenesis and rationale for chemoprevention [14, 15]. Management of CRPC has been recommended by the continued hormone, immunotherapy, chemotherapy palliative radiation and/or bone-targeted therapy [14-18]. Recent review of 303 studies published from 1996 through 2013 indicated others have advocated use of androgen antagonist [16-18]. The treatments of PC and/or CRPC have utilized variations of androgen deprivation straggles. Our review indicates that indicates the sub-type of androgen-insensitive PC involves estrogen. We hypothesize that the sub-type of PC is estrogen-dependent that can be treated by antiestrogen(s). The ADT alone (including inhibition of hypothalamic releasing hormone, LHRH, and/or anti-androgen) does not treat estrogen-dependent PC.Emergence of Castration-Resistant Prostate Cancer (CRPC) sums-up the failure of the ADT and other treatments.
Materials and Methods
The author utilized previously published literature and new studies in preparation of this manuscript. We published a series of papers that have provided the details of materials and methods, including dilutions of the antibodies, buffers, and controls [1, 7, 13, 16-20]. Negative controls did not receive the antibody or were incubated with the serum of animal used for the antibody [1, 19- 20].Unpublished data include localization of estradiol antibodies using 10-15 nm gold particles conjugated with protein gold as detailed [20]. Radiation treated biopsy sections were not published before and are being used as a control to compare cell death in DES-treated cases.Urologic surgeon Dr. Clyde E. Blackard and his associates at the Minneapolis VAMC selected untreated and DES- treated patients for biopsies as reported before [13]. The former and current VAMC urology surgeons Drs. Pratap K. Reddy and Eduardo T. Fernandes and associates selected radical prostatectomy patients at the Minneapolis VAMC. Radiation cases were selected by Dr. Arthur D. Smith of the Minneapolis VAMC. Most of the patients had moderately differentiated PC before radiation. Patients were biopsied between 37 days and 5.6 years [13]. After external beam rotation of about 7000 rad, patients were biopsied. All prostate specimens were submitted to the VAMC Pathology Service, and the extra specimens were provided for the research. All samples were collected after obtaining the approval of the Institutional Review Board (IRB) guidelines in place at the VA and the University of Minnesota as reported before [7, 13, 18-20]. These patients were not treated with any hormone therapy or chemotherapy prior to biopsy and prostatectomy. Biopsy specimens were collected between 1972 and 1979 and prostatectomy specimens were collected between 1983 and 1993. For this study, we collected untreated biopsy (#14), DES-treated (#8) prostatectomy (#55), and radiation cases (#7) samples. They were processed for histology and localization of antibodies, isotopic estrogen and, estrogen antibodies and examination by light and electron microscopy [1, 7, 13, 18-20]. Paraffin sections used in diagnosis of cancer were also available for study. After removal of paraffin, specimens were refixed in a combination of 2% paraformaldehyde and 2% glutaraldehyde [1, 6, 17-19]. After washing, pieces were post-fixed in 1% to 2% buffered osmium tetroxide washed and dehydrated in graded ethanol, and then embedded in resin (Epon 812) as previously described [1,7, 13, 18-20]. Prostate pieces were incubated with 3H isotopic estrogen for 2 to 24 hours at 37˚C and embedded in Epon. Thin sections were coated with Illford-L-4 emulsion for 2 to 4 weeks before processing for autoradiography and electron microscopy [18]. Stem cells, androgen receptors and estrogen receptors were localized as detailed before [1, 7, 13, 18-20]. LR White resin (Poly- sciences, Inc., Warrington, PA)-embedded sections were used for localization of primary antiestrogen antibody IgG (dilution 1:10) Aldrich-Sigma, St Louis, Missouri, USA) and protein A gold, as a secondary antibody to visualize localization was detailed before [1, 7, 13, 18-20].The negative and positive controls have been reported with each published study and were reviewed recently [19-20]. Former staff pathologists Drs. Donald F. Gleason, Nancy A. Staley, and Stephen L. Ewing graded Epon and/or LR White embedded and paraffin sections, CD 133 antibody and the Gleason grading system [22, 23].Addition details on prostate classification, CD 133 antibodies and localization techniques are [23, 24].
Results: We have identified estrogen and androgen-dependent Stem cells by localization of CD 133 in PC cells. CD 133 antibodies do not distinguish between the two types of stem cells [19]. However, localization of antibodies against androgen and estrogen receptors distinguishes stem cells [7]. Control prostate pieces incubated without isotopic estrogen did not show any localization (Figure 1A) Prostate sections incubated with the isotopic estrogen localized grains in the nucleus of invasive cells in the prostatic stroma and not in the cytoplasm (Figure 1B). Another control section localized isotopic thymidine in the peripheral chromatin of the nucleus was published before [20].These figures show that localization of estrogen was specific in prostate. Localization of antibody IgG against estrogen was in the nucleus and not in the cytoplasm by protein A gold particles indicating presence of estrogen in the prostate (Figure 1C).Acinar lumen does not show any gold particles indicating specificity of localization.Figures 1A, 1B, and 1C show the specificity of estrogen localization in the prostate. Removal of androgen (testosterone) by DES or ADT unmasked estrogen that allowed localization of estrogen receptors (Figure 2A).Stem cells were identified in the early 21st century [25-27]. The earlier studies labeled them as light and dark basal cells (Figure 3A) [1, 13].CD 133 does not distinguish between androgen-dependent and estrogen- dependent stem cells whereas androgen receptors and estrogen receptors do distinguish them [1, 13, 19]. Immunogold localization of Estrogen Receptors (ERB, H-50) is shown in nuclei of dark basal cells (or androgen-insensitive cells) and occasional invasive cells in DES-treated sections (Figures 2 A and B). Nuclei of light basal cells also localized estrogen receptors. Figure (2B) also shows a group of unlabeled led invasive cells in the stroma. Light microscopy section of PC showed androgen-sensitive cells (type 1 or b-1 light basal cells) and androgen-insensitive cells (type II or basal cells as dark cells), aggregate of basal type 1 cells (ag), acinar cells around the lumen (l) (Figure 3A). Figure also showed degeneration of androgen-sensitive acinar cells around lumen (l). The androgen- insensitive cell nucleus did not degenerate in untreated and DES- treated cases. The later was followed from 67 days to 5.6 years [13]. Biopsy of radiation treated cases showed condensation of nuclear chromatin, nuclear membrane, cytoplasm, including sloughing of cells in the acinar Lumen (LU) (Figure 3B). Electron microscopy of radiation-treated section showed damage in the nuclei and cytoplasm, including acinar lumen at the upper left corner of the micrograph (Figure 3C).

Figure 1: (A). Prostate tissue incubated as control does not show any localization of grains in the two nuclei or cytoplasm. Bar indicates magnification. (B). Prostatic tissue incubated with 3h estradiol and processed for the localization of the isotope for electron microscopy autoradiography shows any localization of grains in the two nuclei of invasive cells in the stroma. Stroma also shows a portion of the Smooth Muscle (SM). Bar indicates 500 um magnification. (C). Localization of antibody IgG against estradiol shows nuclear localization of the silver grains after processing with the protein gold. Cytoplasm and acinar lumen do not show any localization. Magnification is shown by the bar.
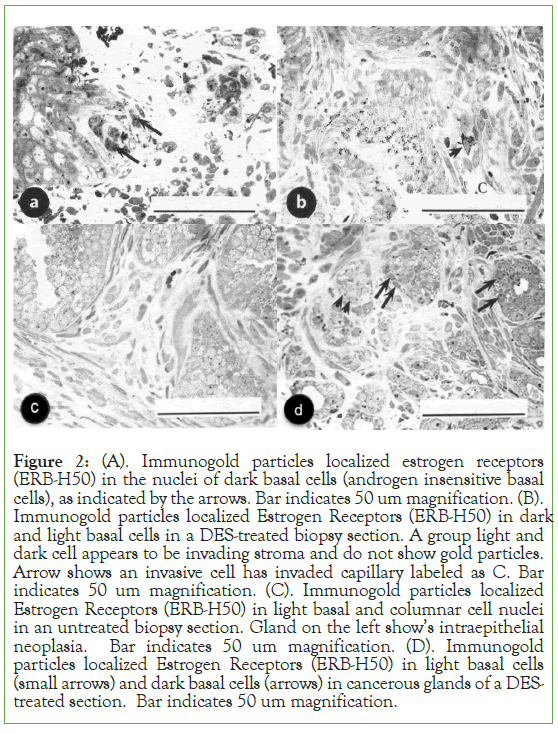
Figure 2: (A). Immunogold particles localized estrogen receptors (ERB-H50) in the nuclei of dark basal cells (androgen insensitive basal cells), as indicated by the arrows. Bar indicates 50 um magnification. (B). Immunogold particles localized Estrogen Receptors (ERB-H50) in dark and light basal cells in a DES-treated biopsy section. A group light and dark cell appears to be invading stroma and do not show gold particles. Arrow shows an invasive cell has invaded capillary labeled as C. Bar indicates 50 um magnification. (C). Immunogold particles localized Estrogen Receptors (ERB-H50) in light basal and columnar cell nuclei in an untreated biopsy section. Gland on the left show’s intraepithelial neoplasia. Bar indicates 50 um magnification. (D). Immunogold particles localized Estrogen Receptors (ERB-H50) in light basal cells (small arrows) and dark basal cells (arrows) in cancerous glands of a DES- treated section. Bar indicates 50 um magnification.
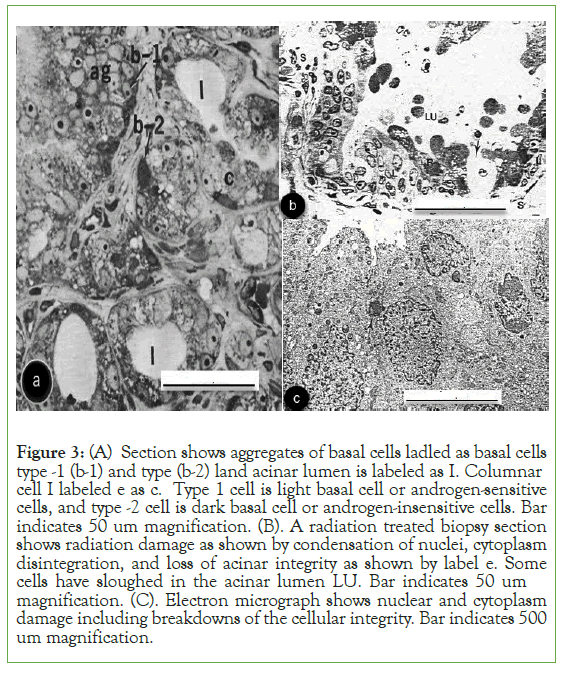
Figure 3: (A) Section shows aggregates of basal cells ladled as basal cells type -1 (b-1) and type (b-2) land acinar lumen is labeled as I. Columnar cell I labeled e as c. Type 1 cell is light basal cell or androgen-sensitive cells, and type -2 cell is dark basal cell or androgen-insensitive cells. Bar indicates 50 um magnification. (B). A radiation treated biopsy section shows radiation damage as shown by condensation of nuclei, cytoplasm disintegration, and loss of acinar integrity as shown by label e. Some cells have sloughed in the acinar lumen LU. Bar indicates 50 um magnification. (C). Electron micrograph shows nuclear and cytoplasm damage including breakdowns of the cellular integrity. Bar indicates 500 um magnification.
Results
Steroid hormone-dependent prostate and breast have sub-types of cancers [1, 5-6, 33-36].Different strategies have been used in diagnosis and treatments of these steroid-dependent cancers.
The presence of estrogen and estrogen-dependent stem cells in prostate and its cancer requires antiestrogen treatment along with ADT. In contrast, strategies for Breast Cancer (BC) differ.Both diagnosis and treatment utilize receptor-based diagnosis, namely a) estrogen receptor positive, b) estrogen receptor negative, c) double negative and d) triple negative breast cancer. Diagnosis is further modulated by premenopausal and postmenopausal states of the females. Receptor-based di diagnosis of BC has delayed (impeded) steroid hormone-based treatment [34-36].Breast cancer need to be treated using steroid-based therapies. Identification of two sub- types of cancers have led us to postulate that sub-types of cancers can exist in other solid organs (such as, lungs, colon, pancreas, neuroblastoma). Identification of a sub-type of cancers in any solid organ can provide opportunities for new methods of diagnosis and treatments. Initially, a group of dedicated pathologists is needed to identify the cell types in cancers using nuclear and/or cytoplasmic differences, including special staining patterns, localization of markers, etc. The initial funding should be for five to seven years with biannual progress reports. This could be further investigated by cell and molecular biologists, geneticists, and others as necessary. Hopefully, the suggested efforts can make some progress. Many solid organ cancers have increased in the world despite numerous efforts after Precedent Nixon declared War on Cancer in 1971 [36].
Discussion
Brief analysis of steroid hormone pathway indicates that a series of enzymes convert cholesterol to progesterone to testosterone and finally to estrogen [1,7, 19, 28-32]. Specific enzyme aromatase converts testosterone to estrogen before the inhibitor(s) stops the conversion to estrogen gradually, and not like the electric switch. For example, after formation of testosterone aromatase enzymatic actions continue to convert some testosterone to estrogen. This also explains the presence of estrogen in the prostate and PC. The exact amount of estrogen in the prostate is unknown, and it probably varies in males with age (2). Several studies have suggested treatments for CRPC [16-18, 32]. We suggest use of ADT (including inhibition of hypothalamic releasing hormone, LHRH, and/or anti-androgen) and antiestrogen treatments to cure PC or CRPC. The exact sequence of treatment needs to be determined by one or more clinical trials which will also establish the dosages, timing, and duration of treatments. We expect more PC cases can be cured, hopefully just as antibiotics have cured bacterial infections.
Huggins and Associates introduced chemical castration by DES treatment that degenerated only androgen dependent cells, but not estrogen dependent cells [9-12]. Some degeneration of acinar cells occurs in untreated and DES-treated cases [13]. DES-treated studies show condensation of acinar nuclei and cytoplasm.Degeneration of DES-treated and radiation treated cases show the differences. DES-led degeneration of cells is a complex multistep process involving degeneration of cytoplasmic organelles, nucleus, or both. For example, mitochondrial cristae will show signs of degeneration, nuclear membrane loose the shape and chromatin distribution are rearranged in the nucleus. All of these can be assessed by electron microscopy.These criteria were used to identify degenerating androgen-sensitive and androgen-insensitive cells. [13]. Our follow- up of the post DES-treated cases ranging from 37 days to 5.6 years indicated that some androgen-insensitive populations of cells survived.In contrast, radiation treated cases showed nuclear and cytoplasmic radiation damage.
Conclusion
The presence of estrogen-dependent PC has been demonstrated by a) localization of isotopic estrogen, b) localization of antibody IgG against estrogen, c) identification of estrogen-dependent stem cells, and d) estrogen receptors. Androgen-insensitive cells have been identified in DES treated and untreated prostate. We have shown the presence of the sub-type of PC that is CRPC. The possibility of progesterone dependent PC exists. Treatment of castration- resistant prostate cancer is expected to save lives of thousands of patients in the world.
Postscript
The author dedicates his work to the memories of patients who die due to prostate cancer and to their families.
Conflicts of Interest
The author has no conflict of interest. No grant funds were used to complete this manuscript.
Acknowledgement
The Research Service of Minneapolis Veterans Affairs Medical Center provided laboratory and other research facilities.The Author is grateful to Dr. Donald F. Gleason, Nancy A. Staley, and Stephen L. Ewing former staff pathologists at the VAMC for grading the prostate cancer sections. We are also grateful to Dr. Clyde E. Blackard and his associates for biopsy and Drs. Pratap K. Reddy and Eduardo T. Fernandes for prostatectomy specimens.We thank Drs. John Ohlfest and S.K. Swaminathan (University of Minnesota, Minneapolis, MN) for providing the CD133 antibody. We are grateful to Mr. Francis F. Pomroy, Jr., for immunohistochemical and for immunogold localization study. The author is grateful to Mr. Don Frederiksen for the use of Photoshop in making the figure composites and help as a technology consultant.The author thanks Ms. Dorothy Sinha for copy editing.
Disclaimer
The opinions expressed in this article are that of the author and not of the U.S. Government, Department of Veterans Affairs, the Research Service of Minneapolis Veterans Affairs Medical Center, or the University of Minnesota.
References
- Sinha AA. Cell proliferation in the gleason scores, immunogold localization of cd133 in the stem cells of human prostate, and analysis of embryonic stem cells, including organogenesis.Intern J. Beachem. Physiol. 2022;7(2):1-10.
[Crossref], [Google Scholar]
- Vermeulen A, Kaufman JM, Goemaere S, Pottelberg IV. Edtrodial in elderly male. Aging Male. 2002;5:98-102.
[Crossref], [Google Scholar], [Pub Med]
- Boice JD, Curtis RE, Kleinerman RA, Flannery JT, Fraument JF. Multiple primary cancers in Connecticut, 1935-82. Yale J Biol Med. 1986;59(5):533-545.
- Vogt A, Schmid S, Heinimann K, Frick H, Hermann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2(2): e000172.
[Crossref], [Google Scholar], [Pub Med]
- Bashirelahi N, Yamanaka H, Young JD, Yoshikazu I, Shida K, Harada M. Androgen, estrogen, and progesterone receptors in peripheral and central zones of human prostate with adenocarcinoma. Urology. 1983;21:530-535.
[Crossref], [Google Scholar], [Pub Med]
- Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of estrogens and endocrine disrupting chemicals on human prostate and prostate cancer risk. Mol Cell Endocrinol. 2012;354:63.
[Crossref], [Google Scholar], [Pub Med]
- Sinha AA, Pomroy FE, Wilson MJ. Concurrent androgen and estrogen ablation and inhibition steroid biosynthetic enzyme treatment of castration resistant prostate cancer. Anticancer Res. 2016;36:3847-3854.
[Google Scholar], [Pub Med]
- White JW. The results of double castration in hypertrophy of the prostate. Ann Surg. 1895;22:1-80.
[Crossref], [Google Scholar], [Pub Med]
- Huggins C, Hodges CV. Studies on prostatic cancer. 1. The effect of castration of estrogen and of androgen on injection on serum phosphatase in metastatic carcinoma of the prostate 1941. Cancer Res. 2002;1:293-297.
[Crossref], [Google Scholar], [Pub Med]
- Huggins C. The physiology of prostate gland. Physiol Rev. 1945;25:281-285.
[Google Scholar], [Pub Med]
- Huggins C, Scott WW. Bilateral adrenalectomy in prostatic cancer. Ann Surg. 1945;122:1031-1041.
[Crossref], [Google Scholar], [Pub Med]
- Huggins C. Endocrine-induced Regression of cancers. Science. 1972;53-61.
[Crossref], [Google Scholar], [Pub Med]
- Sinha AA, Blackard CE, Seal US. A critical analysis of tumor morphology and hormone treatments in the untreated and estrogen-treated responsive and refractory human prostatic carcinoma. Cancer. 1977;40:2836-2850.
[Crossref], [Google Scholar], [Pub Med]
- Maarten C, Bosland D. The role of estrogens in prostate carcinogenesis: A rationale for chemoprevention. Rev Urol. 2005;7(3):sx4- S10.
[Google Scholar], [Pub Med]
- Hotre SJ, Saad F. Current management of castration-resistant prostate cancer.Current Oncol. 2010;17(2):S72-S79.
[Crossref], [Google Scholar], [Pub Med]
- Saad F, Hotte S, Catton C, Drachenberg D, Finelli A, Fleshner N, et al. CUA-CUOG guidelines for the management of castration-resistant prostate cancer (CRPC): 2013 update. Can Urol Assoc J. 2013;7(7-8):231.
[Crossref], [Google Scholar], [Pub Med]
- Lowrance WT, Roth BJ, Kirkby E, Murad MH, Cookson MS. Castration-resistant prostate cancer: AUA guideline amendment 2015. J Urol. 2016;195(5):1444-1452.
[Crossref], [Google Scholar], [Pub Med]
- Sinha AA, Wilson MJ. Identification of two types of stem cells in methylene blue-stained sections of untreated and diethylstilbestrol-treated human prostate cancer and their characterization by immunogold localization of CD133. Anticancer Res. 2018;38(10):5725-5732.
[Crossref], [Google Scholar], [Pub Med]
- Sinha AA, Blackard CE, Doe RP, Seal US. The in vitro localization of H3 estradiol in human prostatic carcinoma. An electron microscopic autoradiographic study. Cancer. 197;31(3):682-688.
[Crossref], [Google Scholar], [Pub Med]
- Sinha AA, Wilson MJ, Gleason DF. Immunoelectron microscopic localization of prostatic‐specific antigen in human prostate by the protein A‐gold complex. Cancer. 1987: 15;60(6):1288-1293.
[Crossref], [Google Scholar], [Pub Med]
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125-128.
[Google Scholar], [Pub Med]
- Veterans Administration Cooperative Research Group. Histologic grading and clinical staging of prostatic carcinoma. Urologic oncology: the prostate. Philadelphia (PA): Lea and Fibiger. 1977:171-174.
- Ellis WJ, Lange PH. Prostate cancer. Endocrinol Metabolism Clin North Am. 1994:23809-23824.
[Crossref], [Google Scholar], [Pub Med]
- Swaminathan SK, Olin MR, Forster CL, Santa Cruz KS, Panyam J, Ohlfest JR. Identification of a novel monoclonal antibody recognizing CD133. J Immunol Methods. 2010;361(1-2):110-115.
[Crossref], [Google Scholar], [Pub Med]
- Collins AT, Maitland NJ. Prostate cancer stem cells. Eur J Cancer. 2006; 42(9):1213-1218.
[Crossref], [Google Scholar], [Pub Med]
- Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4(3):193-201.
[Crossref], [Google Scholar], [Pub Med]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946-10951.
[Crossref], [Google Scholar], [Pub Med]
- Leake R. Steroid receptors in normal and cancer tissue. In: Hormonal Management of Endocrine-related cancer. Stoll BA, editor, Loyd-Luke (Medical Books) Ltd. London, U.K. 1981:3-12.
[Crossref], [Google Scholar]
- Hilborn E, Stal O, Jansson A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget. 2017; 8(18):30552.
[Crossref], [Google Scholar], [Pub Med]
- De Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011;9(1):1-7.
[Crossref], [Google Scholar], [Pub Med]
- Bosland MC. The role of estrogens in prostate carcinogenesis: a rationale for chemoprevention. Rev Urol. 2005;7(3):S4.
[Crossref], [Google Scholar], [Pub Med]
- Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010;4(6):380.
[Crossref], [Google Scholar], [Pub Med]
- Landry I, Sumbly V, Vest M. Advancements in the treatment of triple-negative breast cancer: A narrative review of the literature. Cureus. 2022;14(2):e21970.
[Crossref], [Google Scholar], [Pub Med]
- Fultang N, Chakraborty M, Peethambaran B. Regulation of cancer stem cells in triple negative breast cancer. Cancer Drug Resist. 2021;4(2):321.
[Crossref], [Google Scholar], [Pub Med]
- El Baba R, Pasquereau S, Haidar Ahmad S, Diab-Assaf M, Herbein G. Oncogenic and stemness signatures of the high-risk hcmv strains in breast cancer progression. Cancers. 2022; 14(17):4271.
[Crossref], [Google Scholar], [Pub Med]
- Penberthy LT, Rivera DR, Lund JL, Bruno MA, Meyer AM. An overview of real‐world data sources for oncology and considerations for research. CA Cancer J Clin. 2022;72(3):287-300.
[Crossref], [Google Scholar], [Pub Med]
Citation: Sinha AA (2023) Antiestrogen Treatment for Androgen-Insensitive or Castration-Resistant Prostate Cancer, Identification of Estrogen Stem Cells, and Estrogen Receptors. J Carcinog Mutagen. 14:409
Copyright: © 2023 Sinha AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


