Indexed In
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2025) Volume 9, Issue 2
Ambulatory Percutaneous Endovascular Abdominal Aortic Aneurysm (EVAR) Repair: experience of a British tertiary vascular centre
Reda El Bayoumy1*, Ankur Thapar2 and Vijay Gadhvi22Department of vascular surgery, Basildon university NHS hospital, Basildon, UK
Received: 26-Jun-2025, Manuscript No. JSA-25-29148; Editor assigned: 30-Jun-2025, Pre QC No. JSA-25-29148 (PQ); Reviewed: 14-Jul-2025, QC No. JSA-25-29148; Revised: 21-Jul-2025, Manuscript No. JSA-25-29148 (R); Published: 28-Jul-2025, DOI: 10.35248/2684-1606.25.9.285
Abstract
Background: Enhanced Recovery after Surgery (ERAS) is a comprehensive, multidisciplinary perioperative care pathway that employs evidence-based practices across specialties to promote early recovery following major surgery. It aims to improve patient outcomes, enhance the patient experience, and reduce length of hospital stay through optimized perioperative care bundles. Over the past two decades, ERAS protocols have been successfully implemented across various surgical specialties including cardiac, colorectal, hepatobiliary, urological, gastric, and gynecological procedures to standardize care and ensure the delivery of evidence-based, coordinated treatment. This multidisciplinary approach has significantly reduced recovery times. While each ERAS pathway is tailored to its specific surgical specialty, several core principles are consistent across them. These include preoperative patient and family education and expectation setting, medical and nutritional optimization, pre-emptive multimodal analgesia, intraoperative goal-directed fluid management, postoperative opioid minimization, antiemetic and bowel regimens, early removal of urinary catheters and lines, early mobilization, and prompt resumption of a regular diet. Although enhanced recovery pathways have proven effective across many surgical disciplines, a coordinated, multidisciplinary care pathway specifically for vascular surgery remains undeveloped. The advanced age and multiple comorbidities typical of vascular surgery patients, combined with risks of blood loss and challenges in preoperative optimization, contribute to high rates of postoperative complications. These complications often lead to prolonged hospital stays, chronic pain, increased rehabilitative needs, and higher readmission rates. This article outlines the comprehensive multidisciplinary perioperative management of patients undergoing endovascular aortic surgery at our institution, focusing on the following key areas: Since its introduction over two decades ago, Percutaneous Endovascular Aneurysm Repair (pEVAR) has undergone substantial refinement and is now more commonly performed than open surgical repair for infrarenal abdominal aortic aneurysms. This shift in practice has led to the development of Same-Day Discharge (SDD) protocols. Eligibility for SDD has typically been based on factors such as low preoperative risk, anatomically straightforward infrarenal aneurysms, availability of a responsible caregiver for the first 24 hours post-discharge, and patient residence within close proximity to the hospital (generally within 40 miles). However, discharge decisions have often relied on early postoperative assessment. It has been demonstrated that early discharge (≤6 hours postoperatively) is achievable in over 40% of patients. To safely expand this model, especially into freestanding Ambulatory Surgery Centers (ASCs), validated preoperative risk stratification tools must be developed. These would mirror the safety protocols in place for other minimally invasive procedures already performed in such settings. Multiple large randomized controlled trials have shown that long-term survival following percutaneous EVAR is superior to that of open repair primarily due to significantly reduced perioperative mortality. Additional perioperative advantages of percutaneous EVAR include shorter operative times, reduced blood loss, lower transfusion rates, fewer ICU admissions, and decreased hospital length of stay. While one randomized clinical trial comparing endovascular and open repair reported an average hospital stay of three days for EVAR patients, a more recent analysis of National Surgical Quality Improvement Program (NSQIP) data indicates that the average length of stay following EVAR remains approximately two days. However, with continued advancements in technique and perioperative care, more recent studies have shown that carefully selected patients with uneventful intraoperative and immediate postoperative courses can be safely discharged on postoperative day one. Early complications, when they occur, typically present in the immediate postoperative period, supporting the feasibility of early discharge. A separate study involving a larger European cohort further suggested that same-day discharge may be appropriate for approximately one-third of patients. In the UK, the National Vascular Registry recorded 2,907 infrarenal EVAR procedures in 2017, with a median hospital stay of three days. Reducing the median stay to 1.5 days-by implementing a national short-stay EVAR protocol that discharges 50% of patients on postoperative day 0 or 1-could result in a potential savings of 4,361 hospital bed-days annually. At an estimated cost of £400 per bed-day, this equates to a potential annual cost saving of approximately £1.8 million, particularly if the need for postoperative level 2 or 3 care is reduced by identifying low-risk patients preoperatively.
Study Aim: We aimed to evaluate the safety and feasibility of outpatient percutaneous EVAR in a selected patient cohort, and to validate a preoperative risk profiling strategy for identifying candidates suitable for same-day discharge (≤6 hours post-procedure) or treatment in freestanding ambulatory surgery centers.
Objectives: To identify patient characteristics predictive of successful early discharge (≤6 hours) following elective endovascular repair of asymptomatic infrarenal Abdominal Aortic Aneurysms (AAA). To assess the safety and clinical feasibility of performing EVAR in ambulatory (outpatient) settings. To validate proposed risk stratification criteria for safe patient selection.
Keywords
Endovascular Aneurysm Repair (EAR); Same-Day Discharge (SDD); Risk Factor Profiling (RFP); Multidisciplinary Team (MT); Clinical Protocols/Critical Pathways (CP); Abdominal Aortic Aneurysm (AAA); Enhanced Recovery After Surgery (ERAS); Vascular Surgery (VS); Peripheral Vascular Disease (PVD); Thoracoabdominal Aortic Disease (TAAD); Operating Room (OR); Postoperative Day (POD); The Post-Anesthesia Care Unit (PACU); Perioperative Management (PM); Intraoperative Complications (IC); Postoperative Complications (PC); Estimated Blood Loss (EBL); Post-Implantation Syndrome (PIS); Total Intravenous Anesthesia (TIVA); External Iliac Artery (EIA); Internal Iliac Artery (IIA); Femoral Endarterectomy (FEA); The British Society for Vascular Surgery (BSVS).
Definitions
Patient selection criteria and definitions
Potential candidates for early discharge or outpatient EVAR in a freestanding ambulatory surgery center were defined as patients who required only routine postoperative monitoring or experienced self-limiting minor Adverse Events (AEs) that were identified, managed, and fully resolved within six hours of surgery [1-4].
Functional capacity was considered poor if the patient’s estimated exercise tolerance was less than 4 Metabolic Equivalents (METs) during preoperative evaluation, as assessed using the Duke Activity Status Index and the American Heart Association (AHA) exercise index [2-4].
Post-Implantation Syndrome (PIS) was defined as any combination of the following: Body temperature >37.8 °C, white blood cell count >10,000/mm³, abdominal and/or back pain, or nonspecific systemic symptoms such as malaise or loss of appetite [3-5].
Renal insufficiency was defined as an Estimated Glomerular Filtration Rate (eGFR) <60 mL/min/1.73 m² [4].
Definitions for end leak, technical success, and other aneurysm-related events followed the reporting standards established by the Committee for Standardized Reporting Practices in Vascular Surgery of the British Society for Vascular Surgery (BSVS) [5].
The Instructions for Use (IFU) for proGlide vascular closure devices, as specified by the manufacturer, require a 2-cm segment of the common femoral artery with the puncture site located 10mm above the femoral bifurcation and 10mm below the inferior epigastric artery [5].
Absence of fluoroscopically visible intraoperative arterial calcification, or only non-circumferential calcification involving less than 50% of the posterior luminal diameter on preoperative CT imaging [5]. A non-aneurysmal femoral artery with a diameter >5 mm. A sheath size <21F. Patients should not be morbidly obese, per standard BSVS exclusion [5, 6].
Methods
Eligibility and patient selection
Patients were screened for eligibility based on technical, medical, and social criteria:
- Technical exclusions included emergency procedures, use of fenestrated EVAR devices, and other anatomically complex repairs.
- Medical exclusions encompassed unstable comorbidities or poor functional status [7].
- Social exclusions included the absence of a caregiver available for the first 24 hours post-discharge.
We developed preoperative criteria to identify patients suitable for same-day discharge (SDD), these included:
- Elective infrarenal AAA with favourable anatomical characteristics (appropriate aneurysm size, neck diameter and length, minimal neck angulation, absence of circumferential calcification or mural thrombus, low iliac tortuosity, and suitable iliac diameter) [8].
- Low perioperative risk based on clinical evaluation.
- Patients who were functionally independent and had a responsible adult available to accompany them for 24 hours post-procedure.
The possibility of SDD was discussed during the preoperative clinic visit. Patients meeting criteria were offered discharge on the evening of surgery following 6 hours of postoperative bed rest, provided the procedure was uneventful [9].
Major adverse operative events were defined as:
- Intraoperative bleeding >500 mL or need for transfusion.
- Arterial injury requiring surgical or endovascular repair.
- Thrombosis or arterial dissection.
- Unresolved type I or III endoleaks.
Postoperative Follow-up Protocol:
- Clinical review at 2 weeks.
- Contrast-enhanced CT at 6 months.
- Annual imaging (CT without contrast or duplex ultrasound) if no endoleak or aneurysm sac expansion.
- Patients with persistent endoleak or sac growth underwent CT imaging every 6 months.
- Femoral access sites were assessed via CT and clinical examination [10].
Data collection and outcomes
Collected variables included:
- Operative details and intraoperative complications.
- Postoperative length of stay, perioperative complications, and readmissions.
- Emergency Department (ED) visits, reinterventions, and mortality.
The primary outcome was the 30-day postoperative complication rate. Secondary outcomes included ED visits, unplanned readmissions, and need for reintervention.
Approach
Development and implementation of same-day discharge protocol: The first patient in this series was discharged on the day of surgery without prior planning or protocol discussion, based on a favorable intraoperative course and excellent postoperative recovery. Following this positive outcome, we began to systematically evaluate patients for planned Same-Day Discharge (SDD) based on clearly defined criteria [11].
Eligibility criteria for SDD included:
- Favourable anatomical characteristics for EVAR.
- Preserved renal function with no planned postoperative intravenous hydration.
- Absence of high-risk medical comorbidities: e.g. severe chronic Obstructive Pulmonary Disease (COPD) requiring home oxygen, congestive heart failure and advanced age (>80 years).
- Adequate functional capacity and ability to live independently.
- Presence of a responsible caregiver available overnight.
- Residence within 40 miles of the hospital and in a non-rural location.
To facilitate same-day discharge, SDD EVAR procedures were preferentially scheduled before 12:00 PM, allowing sufficient postoperative observation time. The option for SDD was revisited in the preoperative holding area, where patients were reassessed, and baseline pedal pulses were documented [12].
Patients were counselled that they could be discharged later the same day if:
- The EVAR procedure was technically successful and uneventful.
- Hemostasis was achieved with no access site complications.
- There were no intraoperative or immediate postoperative concerns.
- Their medical status remained stable.
A Foley catheter was placed intraoperatively in all patients and removed in the Post-Anesthesia Care Unit (PACU). Patients were observed in the PACU for approximately four hours, after which they were transferred to the vascular ward for continued monitoring [13].
Discharge was permitted if the patient:
- Was able to ambulate independently.
- Had voided spontaneously.
In addition to standard discharge instructions, patients were advised to take paracetamol for mild back pain or low-grade fever, maintain adequate hydration and monitor for signs of complications and contact the care team as instructed.
Anesthetic and intraoperative management
All patients underwent Total Intravenous Anesthesia (TIVA) using a combination of remifentanil and 1% propofol, with basic physiological monitoring, Bispectral Index (BIS) for depth of anesthesia, and invasive arterial blood pressure monitoring. Intraoperatively, Mean Arterial Pressure (MAP) was maintained between 80 and 100 mmHg using a phenylephrine infusion, while ensuring a negative fluid balance to minimize hemodilution and tissue edema.
Operative technique
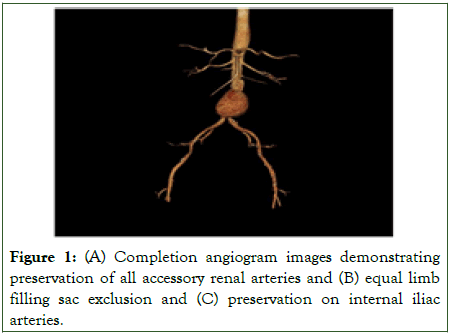
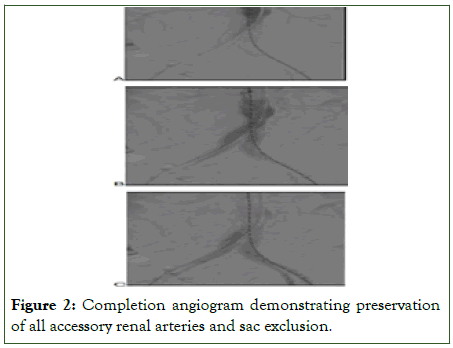
Percutaneous femoral access was utilized whenever anatomically feasible and was more commonly employed in patients selected for Same-Day Discharge (SDD), due to its association with shorter recovery times and lower complication rates compared to open surgical access (Figures 1, 2).
Figure 1:(A) Completion angiogram images demonstrating preservation of all accessory renal arteries and (B) equal limb filling sac exclusion and (C) preservation on internal iliac arteries.
Figure 2: Completion angiogram demonstrating preservation of all accessory renal arteries and sac exclusion.
Complications and discharge
Patients who successfully underwent early discharge exhibited a readmission rate between 0% and 5%. Common reasons for readmission included severe post-implantation syndrome, access vessel complications, acute kidney injury, ischaemic cardiomyopathy, Chronic Obstructive Pulmonary Disease (COPD) exacerbations, and diverticulitis. Moscato et al. reported no significant difference in 30-day readmission rates when comparing early discharge with longer hospital stays [6]. Notably, patients discharged early tended to have smaller aneurysms, which likely reflects the overall presence of favourable anatomical features and better physiological fitness, rather than aneurysm size alone influencing discharge timing. This interpretation is further supported by the observation that operative time and intraoperative blood loss were greater among patients with prolonged hospital stays, although it remains unclear whether these factors are causes or consequences of delayed discharge.
Cost analysis
Moscato et al. reported that operative costs accounted for 80.3% of the total expenses associated with EVAR, with 58% of these costs attributable to the endograft device [6-11].
Al-Zuhir et al. demonstrated a substantial cost benefit associated with early discharge, showing a statistically significant reduction in hospital costs from £13,360 for a Length of Stay (LOS) of four days to £9,844 for an LOS of one day [14]. This finding was corroborated by Lachat et al., who also observed significant savings with early discharge protocols [5].
Furthermore, Al-Zuhir et al. found that increasing the proportion of patients managed under a Same-Day Discharge (SDD) protocol from 30% to 45% resulted in a highly significant (p<0.001) reduction in average EVAR costs, decreasing from £12,102 to £10,330 [14].
Table 1: These results underscore the potential for considerable cost savings with broader adoption of early discharge pathways.
Discussion
Minimally invasive techniques have profoundly transformed various surgical fields, with Endovascular Aneurysm Repair (EVAR) revolutionizing the management of aortic aneurysms. Enhanced Recovery after Surgery (ERAS) pathways have evolved alongside these innovations, aiming to reduce hospital Length of Stay (LOS) while maintaining low complication and readmission rates [1-4]. However, adoption of ERAS protocols in vascular surgery has been slow, as evidenced by only a modest reduction in LOS following EVAR-approximately one day over the past decade [5,6].
Several cohort studies suggest that outpatient EVAR is feasible in carefully selected patients, yet robust safety data remain scarce, and criteria for candidate selection lack precision [7-9]. Our study demonstrates that outpatient EVAR can be safely performed in the majority of appropriately selected patients without increasing perioperative complications, readmission, reintervention, or mortality rates. While Emergency Department (ED) visits were more frequent in the outpatient EVAR group, this did not translate into higher complication or readmission rates. Early postoperative follow-up via telephone could help reduce unnecessary ED visits, especially considering that most visits occurred around postoperative day 10.
It is important to clarify the definition of outpatient or day surgery, as this term can sometimes include 23-hour observation stays. At our institutions, overnight admissions are classified separately, and outpatient procedures require discharge directly from the Post-Anesthesia Care Unit (PACU). Our pathway includes approximately a 4-hour PACU stay followed by either discharge or admission, with all same-day discharges occurring on the day of the procedure.
When surgeons excluded patients from outpatient EVAR, their reasons fell into medical (44%), technical (52%), and social (4%) categories [7,8].
Our findings indicate that about two-thirds of patients undergoing EVAR can be safely discharged home after a 6-hour observation period-even with general anesthesia. Despite the inherent frailty of the EVAR population, severe Congestive Heart Failure (CHF) was the only comorbidity independently associated with prolonged LOS [8,9]. These patients exhibited severe cardiac symptoms at rest and poor exercise tolerance. The association between preoperative use of ACE inhibitors (ACE-I) and prolonged LOS likely reflects the prevalence of ACE-I use in CHF management [9]. While consideration should be given to temporarily withholding ACE-I preoperatively, further research is needed before formal recommendations can be made [9]. Similarly, low hemoglobin levels-likely a marker of chronic illness-were associated with longer hospitalization [8,9].
Our results reinforce the importance of optimizing medical comorbidities before surgery and resuming essential medications promptly postoperatively. Notably, patients discharged on ACE-I or new anticoagulation therapy were more likely to have prolonged LOS, the latter likely due to in-hospital complications such as deep vein thrombosis, pulmonary embolism, atrial fibrillation, or thromboembolism.
Acute Kidney Injury (AKI) following contrast exposure remains a significant modifiable risk factor linked to increased morbidity, mortality, and extended LOS [15]. Identifying and optimizing patients with chronic kidney disease preoperatively including the use of alternative contrast agents like CO2 and minimizing iodinated contrast volume are critical strategies to mitigate AKI risk [15].
Procedure-related factors also influence LOS. Prolonged operative times, increased blood loss, larger volumes of iodinated contrast, and crystalloid administration correlated with extended hospital stays, likely reflecting case complexity but also representing potential targets for improvement [12,13]. Since its introduction in the late 1990s, percutaneous EVAR has grown in popularity [12]. The Preclose/double Proglide technique, first described in 2007, offers advantages including lower cost and use of a nonbraided suture [13]. However, there is a steep learning curve with EVAR, and ambulatory EVAR should be reserved for surgeons with adequate experience to ensure patient safety [11-13].
Percutaneous EVAR may also reduce readmission rates [6,7]. Like open AAA repair, the most common reason for readmission after EVAR is wound complications, observed in about 5% of a random sample [7,8].
We found that the most effective way to avoid unnecessary discharge delays is to send patients home on the day of surgery with instructions for paracetamol or ibuprofen and oral hydration. Few patients require readmission for severe Post- Implantation Syndrome (PIS).
Anesthesia and impact on outcomes
Anesthetic technique influences postoperative morbidity and LOS [5,6]. Analysis of 6009 EVAR patients between 2015 and 2018 found that general anesthesia was associated with increased pulmonary complications and longer LOS compared to spinal or local anesthesia [9-11]. Notably, percutaneous procedures were more frequently performed under general anesthesia (46%) and local anesthesia (40%), compared to spinal (38%) and epidural anesthesia (28%). A review of 10 studies involving 13,459 patients also found that local anesthesia was associated with shorter operative times, reduced LOS, and fewer complications, despite percutaneous procedures being more common under general anesthesia [9-11].
In our series, Total Intravenous Anesthesia (TIVA) with general anesthesia was preferred for high-risk patients. The majority (93%) of Same-Day Discharge (SDD) patients received general anesthesia. While local anesthesia with sedation is feasible, challenges such as inability to suspend breathing reliably during graft deployment and patient discomfort during sheath manipulation or limb ischemia make general anesthesia preferable. This preference did not adversely affect LOS. It appears that the invasiveness of the procedure rather than the anesthesia type determines SDD suitability.
Safety and complications of early discharge
A key concern with SDD is the risk of life-threatening bleeding or limb ischemia after discharge [7,8]. In our experience, all percutaneous failures occurred intraoperatively, with no patients requiring reoperation in the early or late postoperative periods, consistent with prior reports. For example, Lee et al. reported that 15 of 16 early failures happened during the procedure, with only one late complication (POD 27) due to a necrotizing groin infection [17]. Similarly, Borner et al. found that 15 of 17 failures were addressed intraoperatively; two patients returned within 24 hours for bleeding, which were attributed to inadequate hemostasis in early cases [17]. This underscores the critical importance of confirming complete hemostasis and pedal perfusion before considering SDD. Performing EVAR in outpatient ambulatory centers may be challenging because hemostasis and distal perfusion cannot be reliably confirmed before procedure completion, even by experienced surgeons [16,17].
Cost considerations
Device cost remains the largest contributor to EVAR expenses [11-13]. Early studies suggested that reduced operative time, intensive care use, LOS, blood product consumption, and lab tests do not fully offset these costs [11-13]. Since device cost is beyond surgeons' control, reducing LOS is the main avenue for cost savings. Al-Zuhir et al. reported that increasing short-stay EVAR cases from 30% to 45% decreased overall costs by approximately £2,000 [14]. The OVER trial showed hospital costs were $5,900 less for EVAR than open repair despite a mean LOS of 5 days (vs. 10.5 days for open repair; p<0.001) [15]. Even accounting for surveillance and reinterventions, EVAR remained more cost-effective during follow-up [11,12]. The adoption of less frequent surveillance and use of ultrasound-based monitoring may further improve EVAR’s cost-efficiency [11-13].
Feasibility and limitations of SDD
We estimate that approximately 60% of EVAR patients could be candidates for SDD if preoperative transportation and home care issues are addressed. The remaining 40% are unlikely to qualify due to unmodifiable or unpredictable factors, such as inability to void post-procedure. While identifying patients at risk for failure to void might aid discharge planning, it would not substantially impact overall LOS.
Limitations of our study include the lack of a strict protocol for patient selection and the relatively small cohort size. Nevertheless, patients were managed uniformly, and our results should be reproducible in centers experienced with percutaneous EVAR. Although we did not perform a formal cost analysis, the potential savings from decreased LOS and reduced laboratory testing are evident. Our approach helped alleviate hospital bed shortages, reducing patient wait times for admission or diversion from emergency rooms-a critical factor in resource-limited settings (Figure 3).
In an era of value-based healthcare reimbursement, metrics like LOS have become increasingly important [11-13]. For EVAR specifically, a hospital stay longer than 3 days is associated with increased morbidity and mortality, and each additional day can increase costs by approximately 8% [11-13].
Figure 3: Our first day-case EVAR featured in the local press.
Conclusion
As endovascular techniques advance, ambulatory percutaneous EVAR has proven feasible and safe in about two-thirds of elective EVAR patients without excessive medical risk, good functional capacity, and uneventful procedures [11-13]. While the impact of SDD on cost-effectiveness requires further study, it may not be feasible in hospitals reimbursed based on admission status [14].
Although the overall cost of EVAR is higher than open repair—mainly due to device costs, surveillance imaging, and reinterventions—the OVER trial demonstrated lower in-hospital costs for EVAR persisting over 2 years [11-13]. Reducing ICU and hospital LOS and minimizing wound complications offer further cost-saving potential. Al-Zuhir et al. demonstrated that implementing short-stay EVAR protocols enabling discharge on POD 1 in about 27% of patients resulted in significant cost reductions [14].
We also observed that moderate to severe Post-Implantation Syndrome (PIS) prolongs LOS despite requiring only supportive care. Consequently, starting in March 2025, we have been offering same-day discharge after percutaneous EVAR, expanding the options for patient-centered, cost-effective care.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Funding
No funding was utilized in the production of this article.
Data was collected retrospectively from our first 4 cases from the hospital electronic record system. The study was approved as a service evaluation by the institutional research board.
Acknowledgment
We acknowledge the outstanding work by the Frederick Banting Ward staff at Basildon University NHS Hospital.
Special acknowledgment to Mrs. Lindsey Harris and Mrs. Ann Emmanuel for their exceptional dedication and compassionate care.
References
- Montross BC, O'Brien-Irr MS, Koudoumas D, Khan SZ, Rivero M, Harris LM, et al. The selection of patients for ambulatory endovascular aneurysm repair of elective asymptomatic abdominal aortic aneurysm. J Vasc Surg. 2020;72(4):1347-1353.
[Crossref] [Google Scholar] [PubMed]
- Dosluoglu HH, Lall P, Blochle R, Harris LM, Dryjski ML. Ambulatory percutaneous endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2014;59(1):58-64.
[Crossref] [Google Scholar] [PubMed]
- El Bakbachi O, Antoniou GA, Breakton C, Torella F, Neequaye S. Can standard endovascular abdominal aortic aneurysm repair be performed as a day case in selected patients? Int Angiol. 2016;35(1):108-10.
[Google Scholar] [PubMed]
- Hanley SC, Steinmetz O, Mathieu ES. Safety and feasibility of endovascular aortic aneurysm repair as day surgery. J Vasc Surg. 2018;67:1709-1715.
[Crossref] [Google Scholar] [PubMed]
- Lachat ML, Pecoraro F, Mayer D. Outpatient endovascular aortic aneurysm repair: experience in 100 consecutive patients. Ann Surg. 2013;258:754-758.
[Crossref] [Google Scholar] [PubMed]
- Moscato VP, O'Brien-Irr MS, Dryjski ML. Potential clinical feasibility and financial impact of same-day discharge in patients undergoing endovascular aortic repair for elective infrarenal aortic aneurysm. J Vasc Surg. 2015;62:855-861.
[Crossref] [Google Scholar] [PubMed]
- Mehaffey JH, LaPar DJ, Tracci MC. Targets to prevent prolonged length of stay after endovascular aortic repair. J Vasc Surg. 2015;62:1413-1420.
[Crossref] [Google Scholar] [PubMed]
- Shaw Sarah E. Short stay EVAR is safe and cost effective. Eur J Vasc Endovasc Surg. 57;3:368-373.
[Crossref] [Google Scholar] [PubMed]
- Gotlib Conn L, Rotstein OD, Greco E. Enhanced recovery after vascular surgery: protocol for a systematic review. Syst Rev. 2012;1;52
[Crossref] [Google Scholar] [PubMed]
- McGinigle KL, Eldrup-Jorgensen J, McCall R, Freeman NL, Pascarella L, Farber MA, et al. A systematic review of enhanced recovery after surgery for vascular operations. J Vasc Surg. 2019;70(2):629-640.e1.
[Crossref] [Google Scholar] [PubMed]
- Brustia P, Renghi A, Aronici M, Gramaglia L, Porta C, Musiani A, et al. Fast-track in abdominal aortic surgery: experience in over 1,000 patients. Ann Vasc Surg. 2015;29(6):1151-9.
[Crossref] [Google Scholar] [PubMed]
- Giacomelli, Elena. A pilot study of the enhanced recovery after surgery protocol in aortic surgery. J Vas Sur. 2021;74:90-96.e2
[Crossref] [Google Scholar] [PubMed]
- Pasin L, Nardelli P, Landoni G. Enhanced recovery after surgery program in elective infrarenal abdominal aortic aneurysm repair. J Cardiovasc Surg. 2019;60:369-374.
[Crossref] [Google Scholar] [PubMed]
- Al-Zuhir N, Wong J, Nammuni I, Curran G, Tang T, Varty K. Selection thirty day outcome and costs for short stay endovascular aortic aneurysm repair (SEVAR). Eur J Vasc Endovasc Surg. 2012;43:662-665.
[Crossref] [Google Scholar] [PubMed]
- Kim M, Brady JE, Li G. Anesthetic technique and acute kidney injury in endovascular abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth. 2014;28:572-578.
[Crossref] [Google Scholar] [PubMed]
- Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008; 47(5):919-923.
[Crossref] [Google Scholar] [PubMed]
- Borner G, Ivancev K, Sonesson B, Lindblad B, Griffin D, Malina M. Percutaneous AAA repair: is it safe? J Endovasc Ther. 2004;11(6):621-626.
[Crossref] [Google Scholar] [PubMed]
Citation: Bayoumy RE (2025). Ambulatory Percutaneous Endovascular Abdominal Aortic Aneurysm (Evar) Repair: Experience of a British Tertiary Vascular Centre. J Surg Anesth. 9:285.
Copyright: © 2025 Bayoumy RE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.