Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2024) Volume 15, Issue 2
Advancements in Nanoparticles for Drug Delivery, Therapeutic Applications and Computational Modeling: A Comprehensive Review
Souhrid Sarkar* and Akshita DebnathReceived: 01-Mar-2024, Manuscript No. jnmnt-24-24949; Editor assigned: 04-Mar-2024, Pre QC No. jnmnt-24-24949(PQ); Reviewed: 18-Mar-2024, QC No. jnmnt-24-24949(QC); Revised: 25-Mar-2024, Manuscript No. jnmnt-24-24949(R); Published: 31-Mar-2024, DOI: 10.35248/2157- 7439.24.15.715
Abstract
Nanoparticles have shown promise as both drug delivery vehicles and direct antitumor systems, but they must be properly designed to maximise efficacy. Addressing the recent developments in nanomedicines and nano-based drug delivery systems, this review opens up a world of possibilities for treating a variety of illnesses, including cancer. With its ability to operate at the minuscule scale of nanoparticles, nanotechnology offers the prospect of precise drug delivery. We can optimise therapeutic outcomes while avoiding negative effects by customising these materials to target specific body regions. This research explores the adaptability of nanotechnology and demonstrates how it might improve the efficacy of conventional and new medications, including natural products. Chemotherapeutic, biological, and immunotherapeutic agents are only a few of the many uses. A noteworthy feature is how nanomaterials are enhancing medicine delivery, a development that has the potential to completely change the way we use disease marker molecules for targeted diagnosis. In examining the field of nanomedicine, this paper tackles the difficulties in transferring these advancements from laboratory to clinical settings in addition to highlighting areas of potential interest. The journey of nanoparticles are explored, providing a window into their possible influence on the treatment of cancer. This work highlights the revolutionary potential of precision drug delivery by encapsulating the current trends and viewpoints in nanomedicine, signalling the start of a new chapter in the search for more potent and focused cancer treatments.
Keywords
Nanomedicine; Nanoparticles; Drug delivery systems; Precision medicine; Cancer treatment; EPR effect; Modelling
INTRODUCTION
Cancer, a persistent global health challenge, claimed 10 million lives in 2020 as reported by the World Health Organization (WHO). The most prevalent malignancies are those of the breast, lungs, colon, rectum, and prostate. Approximately one-third of cancerrelated deaths are associated to tobacco use, a high body mass index, alcohol usage, a low intake of fruits and vegetables, and lack of exercise. This disease, which is distinguished by its heterogeneity and rapid progression, frequently becomes uncontrollable after onset. While alternative treatments such as immunotherapy, photothermal therapy, photodynamic therapy, and gene and hormone therapy show promise in preclinical studies, conventional approaches such as surgery, radiation, and chemotherapy remain the primary treatment options for the majority of cancer types. However, in the case of metastatic tumours that have spread to distant organs, these methods frequently fall short.
The non-specific nature of conventional chemotherapy, a cornerstone of cancer treatment, is a hindrance. It frequently fails to target cancer cells efficiently, resulting in undesirable side effects in healthy tissues [1]. Despite their widespread use in the treatment of recurrent cancers, cytotoxic agents face a number of obstacles, including poor aqueous solubility, non-specific distribution throughout the body, severe toxicity to normal cells, insufficient drug concentrations at tumour sites, and the development of multiple drug resistance [2]. As a result, the search for alternative therapeutic approaches has become an urgent necessity.
Nanotechnology has emerged as a promising avenue for a variety of biomedical applications, most notably drug delivery. Nanomedicines present an appealing solution due to their ability to improve drug solubility, reduce drug metabolism, extend plasma half-life, and provide a distinct biodistribution profile when compared to conventional chemotherapy. Most importantly, these nanocarriers can specifically target tumour tissues. Nanomedicines effectively reach tumour sites by navigating through the irregular openings in the vascular endothelial layer of tumours, which can range in size from 300 to 4700 nanometers.
Furthermore, tumours frequently have poor lymphatic drainage due to dysfunctional lymph angiogenesis and lymphatic vessel compression by rapidly proliferating cancer cells. These factors allow nanomedicines to accumulate preferentially in tumour interstitial fluid, minimising toxicity to nearby healthy tissues. This is known as the enhanced permeability and retention (EPR) effect.
While passive targeting via the EPR effect has had some success, there is still significant room for improving nanocarrier precision in reaching tumours. To improve this precision, various active targeting strategies have emerged. This review will look at the challenges posed by the EPR effect as well as active targeting strategies that have the potential to improve the selectivity of nanocarriers in cancer therapy. Furthermore, nanocarriers loaded with small molecule chemotherapeutics will be studied, as they have shown therapeutic efficacy in both preclinical and clinical settings. A special emphasis will be placed on the strategies used for active tumour targeting.
Significant progress has been made in this field in recent years, with exciting new developments and innovations holding enormous promise for improving cancer treatment. These advancements will be discussed further in the following sections, emphasising the transformative potential of nanobiotechnology in the fight against cancer.
Types of carriers
Liposomes are spherical vesicles made up of one or more lipid bilayers. These lipid-based structures, which encapsulate a wide range of therapeutic substances, provide an efficient means of drug delivery [3]. Liposomes can merge with cell membranes due to their amphipathic nature, facilitating the transport of their payload into cells. Liposomes are mostly made of phospholipids and cholesterol, but other lipids can be added to improve endocytosis and tissue compatibility. Liposomes of varying composition have been formed using a range of methodologies. The thin-layer hydration method, also known as the Bangham method, is one of the most widely used. This method entails dissolving lipids in an organic phase, evaporating the solvent to form a lipid film, and dispersing this film in an aqueous medium containing the drug [Table-1].
| Types of Nanocarrier | Size (nm) | Function | Reference |
|---|---|---|---|
| Liposomes | 50-500 | Efficient drug delivery, amphipathic, merge with membranes | -3 |
| Micelles | <100 | Transport hydrophobic drugs, various routes of administration | -4 |
| Solid-Lipid Nanoparticles | <200 | Increased stability, controlled release, versatile routes | -5 |
| Gold Nanoparticles (AuNPs) | Up to 100 | Accumulate in tumors, antiangiogenic, direct drug conjugation | -6 |
| Magnetic Nanoparticles (MNPs) | 10-100 | Targeted drug delivery, non-invasive monitoring via MRI | -7 |
| Dendrimers | <10-100 | Encapsulate hydrophobic drugs, targeted cancer therapy | -8 |
| Albumin-Based Nanoparticles | <100 | Utilizes albumin's biocompatibility, NAB technology | -9 |
| Porous Materials | <100 | Cavities for drug carriers, controlled release | -10 |
| Carbon Nanoparticles | <100 | High specific surface area, hydrophobic, various forms | (11,12) |
| Quantum Dots | <10 | Optical and electronic properties, visualization in cancer cells | -13 |
| Calcium Phosphate (CaP) Nanoparticles | <200 | Crystalline structures, pH-sensitive solubility | (14,15) |
| Oligo- and Polysaccharide-Based Drug-Delivery Systems | Variable | Biodegradable, various forms (e.g., chitosan, cyclodextrins, pectins) | (14,16,18) |
| Liposomes | 50-500 | Efficient drug delivery, amphipathic, merge with membranes | -3 |
| Micelles | <100 | Transport hydrophobic drugs, various routes of administration | -4 |
| Solid-Lipid Nanoparticles | <200 | Increased stability, controlled release, versatile routes | -5 |
| Gold Nanoparticles (AuNPs) | Up to 100 | Accumulate in tumors, antiangiogenic, direct drug conjugation | -6 |
| Magnetic Nanoparticles (MNPs) | 10-100 | Targeted drug delivery, non-invasive monitoring via MRI | -7 |
Table 1:Types of nanocarriers in drug delivery-size and function.
Liposomes are formed by vigorous stirring of sealed spherical structures. Liposomes, on the other hand, have a short elimination half-life due to opsonization, which occurs primarily in the liver and spleen. To address this limitation, liposome surfaces have been modified with functional ligands such as polyethylene glycol (PEG). PEG coating reduces interactions with blood components, allowing liposomes to stay in the bloodstream for longer. Physical adsorption, covalent attachment, or inclusion of a PEG-lipid conjugate in liposome preparations can all be used to attach PEG to liposome surfaces. PEG is commonly anchored to the liposome surface by a cross-linked lipid. Aggregation is reduced, and targeted drug delivery is improved. For decades, liposomal delivery of anticancer drugs has been used successfully.
Micelles are nanoparticles with a hydrophobic core and a hydrophilic surface. They are primarily used to transport hydrophobic drugs and can be administered through a variety of routes, including the respiratory tract or the skin. Micellar structures spontaneously form in amphiphilic block copolymers, which frequently contain PEG as the hydrophilic component [4]. These copolymers are made up of two or three distinct blocks. Other hydrophilic block-forming polymers that can be used include chitosan, polyvinylpyrrolidone, and poly (N-isopropyl acrylamide). Micellar cores with functional groups such as -NH2 and -COOH enable chemical drug modification as well as physical encapsulation. In both in vitro and in vivo studies, various cytostatic drug-loaded micelles demonstrated significant results. Paclitaxel encapsulated in micelles, for example, has shown promising results in clinical trials, with lower toxicity and sustained antitumor activity when compared to free paclitaxel.
Solid-Lipid Nanoparticles are colloidal nanoparticles stabilised by surfactants and composed of mono-, di-, and triglycerides, solid fats, and waxes. They are a viable alternative to liposomes,with benefits such as increased stability, controlled drug release, cost effectiveness, and simplified manufacturing. In contrast to liposomes, which are typically administered intravenously, intraperitoneally, subcutaneously, or orally, solidlipid nanoparticles can be administered via a variety of routes, including inhalation, intranasally, and intra-vesically, allowing for localised drug targeting. Recent experiments have shown that solid-lipid nanoparticles loaded with cytostatic drugs outperform conventional drug solutions in terms of efficacy, pharmacokinetics, and bioavailability. Although clinical studies in this area are limited, the future holds promise for more research [5].
Gold Nanoparticles (AuNPs) boast different physical and chemical properties compared to other biomedical nanotechnologies. Because of their ease of production, low toxicity, and antiangiogenic properties, they are appealing candidates for cancer treatment [2, 6]. AuNPs have a size of up to 100 nm and a significant enhanced permeability and retention (EPR) effect, which causes them to accumulate preferentially in tumours. Typically, these AuNPs are synthesised using colloidal methods, which involve the reduction of gold salts in the presence of surface stabilisers. Citric acid is the most commonly used stabilising agent, and it determines the overall charge of the AuNP surface. The choice of a stabilising agent allows for the electrostatic or covalent attachment of various biomolecules to the AuNP surface, such as DNA, antibodies, and polypeptides. Unlike liposomes and micelles, drug molecules are directly conjugated to the surface of AuNP. In in-vitro and invivo studies, this conjugation demonstrated increased antitumor potential, making AuNPs promising for targeted drug delivery.
Magnetic Nanoparticles (MNPs) have ignited significant interest in the treatment of malignant tumours due to their ability to deliver targeted drugs and monitor drug accumulation non-invasively using magnetic resonance imaging (MRI). Magnetic nanoparticles (MNPs) are magnetic materials with small particle sizes (10 to 100 nm), large specific surface areas, magnetic responsiveness, and super paramagnetism. Because of their superparamagnetic behaviour, MNPs exist in a single-domain state and exhibit temperature dependent changes in magnetic moment. Iron oxides such as magnetite and maghemite are common materials used in MNP production [7].Typically, these materials are created by precipitating iron salts from an aqueous solution. Surface modifications are required to reduce nanoparticle agglomeration and make drug conjugation easier. PEG, dextran, polyvinylpyrrolidone, polyaniline, alginate, fatty acids, and chitosan are among the polymers used. Because of the EPR effect, MNPs accumulate in tumours and have demonstrated the ability to release drugs in a controlled manner. MNPs have the added benefit of lowering overall drug toxicity, as well as lowering the required drug concentration for therapeutic effects.
Dendrimers are highly branched monomolecular macromolecules. These dendrimers are typically rotationally symmetric and spherical in shape. Dendrimers have a hydrophobic core that branches into terminal functional groups that allow for water solubility. They are appealing for drug delivery due to their ability to encapsulate hydrophobic drugs and increase their concentration in water. Dendrimers have a number of advantages, including biocompatibility, ease of excretion from the body, and enhanced EPR. Dendrimers, on the other hand, have a disadvantage in that their cationic surface can be cytotoxic to normal cells due to the stability of electrostatic interactions with cell membranes [8]. Surface modification with biocompatible polymers such as PEG aids in overcoming this problem by ensuring dendrimer functionality while causing minimal harm to healthy cells. Dendrimers have demonstrated potential in the field of targeted cancer therapy. Dendrimers have the potential to deliver a wide range of drug molecules, including chemotherapy agents and nucleic acid-based therapeutics. Clinical trials have begun for a number of dendrimer based drug delivery systems.
Albumin-Based Nanoparticles takes advantage of albumin's biocompatibility as the most abundant plasma protein in the human body. Albumin is easily modified to form nanoparticles. Abraxane® is a well-known example of nanoparticle albuminbound (NAB) technology, which uses albumin nanoparticles for drug delivery [9]. NAB technology is based on albumin's natural ability to accumulate in tumours due to increased vascular permeability and retention. Nab-paclitaxel is an FDA-approved formulation of paclitaxel bound to albumin.
Porous Materials are nanoscale structures with cavities or pores that can be used as anticancer drug carriers. The most notable examples are zeolites and mesoporous silica particles. Zeolites are microporous crystalline materials with negatively charged open frame cavities. Their distinct structure enables them to absorb a wide range of substances, including drug molecules. Zeolites are suitable for controlled drug delivery due to their high thermal stability and selectivity. The encapsulated drugs can be released in response to changes in pH, temperature, or other external stimuli. Mesoporous silica particles, on the other hand, have adjustable pore sizes that are especially useful for accommodating different drug molecules. These particles also provide a stable matrix for controlled drug release. The porous structure of these materials helps in maintaining a high specific surface area for drug loading, enabling optimal drug delivery [10].
Carbon nanoparticles are available in a variety of allotropic forms, including carbon nanotubes, fullerenes, and nanodiamonds, which have found applications as drug delivery carriers. Notably, they have a high specific surface area and are hydrophobic. Carbon nanotubes and fullerenes have distinct structures with cavities that can encapsulate therapeutic agents. Nanodiamonds, on the other hand, have a rougher surface, which promotes strong interactions with drug molecules. Furthermore, surface modifications, such as carboxyl group formation via acidic oxidation, increase their versatility for drug conjugation [11]. Carbon nanoparticles with specific properties can be produced by tailoring the synthesis method. While carbon nanoparticles are useful in drug delivery, their toxic potential varies with concentration. As a result, when considering their use as drug carriers, extreme caution is advised. For example, studies have shown that fullerene absorption through the respiratory and digestive tracts is relatively low, resulting in low systemic exposure. It is critical to note that inhaled carbon nanotubes can have similar effects to asbestos fibres, necessitating careful consideration of their use [12].
Quantum dots, inorganic semiconductor nanocrystals with sizes typically less than 10 nm, have exceptional optical and electronic properties. This domain is dominated by cadmium based and carbon quantum dots [11, 13]. Beyond drug delivery, quantum dots are useful in cancer cell visualisation due to their unique optical properties derived from quantum phenomena and related effects. Cadmium compounds such as cadmium selenide (CdSe), cadmium sulphide (CdS), and cadmium telluride (CdTe) show promise as drug carriers in core-shell structures. However, it is critical to recognise their non-biodegradability and toxicity due to cadmium compounds. Carbon-based quantum dots, including carbon and graphene quantum dots, on the other hand, have emerged as biocompatible, easily modifiable, and low-toxicity alternatives in a variety of biomedical applications. They are ideal candidates for anticancer drug delivery due to their biocompatibility, large specific surface area, and high photostability.
Calcium Phosphate (CaP) nanoparticles are crystalline structures made primarily of carbonate apatite that can transport drugs both on their surface and within their structure. CaPs, which are biodegradable and capable of releasing non-toxic calcium and phosphate ions upon degradation, are an important component of vertebrate and human bones and teeth [14]. Because of their pH-sensitive solubility, insolubility at physiological pH, and rapid dissolution in acidic biological environments, they are well suited for drug delivery, particularly in endosomes and lysosomes, where they aid in the efficient release of encapsulated agents [15]. The synthesis of CaP-based nanoparticles is a versatile process that allows for particle size and morphology control based on synthesis conditions. While various morphologies for drug delivery have been investigated, spherical nanoparticles are the most commonly used due to their thermodynamic stability.
Oligo- and polysaccharide-based drug-delivery systems
Chitosan, is an amino polysaccharide polymer derived from chitin via deacetylation that is known for its biodegradability, biocompatibility, and low immunogenicity [16]. Some chitosan derivatives, such as carboxymethyl chitosan, sulfated chitosan, and polypyrrole-chitosan, exhibit intrinsic anticancer properties, which may be linked to their antioxidant properties. Because the chitosan backbone contains free amino groups, it is an excellent polycationic material for encapsulating negatively charged drugs such as doxorubicin. To enable versatile drug delivery, various chitosan-based hydrogels and delivery systems, such as microspheres and film capsules, have been developed [17]. The properties of chitosan-based delivery systems are easily adjustable, allowing for control over particle size, toxicity, stability, and release kinetics depending on the preparation method used.
Cyclodextrins, cyclic oligosaccharides derived from enzymatic processing of starch, come in three main types: α-, β-, and γ-CDs [18]. CDs can form complexes with hydrophilic, lipophilic, and amphiphilic substances due to their hydrophilic outer surface and less hydrophilic cavity, increasing the solubility and bioavailability of numerous anticancer drugs [19]. To improve drug stability and reduce toxicity, various cyclodextrin derivatives are used to create drug delivery systems, often in conjunction with other nanoparticles [20]. CD cross-linking produces unique particles such as CD polymers and nano sponges, which can encapsulate a variety of substances due to their porous structure. Natural CDbased drug delivery systems are notable for the efficacy of their oral, mucosal, and transdermal drug formulations [21]. Although more research is needed to validate these associations, cyclodextrin-based macromolecules have the potential to transport oligonucleotides and siRNAs into cells.
Pectins, derived from various plant sources, primarily fruit, have a variety of structures that can be classified as homogalacturonan, rhamnogalacturonan-I, and substituted galacturonans [22]. Because of their unique behaviour in the gastrointestinal tract, pectins and their derivatives have shown promise as anticancer agents, particularly in colon cancer research. Pectin remains undigested until it reaches the colon, where it ferments and releases therapeutic agents that have been encapsulated. Pectin-based microgranules, microspheres, and hydrogels have been developed by researchers for effective drug encapsulation and targeted release, making them a valuable tool in cancer therapy. The carboxyl groups in pectin structures allow for the formation of negatively charged particles, which aid in drug retention via electrostatic interactions [23]. Selforganizing polymer nanoparticles based on pectin have also been used to deliver therapeutic agents.
Targeting to cancer cells
One kind of serum glycoprotein that helps move iron into cells is called transferrin. The majority of solid tumour cells overexpress transferrin receptors, whereas normal cells only express them at low levels. Thus, medications for the treatment of cancer are delivered using transferrin-conjugated NPs as an active targeting technique. Transferrin-modified NPs have been demonstrated to have improved intracellular drug delivery and greater cellular uptake efficiency when compared to unmodified NPs [24]. Furthermore, research suggests that transferrin-conjugated polymeric nanoparticles (NPs) are crucial in defeating chemotherapy that is resistant to drugs.
One kind of vitamin that is necessary for nucleotide synthesis is folic acid. A folate receptor, which is expressed on a small number of normal cell types, internalises it. On the other hand, haematological malignancies express FR-β on their surface, but the alpha isoform of folate receptor (FR-α) is overexpressed in about 40% of human tumours. Consequently, the use of folateconjugated nanomaterials in the folate receptor-targeting approach has been extensively employed in cancer treatments [3, 25].Furthermore, different forms of glycoproteins, such as lectins— non-immunological proteins that identify and bind selectively to certain carbohydrates—are also typically expressed by cancer cells. The direct lectin targeting method involves using lectins attached to NPs to target carbohydrates on the surface of cancer cells; the reverse lectin targeting path involves using NPs' carbohydrate moieties to target lectins on cancer cells in an inverted manner.
The tyrosine kinase receptor ErbB family includes the epidermal growth factor receptor. EGFR has been used as a target for cancer treatment as it is overexpressed in many types of cancer and plays a role in the growth and progression of tumours. For example, human epidermal receptor-2 (HER-2) targeting is a frequent treatment for gastric and breast cancers that are HER-2 positive. Therefore, NPs that have been engineered to include altered ligands that bind to EGFR represent a promising drug delivery strategy for EGFRoverexpressed cancer cells. Moreover, combining two ligands unique to cancer into a single NP is an additional method of active targeting that can enhance target specificity [26].
Limitations of conventional chemotherapy
The primary characteristic of neoplastic cells is their rapid proliferation, which is how conventional chemotherapy drug function. Chemotherapy therefore also causes damage to normal, healthy cells that proliferate quickly, including bone marrow cells, macrophages, digestive tract cells, and hair follicles. The primary limitation of traditional chemotherapy is its inability to target malignant cells specifically. This leads to frequent adverse effects of most chemotherapeutic treatments, such as anaemia or thrombocytopenia, mucositis (inflammation of the lining of the digestive tract), alopecia (hair loss), myelosuppression (decreased production of white blood cells causing immunosuppression), and organ failure. Sometimes these side effects force a change in the prescribed therapy's dosage, a postponement of treatment,or its cessation. When it comes to solid tumours, it is possible to accurately stop cell division close to the centre, rendering chemotherapy-resistant drugs [27]. Moreover, chemotherapy drugs frequently are unable to enter solid tumours and kill the dangerous cells there.
Conventional chemotherapy drugs frequently leave the bloodstream after being ingested by macrophages. As a result, they don't have a long half-life in the blood and are unable to interact with malignant cells, rendering chemotherapy totally ineffectual. Another significant issue with traditional chemotherapy is the medicines' limited solubility, which prevents them from passing through biological membranes. P-glycoprotein, an overexpressed multidrug resistance protein on the surface of malignant cells, is another issue. It functions as an efflux pump to keep pharmaceuticals from building up inside the tumour and frequently causes the emergence of resistance to anticancer medications. As a result, the medications that are given are ineffective or fail to produce the intended results.
Chemotherapy, the conventional cancer treatment, has adverse effects and systemic effects on the body that cause patients to experience significant psychological distress. The neurotoxic side effects of chemotherapy medications, including the cognitive dysfunction known as "chemo brain," cause poor concentration, memory loss, and a decline in executive function. Additionally, there is a link between the oxidative stress and inflammation brought on by chemotherapy and elevated levels of anxiety and depression in cancer patients undergoing treatment.
There may be ways to minimize the psychological impact that chemotherapy takes because of nanomedicine, which is looking very promising as a therapeutic strategy. When precisely engineered, nanoscale drug carriers can maximise the delivery of therapeutic agents to cancer cells while reducing the amount of off-target effects on healthy tissues (34, 36, 43). By minimising systemic exposure and lessening the neurotoxic effect on the central nervous system, this targeted drug delivery may help to mitigate the cognitive deficits linked to chemotherapy.
Furthermore, a sustained and localised therapeutic effect is made possible by the controlled release of drugs formulated with nanotechnology, which may also lessen drug concentration fluctuations that worsen psychological distress. Nanomedicine has the potential to improve treatment efficacy while minimising side effects by raising the therapeutic index of anticancer medications. This could have a positive effect on the psychological health of cancer patients undergoing therapy.
Targeting drugs for cancer treatment
Our growing understanding of the molecular underpinnings of diseases, combined with advances in molecular biology techniques, has revealed a wealth of molecular information in the field of cancer treatment. However, the rate at which these discoveries have been made has far outpaced our current ability to effectively apply this knowledge [28, 32]. As a result, there has been an increase in efforts to identify and develop drugs that can specifically disrupt unique signalling pathways unique to cancer cells. This method holds the promise of tailoring individualised treatments based on the specific molecular targets found in a patient's tumour [29] [Figure 1].
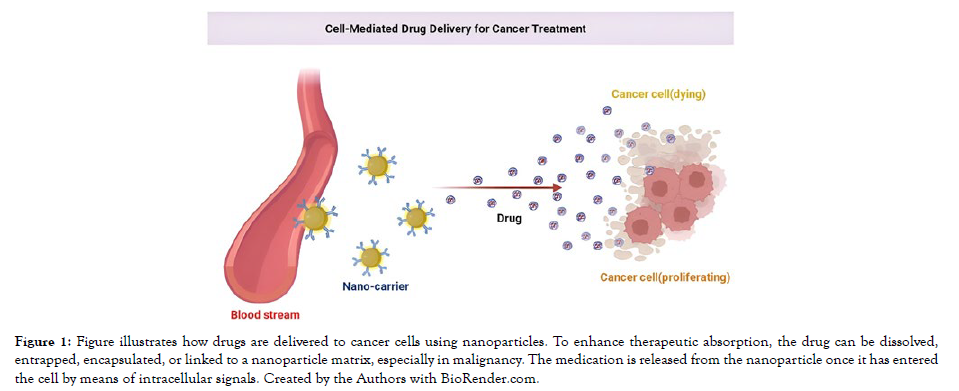
Figure 1: Figure illustrates how drugs are delivered to cancer cells using nanoparticles. To enhance therapeutic absorption, the drug can be dissolved, entrapped, encapsulated, or linked to a nanoparticle matrix, especially in malignancy. The medication is released from the nanoparticle once it has entered the cell by means of intracellular signals. Created by the Authors with BioRender.com.
Direct administration or a targeted drug delivery system designed to pinpoint specific organs where the tumour resides or the cancer cell's surface can be used to deliver drugs to cancer cells. Specific molecular targets, ligands designed to interact with these targets, and various delivery systems conjugated with these ligands comprise a targeted drug delivery system. Furthermore, different cancer types, ranging from hematologic to solid tumours, necessitate different treatment strategies [30]. Solid tumours, in particular, have heterogeneous and dynamic biology that evolves over time, posing unique drug delivery challenges.
Passive targeting
Passive targeting strategies leverage the natural anatomical and functional differences between the vasculature of normal tissues and tumors to allow the selective accumulation of drugs at the tumor site. The Enhanced Permeability and Retention (EPR) effect is used in one critical passive targeting mechanism [31]. The vascular structure of tumours differs from that of normal tissues, making it an ideal site for selective drug accumulation. Tumor vessels are typically more heterogeneous, larger, and permeable. Unlike normal blood vessels, which have tight endothelial cells, tumour microvessels have discontinuous and leaky endothelial cells. Depending on the anatomical location of the tumour, the gaps between these endothelial cells can range from 100 to 780 nanometers.
Tumor vasculature is also linked to increased levels of growth factors like Vascular Endothelial Growth Factor (VEGF) and Basic Fibroblast Growth Factor (bFGF), which cause vasodilation and increased drug extravasation into tumours [32]. When combined with impaired lymphatic drainage in solid tumours, this allows high molecular weight drugs to accumulate within tumours. The Enhanced Permeability and Retention (EPR) effect describes this phenomenon [Figure 2]. The EPR effect is primarily used in solid tumours for passive targeting of drugs with molecular weights greater than 40 kDa and low molecular weight drugs incorporated into drug carriers such as polymeric drug conjugates, liposomes, polymeric nanoparticles, and micellar systems. To fully capitalise on the EPR effect, targeted drugs or drug carriers must have long circulation times while retaining therapeutic activity [26, 33]. Tumor size, tumour vascularization, and angiogenesis are all factors that influence the EPR effect. As a result, the stage of cancer progression is an important factor in passive drug targeting via the EPR effect. In the commercial production of two liposomal formulations, the EPR effect is used. DaunosomeTM liposomes (NeXtar, Inc.) containing daunorubicin and DoxilTM (Sequus Pharmaceuticals) containing doxorubicin are sterically stabilised liposomal formulations with prolonged circulation times. Through passive targeting, they efficiently accumulate within tumour cells, lowering drug levels in the bloodstream and minimising the common cardiac side effects associated with anthracycline anticancer agents [34].
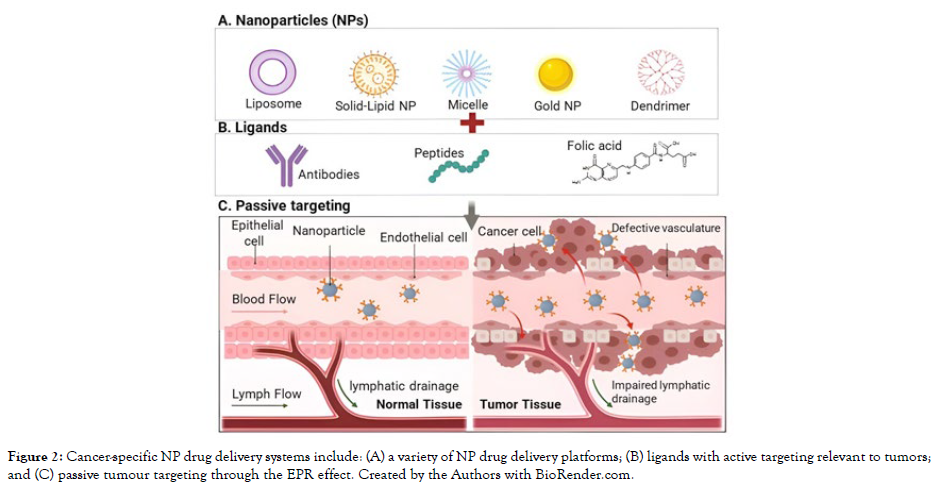
Figure 2: Cancer-specific NP drug delivery systems include: (A) a variety of NP drug delivery platforms; (B) ligands with active targeting relevant to tumors; and (C) passive tumour targeting through the EPR effect. Created by the Authors with BioRender.com.
Low molecular weight cytotoxic drugs have also been conjugated to polymers for passive tumour tissue targeting. This method takes advantage of the EPR effect by attaching therapeutic agents to polymers via hydrolyzable or degradable peptide linkers [35]. Only upon degradation within the tumour cells do these linkers release the active drug. Several polymers have been used to create such polymer-drug conjugates, including block co-polymers, dextran, inulin, polysaccharide B, polyglutamate, and alginic acid.
Maeda's development of SMANCS, a conjugate of polystyrene-comaleic acid half-nbutylate (SMA) and neocarzinostatin (NCS), a potent cytotoxic agent used in the treatment of primary hepatoma, is a notable example of this approach. In general, such systems cause tumour regression at first, followed by remission due to residual cells in the tumor's less accessible regions. This limitation arises from the fact that drug extravasation and permeation are primarily restricted to the tumor's periphery [36]. Interstitial fluid pressure is high in the centre of solid tumours, preventing drug diffusion. Overcoming this barrier requires preventing the outward flow of interstitial fluid, which can transport drugs into normal tissues via convection. As a result, systems that rely on the EPR effect must be optimised for deeper tumour penetration, or adjunctive physiological modulators can be used in conjunction to increase tumour blood flow. Substances such as VEGF, bFGF, and angiotensin-converting enzyme inhibitors like enalapril can increase tumour blood flow and thus promote drug penetration [37]. However, because of their role in tumour growth and metastasis, these growth factors have limitations in their use. Furthermore, none of these theoretical possibilities have been tested in clinical settings.
Localized delivery
The basic principle of localised delivery is the direct delivery of drugs to a localised tumour site while avoiding systemic side effects. This method is highly effective for some cancers but may not be appropriate for all, such as lung cancer.
Early intervention with less invasive localised treatment can be extremely effective in the case of prostate cancer. Prostate cancer management has greatly improved as a result of advances in diagnosis and staging. A comprehensive diagnostic approach is provided by digital rectal examination, serum Prostate-Specific Antigen (PSA) levels, and transrectal ultrasound. Nearly 90% of men diagnosed with prostate cancer in North America have localised disease. This allows for earlier intervention with less invasive localised treatments.
In a subcutaneous mouse model of prostate carcinoma, recent research demonstrated the efficacy of direct intratumoral delivery of paclitaxel using biodegradable nanoparticles conjugated with transferrin (Tf) ligands [33,38]. The key to Tf-conjugated nanoparticles' increased efficacy is their improved cellular uptake and sustained intracellular drug retention, which outperforms drug solutions or unconjugated nanoparticles. While passive targeting takes advantage of physiological differences between tumour and normal vasculature, localised delivery takes a more direct approach by delivering the drug directly to the tumour site. This approach, however, is not universally applicable and must be tailored to the specific characteristics of each cancer type.
Advanced physical targeting for precision drug delivery in cancer treatment
The pursuit of precision in cancer treatment has led to innovative strategies that allow for the targeted release of drugs at specific sites within the body. These novel approaches use external stimuli to achieve this precision, ensuring that therapeutic agents reach their intended destination. Among these novel techniques, ultrasound and magnetic field-based methods have shown great promise in the field of oncology drug delivery.
Ultrasound as a trigger for drug release
Using ultrasound waves to precisely and accurately target tumour tissue is a promising strategy for targeted drug delivery. This method makes use of polymeric micelles, which can encapsulate anti-cancer agents and facilitate their intracellular uptake when activated by ultrasound [39]. Although the precise mechanism of this targeting approach is still being investigated, several factors are thought to be involved.
Ultrasound may promote micelle extravasation into tumour tissue. This may result in improved drug delivery at the tumour site. Second, the ultrasound waves may selectively trigger the release of the drug from the micelles at the tumour site [40, 41]. These mechanisms are highly promising but require further research for a comprehensive understanding.
Researchers have investigated the use of ultrasound-triggered drug delivery in vitro, particularly for the delivery of anthracycline drugs. This strategy has shown promise in targeting both drug-sensitive and MDR ovarian A2780 carcinoma cells. It should be noted that ultrasound provides a non-invasive method of precise targeting. It also has the benefit of penetrating deep into the body while allowing for precise control and focus on specific target sites [42]. However, there have been some concerns raised about the effect of ultrasound radiation on cell membranes. Low ultrasound energies are required to increase intracellular drug uptake because energies above the cavitation threshold can severely damage cell membranes. Ongoing animal studies aim to provide insights into the potential clinical applications of this tumor targeting technology in humans [43].
Magnetic field-responsive drug targeting
Another cutting-edge approach to precision drug delivery involves the use of magnetic fields. In this method, therapeutic agents are either bound or encapsulated in a magnetic drug carrier, enabling their preferential localization in tumor tissues upon the application of an external localized magnetic field.
Magnetic responsive drug carriers frequently contain magnetite, iron, nickel, or cobalt [44]. Magnetic liposomes, microspheres, nanospheres, and colloidal iron-oxide solutions are examples of these carriers (magnetic ferrofluids). Magnetic ferrofluid, for example, is made up of particles with a diameter of about 100 nm that can be coated with a specific carbohydrate capable of reversibly binding drugs. These ferrofluids are designed to desorb the drug carried, which is triggered by specific physiological conditions such as pH or osmolality changes. Magnetic targeting of the drug epirubicin in patients with advanced sarcomas has been tested in clinical trials. These trials discovered that the approach was well tolerated and safe for patients, despite the fact that due to their low magnetic susceptibility, more than half of the carriers ended up in the liver [45, 46]. As a result, improvements are required to improve the targeting system's effectiveness and reduce its reliance on patient or disease related factors. Magnetic drug carriers are currently being studied in preclinical trials for a variety of chemotherapeutic agents, including mitoxantrone, etoposide, and paclitaxel [47].
Researchers also developed a water formulation using oleic acid- Pluronic coated iron-oxide magnetic nanoparticles. This novel formulation is capable of loading high doses of water insoluble anticancer agents such as paclitaxel efficiently [48]. Notably, under in vitro conditions, this nanoparticle formulation maintains the release of the incorporated drug for more than two weeks without affecting the magnetic properties of the core iron-oxide nanoparticles.
One intriguing potential application for magnetic drug carriers is radioisotope delivery. These carriers can be used to deliver radioisotopes to tumour cells, delivering a targeted, high dose of radiation while sparing normal cells in the surrounding area [49]. In this approach, radioisotopes are not released, but the entire magnetic carrier is delivered and held in close proximity to the area that requires irradiation. This approach holds great promise, and its further development necessitates collaboration between biologists and physicists.
The need for a significant magnetic force to overcome the forces exerted by linear blood flow rates in arteries and blood capillaries is a significant challenge in achieving effective targeting via systemic administration. Thus, researchers are focusing their efforts on developing targeting carriers with higher magnetic moments and magnets with stronger magnetic field gradients to precisely direct these magnetic drug carriers to the tumour site [50].
Precision drug delivery has emerged as a critical frontier in the relentless pursuit of more effective cancer treatments. This strategy aims to deliver therapeutic agents selectively to cancerous tissues while sparing healthy cells, minimising side effects, and increasing treatment efficacy.
Active targeting of tumor vascular endothelium
Tumor angiogenesis, the formation of new blood vessels, is a hallmark of solid tumors and a pivotal aspect of cancer progression. It's driven by increased demands for nutrients and oxygen by rapidly dividing tumor cells. This process creates differences between the vascular endothelium in tumors and that in normal tissues, making the former a promising target for drug delivery [Figure 3].
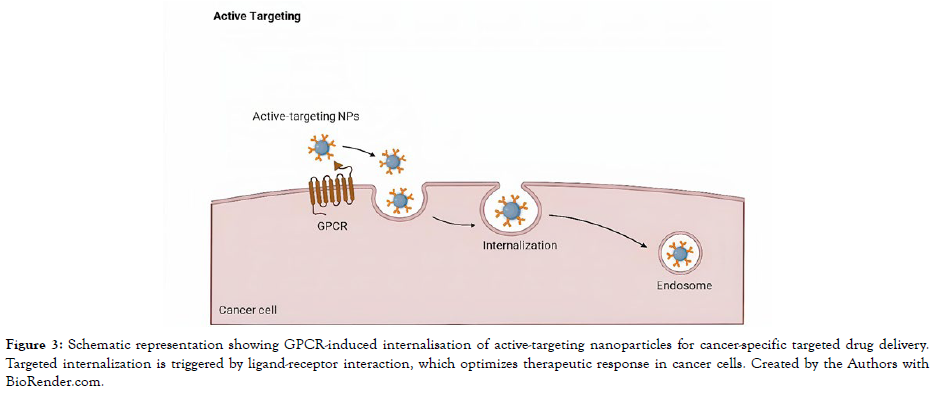
Figure 3: Schematic representation showing GPCR-induced internalisation of active-targeting nanoparticles for cancer-specific targeted drug delivery. Targeted internalization is triggered by ligand-receptor interaction, which optimizes therapeutic response in cancer cells. Created by the Authors with BioRender.com.
Vascular endothelial targets
The vascular endothelium in tumours differs from that in normal tissues. Among these characteristics are increased expression of cell adhesion molecules and proteolytic enzymes. They provide a variety of cancer therapy targets, including endothelial cells and specific stromal components that are easily accessible via the bloodstream [51]. Endoglin (CD105), a tumour growth factor (TGF- α) receptor,has emerged as a promising target for tumour imaging and therapy [52]. Furthermore, vascular endothelial growth factor (VEGF) promotes tumour neo-vasculature and is targeted by anti-VEGF antibodies.
Angiogenesis-related molecules such as matrix metalloproteinases (MMPs), angiopoietins and their receptors (Tie1 and Tie2), plateletderived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and their respective receptors are also potential targets within the vascular endothelium. Vascular endothelial cadherin (VE-cad), a type of endothelial cell adhesion molecule, is essential for vascular integrity and angiogenesis during tumour growth [53, 54]. Anti-VE-cad monoclonal antibodies have shown promise in inhibiting tumour growth and metastasis.
Integrins, particularly v5 integrin, are intriguing molecular targets because they are only expressed during angiogenesis and are noticeably absent in mature blood vessels [55, 56]. Monoclonal antibodies and ligands, particularly peptides rich in Arg-Gly- Asp (RGD), have been shown to bind to integrins, providing an opportunity to effectively target tumour endothelium.
Targeting tumor cells
Cancer cells differ from normal cells in the expression of specific proteins, some of which are novel, while others are overexpressed. These proteins have the potential to be useful biomarkers for cancer progression and drug therapy efficacy. Tumor-associated antigens (TAAs) are one type of protein.
Melanoma antigen E (MAGE)-1, discovered to be overexpressed on tumour surfaces, was one of the first TAAs discovered [57]. Proteomics and bioinformatics advances have sparked a race to find novel TAAs, and an ideal TAA is one that is expressed primarily on the tumour cell surface and plays an important role in cancer cell survival.
Herceptin® (Trastuzumab), an antibody that targets Her-2, a tyrosine kinase found in breast cancer cells, is an example of tumorspecific functional targeting that has been successful [58]. Although highly desirable, such tumor-specific functional targets are currently scarce. However, due to this limitation, alternative approaches have been developed in which therapeutic agents are conjugated to non-functional TAAs, relying on the specificity of these ligands to deliver toxins to tumour cells [59, 60]. Furthermore, several cell surface receptors for peptides, hormones, and essential nutrients such as iron and folic acid are overexpressed in many cancer cells, opening up new avenues for drug targeting.
Promising tumor-specific targets
In the field of active targeting, two specific receptors have received a lot of attention: the folate receptor (FR) and the low-density lipoprotein (LDL) receptor. Over 90% of ovarian carcinoma patients and many other cancer types have high levels of FR expression, while most normal tissues have low to no expression [61]. Targeting the FR with small molecules like folic acid or antibodies against the receptor provides numerous opportunities for precise drug delivery [62]. The LDL receptor is an endocytic receptor that is responsible for transporting cholesterol-rich lipoproteins into cells. Liposomes designed to mimic LDL can interact with LDL receptors on cancer cells, increasing drug uptake via LDL receptor-mediated endocytosis.
Hormone receptors, which are found on the cell surface of many hormone-dependent tumours, offer yet another avenue for precise targeting. GnRH/LHRH receptors, for example, have been identified in some solid tumours and cell lines of hormonedependent tumours such as breast, prostate, endometrial, and ovarian carcinoma. Ligands such as LHRH or its synthetic peptide analogue, as well as antibodies targeting this receptor, can be used to target cancer cells specifically [60].
The discovery and validation of tumor-associated antigens and targets has resulted in a surge in research. To successfully use these targets for drug delivery, however, it is necessary to first understand their presence within the molecular and cellular context of tumour pathophysiology. Tumors are inherently heterogeneous, and this heterogeneity varies according to disease stage, aggressiveness, and even patient to patient.
Additionally, tumor-associated antigens may not be expressed uniformly throughout the tumour mass. Variations in posttranslational modifications and genetic instability in hypoxic regions can both contribute to this heterogeneity. Despite these complexities, antigen expression heterogeneity can be managed using stable cytotoxic drugs, ensuring a "bystander effect" that reaches antigen-negative cells [63].
Surface antigens are frequently released into the bloodstream during the advanced stages of cancer, when tumour cells metastasize. These circulating antigens can compete with bound antigens on tumour cells, reducing therapy efficacy. This phenomenon highlights the difficulties and complexities of active targeting.
Selecting optimal targeting agents
The selection of a targeting agent is a critical determinant of drug targeting success. Because not all tumor-cell-associated surface proteins are receptors with specific ligands, they are not all amenable to antibody targeting. Small molecular ligands, on the other hand, have advantages such as better penetration of solid tumours, availability, and straightforward conjugation chemistry [64]. Their supposed lack of immunogenicity adds to their allure.
Antibodies are commonly used as targeting agents, and the development of monoclonal antibodies (MAbs) and smaller antibody fragments has increased their usefulness [65]. To address their limited tumour penetration, new antibody formats such as Fab fragments, single-chain Fv fragments (scFv), and bispecific antibodies have been introduced [66]. Beyond antibodies, smaller molecules and ligands can also be explored as targeting agents. These molecules have high specificity and affinity for binding to tumor-associated proteins, and their potential as targeting agents is increasingly recognized.
Modeling applications in drug delivery and nanoparticle device design
Precision drug delivery with nanoparticles (NPs) is a complex endeavour that necessitates a thorough understanding of both the drug's pharmacokinetics and the delivery system's properties [67, 68]. A variety of parameters influence NPs, including platform selection (e.g., liposomes, polymeric micelles, polymer drug conjugates, and dendrites), physical attributes such as size and shape, and surface chemistry. Furthermore, NPs encounter a variety of biological barriers on their way to the target site, including barriers within the bloodstream, at the tumour site, on the cell surface, and within the cell. NP effectiveness is highly dependent on individual patient-specific conditions [Figure 4].
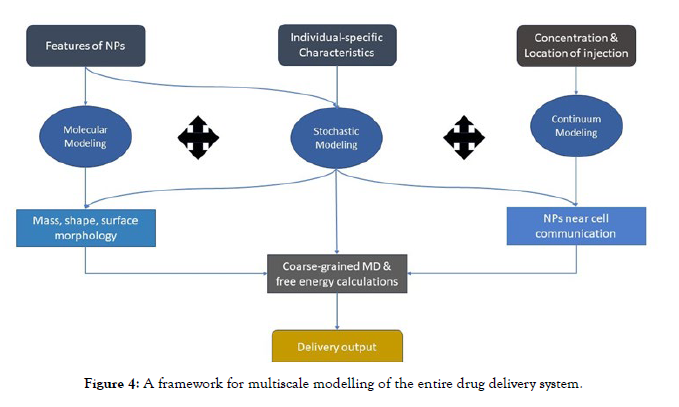
Figure 4: A framework for multiscale modelling of the entire drug delivery system.
Given the complexity of nanoparticle research, which is characterised by numerous variables and costly experimental procedures, computer simulations are well-suited. Simulations can be used to screen potential drug and carrier combinations as well as provide insights for the design of entirely novel NPs. Theoretical and computational modelling techniques can be used to optimise parameters such as geometry, surface chemistry, and other properties in various aspects of drug delivery. These models include processes covered in previous sections, such as drug transport, interactions with biological systems, and dealing with patient-specific variations.
Continuum modeling
Because of the wide range of blood vessel diameters, from centimetres in larger vessels to microns in capillaries, modelling the transport of NPs through the vascular network presents challenges. Transport in larger vessels can be described using advectiondiffusion models, which treat blood as a simple Newtonian fluid.To study NP concentration in microvasculature, a more complex convection-diffusion-reaction model is required [69]. To investigate NP distribution in capillaries, recent studies have used large-scale agent-based modelling in conjunction with computational fluid dynamics. These simulations revealed that red blood cells play a role in dispersing NPs along vessel walls and altering the Enhanced Permeability and Retention (EPR) effect [70]. Larger NPs have been shown to adhere to vessel walls more readily, increasing their chances of penetrating diseased vasculature and reaching target cells.
The process of NP adhesion to endothelial cells can be modelled to understand how NP shape influences the process. For example, in the simulation of NP binding probability, nanoparticles are assigned initial positions at the channel inlet, a Brownian adhesion dynamics model is used, multiple trials are run, the average number of bonded nanoparticles is calculated, and the total number of nanoparticles is normalised based on depletion layer thickness and shear rate [69]. Additionally, NP deposition and distribution within the blood vessel network can be simulated using continuum models.
Diffusion-based kinetics models have been developed to study drug release in brain tissue. These models take into account pressure, velocity, and blood chemistry, all of which can have an impact on NP transport, deformation, and release. Accurate parameter predictions are critical for designing stable NPs. When modelling NPs, swelling and erosion during circulation must also be taken into account, necessitating dynamic boundary assumptions during the release process. These complex models are solved using finite element analysis [71].
Molecular modeling
Molecular modeling offers insights into the size, shape, and charge distribution of NPs. It focuses on interactions between atoms and molecules over a defined period, employing free energy minimization to generate numerical solutions for system properties. This approach can simulate molecular motion and elucidate the NP uptake process and the influence of NP characteristics. Molecular modelling provides information about the size, shape, and charge distribution of nanoparticles. It focuses on atoms and molecules interacting over time, using free energy minimization to generate numerical solutions for system properties. This method can simulate molecular motion and shed light on the NP uptake process as well as the impact of NP characteristics.
Empirical methods, ab initio quantum mechanical methods, classical molecular dynamics, and coarse-grained molecular dynamics are some of the molecular modelling methods that can be used. Based on experimental data fitting, empirical molecular descriptors can predict physical properties such as drug solubility and diffusion coefficients. However, because they rely on experimental data, they frequently use artificial parameters [72, 73]. In contrast, ab initio quantum mechanical methods consider electronic degrees of freedom to determine electronic behaviour. They are computationally intensive and complex, despite being highly informative.
When compared to ab initio quantum mechanical methods, classical molecular mechanical simulations are less computationally demanding. Because potential energy is relatively simple to calculate, it can be applied to larger systems over longer time periods. Coarse grained molecular dynamics is also used for large systems and simulations with time periods greater than one millisecond. These models require calibration of parameters using experimental data, limiting accuracy based on data availability.
Stochastic modeling
Stochastic modelling is required to account for patient-to-patient and within-patient variability, which is a difficult aspect of NP research. Variations in blood flow, vasculature, and tumours can be significant, necessitating the inclusion of uncertainties in model parameters. Using average values alone can lead to significant errors in NP modelling and design. The Monte Carlo method is a popular method for dealing with parameter uncertainties.
The Monte Carlo method employs random number generators from specific probability distributions to generate samples repeatedly, allowing sample means and variances to be calculated [74]. It has been used to investigate the interactions of ligand bound NPs with both tumour and healthy cells. Many parameter values were considered as inputs to understand their effects, and it was discovered that highly selective binding to target cells can be achieved by binding multiple weak-affinity ligands to the receptors. Furthermore,
Liu et al. used Monte Carlo simulations to understand the effect of NP surface functionalization on endothelial binding [75]. They discovered that antibody coverage is a critical factor in the binding process. Recent advancements include quantifying uncertainty dispersion coefficients of NPs during transport through microvasculature using a Bayesian calibration method.
Linking of multiple length scales in modeling
Individual models can address aspects of NP circulation, endocytosis, and drug release, but drug delivery spans multiple temporal and spatial scales, making simulation of the entire process difficult. Multiscale modelling frameworks have emerged, predicting physical parameters, diffusivity, and solubility using Monte Carlo methods and atomic-level calculations. These models take into account a variety of inputs, such as drug properties, delivery system characteristics, and patient-specific data, such as MRI images for vascular geometry [69, 75]. When information is transferred between scales, however, uncertainties are propagated. Researchers addressed this issue by combining various models, resulting in more accurate simulations and improved drug delivery optimization. This method has the potential to improve our understanding of complex drug delivery systems.
Challenges and constraints in computational modeling for drug delivery
In the pharmaceutical industry, the inherent limitations in predictability and accuracy have hampered the widespread adoption of computational modelling for drug delivery design. Many approaches currently rely on agent-based empirical models, primarily because mechanism-based models struggle to accurately replicate the complex biological and chemical interactions involved in drug delivery [76]. To address this issue, a better understanding of the fundamental mechanisms that govern each stage of drug delivery is required, which can be accomplished through more intelligent experimental designs [72,73,75]. It is critical to recognise that each modelling approach has limitations, frequently focusing on specific aspects of drug delivery while struggling to encompass the entire process across various length and time scales. Although linking models operating at different spatial and temporal scales is indeed a challenge, it holds the promise of delivering higher accuracy and comprehensive insights.To facilitate parameter estimation, model calibration, validation, and error analysis, mechanistic models require robust experimental data. The movement of nanoparticles within cells and tissues is a significant challenge. Using in vitro data can help to create simulations that closely resemble physiological conditions.
Furthermore, these experiments should be replicated to ensure statistical reliability. The importance of data that spans multiple scales cannot be overstated. Because errors in one segment of the simulation can propagate throughout the model, resulting in an unsatisfactory outcome, it serves the dual purpose of updating models and mitigating uncertainties.
Conclusion and future prospects in nanoparticle-based drug delivery
Because of their widespread availability, ease of functionalization, biocompatibility, and stability, nanoparticles (NPs) have undoubtedly emerged as promising candidates for targeted drug delivery. These characteristics pave the way for potent drugs to be encapsulated and delivered precisely to the tumour site. Currently, three main delivery strategies are under consideration:
Passive targeting: This strategy takes advantage of the Enhanced Permeability and Retention (EPR) effect, which allows NPs to accumulate at tumour sites via leaky vasculature. It is a critical step that is currently being implemented.
Active targeting: Active targeting involves the use of ligandreceptor interactions to achieve more selective drug delivery. This method has a lot of potential for improving treatment precision.
Next-generation nps: These cutting-edge nanoparticles are programmed to respond to external stimuli such as heat or magnetic fields, allowing for time and locationcontrolled drug release. Even though they are still in the early stages of development, they offer exciting possibilities.
Furthermore, NPs have an extraordinary ability to self-assemble into a variety of structures, including amphiphilic micelles, liposomes, dendrimers, and metal-core particles. Each of these structures has distinct strengths and weaknesses, making them suitable for delivering specific therapies to various parts of the body. The selection of NP is highly dependent on the intended application, and no universal standard has yet been established. While micelles and liposomes are commonly used, there is a growing body of research investigating alternative NP structures, each of which offers distinct advantages. Rotaxanes, for example, can release cargo in response to customised stimuli, dendrimers maximise surface area and cargo capacity, and metal-core particles are well-suited for a variety of targeting approaches.
Despite their enormous potential, NPs must be meticulously controlled and considered in order to ensure effective drug delivery without triggering side effects. Inadequate size, geometry, or surface charge can all have negative consequences. Environmental factors can also have an impact on the success of NP drug delivery.
Furthermore, before NPs can be used in humans, they must undergo extensive in vitro and in vivo testing to ensure their safety and efficacy in living organisms. Computational modelling has proven invaluable in optimising NP design due to the intricate nature of the drug delivery process and the inherent uncertainties. However, due to their lack of predictability, computational models have been slow to gain traction in the pharmaceutical industry. Most existing models tend to focus on isolated aspects of drug delivery rather than covering the entire process across multiple scales, from the drug to the patient. It is uncommon to find multiscale modelling that spans multiple temporal and spatial scales.
Multiscale modelling, in conjunction with experimental validation, may provide guidance for the future of NP design. Collaborative research that combines the power of computational models with experimental data has the potential to improve the design of more efficient nanocarriers. Recent studies have already demonstrated the efficacy of this strategy. For example, used molecular docking and molecular dynamics simulations to screen building blocks for nanocarrier synthesis, which they then validated experimentally. Similarly, Jiang et al. combined multiscale modeling with experiments to understand the role of building blocks in telodendrimer self-assembly [72,26,78]. Likewise, collaboration between experiments and multiscale computational modelling is the future of drug delivery research. The development of microchips has made it possible to simulate tumour microenvironments and then test the efficacy of nanoparticles for targeted drug delivery. Computational models are crucial in designing these devices, pushing the limits of NP development. Shi [77] used a combination of molecular docking and molecular dynamics simulations to guide the selection of building blocks for nanocarrier synthesis in one of the seminal research studies. The findings were then experimentally validated, emphasising the synergy between computational modelling and real-world application. Correspondingly, Jiang [78] used multiscale modelling in conjunction with experiments to gain a comprehensive understanding of the role of building blocks in telo-dendrimer selfassembly. The study demonstrated effectively how computational modelling can supplement experimental investigations. These ground-breaking studies highlight the importance of combining computational modelling and empirical research, presenting a promising path forward for drug delivery strategies.
REFERENCES
- Longley, D B, Johnston, P G. Molecular mechanisms of drug resistance. J Pathol. 2020; 205(2): 275-292.
- Prabhakar, Xu, L An, D Qian, S West, A C. Nanomedicine as an Emerging Platform for Metabolic Therapy in Cancer. Frontiers in Oncology. 2021: 11: 1622.
- Torchilin, V P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2018;13(11): 813-827.
- Lukyanov, A N, Torchilin, V P. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2016; 56(9): 1273-1289.
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Pharm Sci Res. 2018; 13(4): 288-303.
- Ekambaram P, Abdul A B . Anticancer efficacy of solid lipid nanoparticles loaded with 5-fluorouracil and its hydrophobic derivative: In vitro and in vivo analysis. Eur J Pharm Sci. 2016; 88:178-185.
- Safarzadeh M, Hajialyani M, Nematollahi A, Roshan, M K, Rezaee R. The potential role of nano-curcumin as an anti-inflammatory agent in rheumatoid arthritis. J Cell Biochem. 2018;119(2):1015-1021.
- Kaminskas, L M McLeod, V M Ryan, G M Kelly, B D Haynes, J M Williamson et al . Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J Control Release. 2017; 268:147-158.
- Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. Journal of Controlled Release. 2018; 132(3): 171-183.
- Vallet-Regi M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2019; 24(2):247.
- Li Z, Zhang Y, Fullston D, Shen Y. Advanced carbon-based nanomaterials for tumor photothermal therapy. Nanomaterials. 2021; 11(5):1137.
- Xia Q, Cai Y, Zheng J, Zhang J. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. Journal of Controlled Release. 2021; 330: 75-90.
- Narayanan, K B, Sakthivel N. Green synthesis of biogenic metal and metal oxide nanoparticles and their effect on the bioactivity of pharmaceuticals. Journal of Molecular Liquids. 2020; 300:112202.
- Brown S A, Hansbro, P M, Hansbro, N G. Animal models of asthma: value, limitations and opportunities for alternative approaches. Drug Discovery Today. 2019; 24(1): 206-218.
- Jones, J R, Barrère F, van Blitterswijk, C A. Calcium phosphate ceramics as bone graft substitutes in filling bone tumors. Pharmaceuticals. 2020; 3(3):125.
- Wang Y, Qi X, Lu L, Xu X. Anticancer properties of sulfated chitosan. Biol Trace Elem Res. 2019; 192(2): 205-212.
- Li J, Wang X, Zhang T, Wang C, Huang Z, Luo et al . Polypyrrole/chitosan-coated Fe3O4 nanoparticles for MRI-guided photothermal cancer therapy. Biomedical Materials. 2020; 15(4): 045001.
- Sharma N, Baldi A, Garg S. Cyclodextrins: encapsulation of drugs. Critical Reviews in Therapeutic Drug Carrier Systems. 2019;19(3):185-208.
- Narayanan N, Sudhakumari C C. Cyclodextrin as a tool in enhanced drug delivery. Polym Renew Resour. 2021;10(2): 77-84.
- Smith, K A, Buhro, W E. Synthesis of surface-stabilized beta-cyclodextrin/gold nanoparticle assemblies. Nanoscale Advances. 2020; 2(2): 527-535.
- Dong Y, Li Z, Zhou J. A new class of biocompatible and luminescent carbogenic nanodots for bioimaging. Chemical Communications. 2020; 46(46): 2761-2763.
- Brown, D M, Donaldson K, Stone V, Clouter A. The effects of PM10 particles and oxidative stress on macrophages and lung epithelial cells: modulating effects of calcium signalling agonists and antagonists. Thorax. 2020; 58(4): 271-279.
- Gupta A, Kumar R, Jha A. Preparation, characterization, and application of magnetic nanoparticles for gene delivery and imaging. Journal of Nanoparticles. 2019; 2013: 1-14.
- Soe Z C, Thapa R K, Ou W, Gautam M, Nguyen, H T, Jin et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy in colorectal cancer mouse xenografts. Journal of Controlled Release.2019; 303:1-15.
- Yao V J, D'Angelo S, Butler K S, Theron C, Smith T L, Marchio S, et al. Ligand-targeted theranostic nanomedicines against cancer. Chemical Reviews. 2016; 116(6): 3436-3486.
- Balasubramanian P, Yang L, Lang J C, Jatana K R, Schuller D. Aloe-emodin induces chemo-radiosensitivity in human hepatocellular carcinoma cells via modulating p53-mediated apoptosis. J Exp Clin Cancer Res. 2014;33(1): 1-14.
- Sutradhar K B, Sumi C D, Al-Mahmood A K. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. Journal of Acute Disease. 2014; 3(3):182-185.
- Huang B, Abraham W D, Zheng Y, Bustamante Lopez S C. Breaking Through Tumor Hypoxia: Developing Hypoxia-Targeting Nanoparticles. Crit Rev Ther Drug Carr Syst. 2018; 35(5): 433-469.
- Burrell R A, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2018; 501(7467): 338-345.
- Tong R, Chiang, H H, Kohane D S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proceedings of the National Academy of Sciences. 2019; 110(46): 19048-19053.
- Shi Y, van Steenbergen M J, Teunissen A J P, van der Meel R, Lammers T. Drug delivery strategies for platinum-based anticancer drugs. Expert Opin Drug Deliv. 2020; 17(5): 587-604.
- Ma D, Zhang, H B, Shu W. Integration of a Nanocarrier-Based Platform for Dual-Responsive Cocktail Chemotherapy and Dual-Modal Imaging. Advanced Materials. 2019; 31(12): 1807887.
- Hobbs S K, Monsky, W L, Yuan F, Roberts W G, Griffith L, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci. 2018; 95(8): 4607-4612.
- Dong Y, Love K T, Dorkin J R, Sirirungruang S, Zhang Y, Chen D, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci. 2018; 111(11): 3955-3960.
- Li Y, Wang Y, Huang G, Gao J, Cooper I R, Ji J, et al. Recent progress in smart drug delivery nanosystems using stimuli-responsive polymers. J Mater Chem B. 2019; 7(8): 1139-1161.
- Miao L, Huang L, Exploring N. Nano-enabled delivery of RNAi and CRISPR-Cas9 for cancer treatment. Nano Today. 2020; 32: 100853.
- Wang Z, Li X, Ying Z. Combining Magnetic Nanoparticles and Ultrasound for Drug Delivery in Cancer: A Comprehensive Review. Crit Rev Oncol Hematol. 2018; 131: 34-49.
- Qin B, Pei J, Guo H, Dong X, Zong B, Li D. Bioinspired nanoparticles for direct intratumoral chemotherapy of local cancer. J Mater Chem B. 2019; 7(23): 3646-3652.
- Lentacker I, Geers B, Demeester J. De Smedt, S. C. Advanced Delivery Strategies for Anticancer Nanomedicine. Nanomedicine. 2021; 7(2): 179-196.
- Kieran D, Woods A, Villalta-Cerdas A, Weiner S. Tumor-associated antigens for the induction of antitumor immune responses. Annual Review of Immunology. 2020; 39: 251-272.
- Ngoune R, Peters A, von Elverfeldt D, Winkler K, Pütz G, Plank et al. Ferrous iron-containing liposomal contrast agent with ultrahigh relaxivity for magnetic resonance blood pool imaging. Contrast Media & Molecular Imaging. 2016; 11(1): 37-44.
- Mitragotri S, Lahann, J. Physical approaches to biomaterial design. Nature Materials. 2019; 8(1): 15-23.
- Kang J H, Um E, Muzzio M. On the mechanism of the ultrasonic atomization of highly viscous liquids. AIChE Journal. 2021; 67(8): e17312.
- Safra T, Muggia F, Jeffers S, Tsao-Wei D, Groshen S. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Annals of Oncology. 2019; 11(8): 1029-1033.
- Meng H, Wang M, Liu H. Ultrasmall magneto-plasmonic nanoclusters for magnetic resonance imaging-guided photothermal therapy. Bioconjugate Chemistry. 2020; 29(10): 3385-3392.
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 2016;100(1): 1-11.
- Mikhaylov G, Mikac U, Magaeva A. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nature Nanotechnology. 2011; 6(8): 594.
- Hua M Y, Liu H L, Yang H W, Chen P Y, Tsai RY , Huang C Y, et al. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials. 2020; 31(28): 9165-9173.
- Tang T Y, Howarth S P, Miller S R, Trivedi R A, Graves M J, U-King-Im J M, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2017; 53(22): 2039-2050.
- Schleich N, Sibret P, Danhier P, Ucakar B, Laurent S, Muller R N, et al. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int J Pharm. 2014;466(1-2): 172-185.
- Miller K D, Nogueira L, Mariotto A B, Rowland J H, Yabroff K R, Alfano, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2020; 69(5): 363-385.
- Lee S, Patel T. Tumor-Targeted Drug Delivery Utilizing Engineered T Cells. Frontiers in Immunology. 2018; 9: 2294.
- Garcia J R, Davis J. Probing Targeted Nanoparticle Surface Interactions with Cellular Membranes. ACS Nano. 2019; 13(8): 9137-9151.
- Smith A M, Lee K. Conjugated Polymer Nanoparticles for Imaging and Therapeutics. Bioconjugate Chemistry. 2022; 33(1): 1-13.
- Brown M, Anderson R, Artis D. Intestinal Memory CD4+ T Cells Function to Suppress Inflammation in the Gut. Journal of Immunology. 2018;187:16-187.
- Anderson J C, White K A. Structure of the African swine fever virus major capsid protein p72. Cell Reports. 2021; 36(7): 109454.
- Parker N, Smith A. Multiple Myeloma Antibodies and Targeted Therapies. Hematol Oncol Clin North Am. 2019; 33(1): 37-55.
- Johnson D E, O'Keefe R A, Grandis J R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2022;19(3): 208-222.
- Smith A M, Davis J. Increasing the accuracy of nanoparticle sensing using a multi-level gating approach. Nature Communications. 2019; 10(1): 1-9.
- Davis J, Yaghi O M, Dincă M, Gagliardi L. Contribution of Open Metal Sites to Quantum Spin Liquidity in Molecule-Based Magnets. J Am Chem Soc. 2022; 144(7): 2905-2914.
- Jones L W, Anderson T. Exercise Oncology: Historical Perspective and Future Directions. Cold Spring Harb Perspect Med. 2023;13(6): a037038.
- Lee J H, Wilson W. Virus-Mimicking Phytosomes for Drug Delivery in Cancer. Pharmaceutics. 2020; 12(3): 229.
- White K A, Parker N. Expanding the Scope of Enzyme Specificity for Genome Engineering. J Am Chem Soc. 2021; 143(45):18445-18450.
- Garcia J R, Wilson W. RNA Delivery and Therapeutics for Oncology. Trends Pharmacol Sci. 2021; 42(11): 905-915.
- Lee K, Anderson R. Nanoparticles in Biology and Medicine: Small Structures with Big Potential. Adv healthc mater. 2022; 11(19): 2100713.
- Harrison O J, Smith S J, Sperandio M. Detection and Discrimination of Intact and Cleaved E-Cadherin Fragment Release from Cell Surfaces: Implications for Understanding the Role of E-Cadherin in Angiogenesis and Cancer Progression. J Proteome Res. 2023; 22(1): 683-693.
- Hoshyar N, Gray S, Han H. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanoscale. 2016; 8(13): 6731-6747.
- Kamaly N, Yameen B, Wu J. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chemical Reviews. 2016; 116(4): 2602-2663.
- Zhang S, Bellinger A, Genshaft A. A blood–brain barrier–penetrating gut-microbe-derived molecule that enhances amyloid beta deposition and cognitive impairment in a mouse model. Science Advances. 2021; 7(23): eabf1773.
- Tong R, Chiang H H, Kohane D S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc Natl Acad Sci. 2020; 110(46): 19048-19053.
- Sarvi F, Mahmoodzadeh A, Kiani M. A novel mathematical model for the effective drug administration and the drug concentration in brain tumor. J Drug Deliv Sci Technol. 2021; 61:102275.
- Bai Y, Li Y, Zhang G, Hou X. Understanding the mechanisms of graphene-based nanomaterials as drug carriers: a theoretical perspective. Phys Chem Chem Phys. 2020; 22(29): 16544-16556.
- Garg S, Liao W, El-Kadi A O. Understanding the interaction of dietary polyphenols with mammalian drug metabolizing enzymes. Curr Drug Metab. 2020; 21(6): 416-429.
- Zhang Y, Zhang R, Su H. Interaction of ligand-bound nanoparticles with cell membranes: from specific binding to nonspecific penetration. Langmuir. 2019; 35(3): 677-688.
- Li C, Li Q, Zeng Z, Zhou X. Dispersions of hydrophobic magnetite nanoparticles in nonpolar solvents with different surfactants: the effect of surfactant chain length and surfactant concentration. J Colloid Interface Sci. 2019;539: 567-577.
- Ranade A R, Zhang H, Drueckhammer D G. 3D-QSAR Models for Arylcarboxamides as Potent and Selective Human Beta 3 Adrenergic Receptor Agonists. J Chem Inf Model. 2018; 58(7): 1476-1487.
- Shi Y, Zhao R, Zhang Q, Wei Y, Zhao Y. Molecular Dynamics Simulation-Guided Rational Design of Polymer Nanocarriers for Drug Delivery. Nanoscale Res Lett. 2019; 14(1): 372.
- Jiang H, Du L, Li L. Multiscale modeling and experimental study on the self-assembly of telo-dendrimers. J Mol Model. 2021; 27(1): 1-13.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sarkar S, Debnath A (2024) Advancements in Nanoparticles for Drug Delivery, Therapeutic Applications and Computational Modeling: A Comprehensive Review. J Nanomed Nanotech. 15: 716.
Copyright: ©2024 Sarkar S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


