Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2025) Volume 10, Issue 1
Accumulation of Pigment Particles Used in Tattooing in Human Skin-Results of Histological Studies
Anna Charuta1*, Agnieszka Paziewska1, Iwona Luszczewska-Sierakowska2, Marta Gajewska Justyna Olszewska3, Michalina Kosowska1 and Agata Wawrzyniak42Department of Normal Anatomy, Siedlce University of Natural Sciences and Humanities, Lublin, Poland
3Department of Dermatology, Warsaw Medical University, Warszawa, Poland
4Department of Histology and Embryology, University of Rzeszow, Rzeszow, Poland
Received: 14-Aug-2023, Manuscript No. JOD-23-23867; Editor assigned: 16-Aug-2023, Pre QC No. JOD-23-23867 (PQ); Reviewed: 31-Aug-2023, QC No. JOD-23-23867; Revised: 06-Jan-2025, Manuscript No. JOD-23-23867 (R); Published: 14-Jan-2025, DOI: 10.35248/2684-1436.25.10.261
Abstract
The reports of the dangers of tattoos and the observed side effects have increased interest in the toxicity of tattoo pigments. The effects of tattoo pigments on skin homeostasis remain largely unknown. This is why research on the accumulation of pigment particles in the layers and cells of the skin (macrophages, fibroblasts) is so important. The main aim was to analyze which cells phagocyte tattoo ink in human skin. The accumulation of pigment particles used in tattooing in individual skin layers and in skin cells such as macrophages and fibroblasts was analyzed. The test material was human skin fragments with a tattoo, measuring 3 × 3 cm (including subcutaneous tissue), taken from human cadavers from the deltoid region, thorax, abdomen and the lateral aspect of the arm. Immunohistochemical staining was performed to identify Leukocyte Common Antigen (LCA) leukocytes without T and B lymphocyte divisions and inflammatory mediator cells–CD68 macrophages. Basic topographical staining allowed observing the distribution and penetration of tattoo ink particles in human skin. Tests performed showed the presence of inflammation around the tattoo dye papules, as confirmed by the presence of leukocytes and in macrophages visible in microscopic images. Pigment particles were also found to accumulate in macrophages and fibroblasts. The study shows that macrophages and fibroblasts accumulate dye molecules used in tattooing processes.
Keywords
Skin; Tattoo; Macrophages; Fibroblasts
Introduction
Reports of the dangers of tattoos have increased interest in the toxicity of pigments contained in tattoo ink [1]. The effects of tattoo pigments on skin homeostasis and human health remain largely unknown.
That is why research on the accumulation of tattoo ink pigment particles in individual layers and cells of the skin, such as macrophages, fibroblasts, is so important. It is also important to determine the size of pigment particles accumulated in skin cells, as histopathologicmacrophages loaded with pigment particles can cause tattooed skin to look similar to skin with melanoma regression, potentially misleading surgeons and pathologists [2,3].
Macrophages are part of the skin's immune system. They constitute a population of cells in the immune system. Macrophages are part of the body's phagocytic system acting as the first line of defense against pathogens. They are responsible for the elimination of abnormal cells in the body. Furthermore, they are involved in the production of substances involved in immune reactions. What is more, they release chemokines that drive the recruitment of neutrophils that are integral to repair processes [4,5]. Having considered the foregoing, with such important skin cell functions in mind, it is necessary to study the number, structure and accumulation of pigment particles in the cells, as well as the size of the pigment particles. Therefore, many researchers recommend undertaking targeted studies of tattoo particle overaccumulation in skin cells, i.e., macrophages and fibroblasts [6,7].
Substances contained in tattoo dyes and their decomposition products can pose a risk to human health. That is why it is so important to identify the sites of pigment accumulation in cells and organs, and to analyze the impact of these molecules on the human body. It is worth mentioning that the energy of laser light absorbed by the pigment causes a number of changes. It is converted to heat and the photothermal effect results in the internal breaking of chemical bonds, causing the breakdown of pigment molecules (photothermolysis). Small pigment particles, unknown products of their decomposition and newly produced chemical compounds are then removed from the skin by the blood and lymphatic system. They can pose a threat to the proper functioning of the body [8,9]. Animal experiments have shown that about a third of the injected tattoo ink disappeared from the skin within a few weeks after tattooing. It is assumed that some of the tattoo pigments remain in the skin because the pigment particles are insoluble and too large to be transported, such as to the lymphatic or bloodstream. Some tattoo dyes appear in the lymph nodes next to the tattoo. Thus, the accumulation of tattoo pigments in human body cells (lymphocytes, fibroblasts, macrophages) should be constantly monitored in terms of their negative effects on the human body.
In his study, Sepehri points to the accumulation of tattoo nodules in distant organs of the human body such as the liver in Kupffer cells [10]. According to Strandt, the injected pigment can accumulate in the cytoplasm of various cells, such as keranocytes, macrophages, mast cells or fibroblasts [11]. Fujita showed that ink particles (ranging in size from about 0.5 to about 4 micrometers) were endocytosed by fibroblasts and macrophages in the dermis and subcutaneous tissue [12]. It appears that after the ink is injected into the tattoo, bound pigment particles can leave the skin through the vascular system and migrate to the lymphatic system (lymph nodes) [13]. Because of that, it is clear that tattoo inks contain nanoparticles. However, their toxicological potential and the effects of tattoo ink ingredients on cells and collagen fibers are unknown.
Literature Review
The aim of the study was to determine the depth and site of penetration of pigment clumps in relation to skin and epidermal thickness in tattooed men. We evaluated skin cells: Macrophages and fibroblasts, and the depth of penetration of pigment lumps in relation to and thickness of the dermis and epidermis in tattooed men.
Tattooed skin fragments measuring 3 × 3 cm (including subcutaneous tissue), taken from human cadavers from the deltoid, thorax, abdomen and lateral aspect of the arm, were studied. Human skin sections were taken from 26 men ranging in age from 21 to 60. The study was approved by the bioethics committee at the Medical University of Lublin, with the number KE-0254/156/2020. Skin fragments were taken with the approval of the relevant prosecutor, during forensic medical autopsies. The material was obtained through cooperation with the Medical University of Lublin (Department and Laboratory of Forensic Medicine). The study focused on the morphology and morphometry of the different layers of tattooed human skin, and was conducted in cooperation with the University of Rzeszow, faculty of medicine (College of Medical Sciences, Department of Histology).
The first stage of the research was to take fragments of tattooed skin from human cadavers using a scalpel. Immediately after collection, the sections were fixed with a solution of 4% buffered formalin to prevent cellular necrotic and degenerative changes.
The second stage of the study involved the technical preparation of histological preparations so as to obtain a permanent, stained tissue specimen of a thickness that would enable analysis of its structure. To do this, the tissue was embedded in a harder, but easily cut and biochemically inert material-paraffin. Paraffin embedding is the classic method of preparing specimens from biological material for histological examination by light microscopy. The formalin-fixed tissue was subjected to dehydration in a series of alcohols of increasing concentration (60%, 70%, 80%, 90% and 100%) until the water was completely removed. The alcohol was then replaced with organic solvents (mixing with both alcohol and liquid paraffin). Skin specimens prepared in this way were embedded in paraffin, with a temperature slightly above its melting point (typical melting point 56-60°C). After the paraffin blocks prepared in this way coagulated and cooled, the skin specimens were cut on a microtome to a thickness of 4 μm (Figure 2). Cut sections of material were placed centrally on adhesion slides for further study (Figure 3).
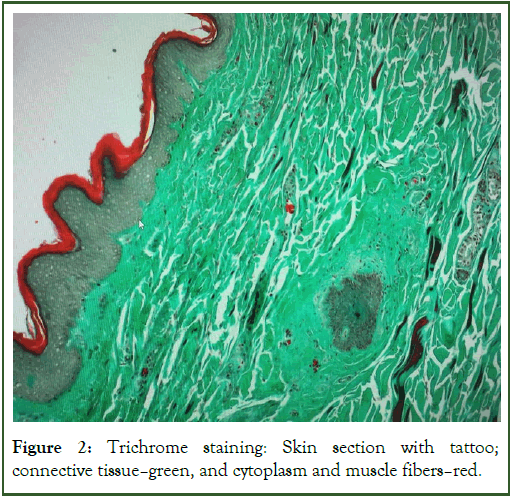
In the next step, a routine Hematoxylin and Eosin (HE) solution staining technique was performed, allowing the analysis of skin layers and their individual cellular structures using an optical microscope. Standard morphological staining with Hematoxylin and Eosin (HE) was performed using a ready to use solution used in routine staining of human material-sigma-aldrich, Germany. Trichrome staining (Masson goldner) was also used, which made it possible to observe the distribution of fat in the tissue, the distribution and thickness of the layer of collagen fibers and infiltrating cells.
First, the specimen paraffin was removed by immersing the specimens in two series of xylene, and then dehydrated in a series of alcohols (ethanol) of decreasing concentration (100%, 95%, and 80%). In the next step, the slides were rinsed with tap water and then stained in the previously mentioned dyes. The specimens were again dehydrated in an alcoholic series to end up in xylene again. In the final stage, the slides were sealed by applying a drop of sealing medium (synthetic resins-DPX in this case-are currently used) to the surface of the specimen and gently placing a coverslip. Once the resin is polymerized, the preparation becomes stable and ready for microscopic analysis. This stage was carried out in co-operation with the University of Rzeszow, Faculty of Medicine (College of Medical Sciences, Department of Histology).
The next stage of the study was microscopic evaluation, which included a general description of the tissue, analysis of morphological and morphometric parameters of the skin layers and its individual structures. The foregoing analyses were performed using an olympus BX43 light microscope equipped with an olympus SC50 digital camera, photographed and measured using olympus CellSence standard software for microscope image acquisition, archiving and conducting morphometric measurements. Measurements (an average of 170 measurements per agent) included the depth of tattoo pigment deposition in the skin, the height of the epidermis and the thickness of the dermis
In the next stage of the study, immunohistochemical staining was performed to identify leukocytes containing Leukocyte Common Antigen (LCA) without T and B lymphocyte divisions and inflammatory mediator cells-CD68 macrophages.
Immunohistochemical (IHC) staining is a routinely used technique to determine the localization and evaluation of cellular macromolecules in fixed preparations. The method is based on selective binding of the antibody to a specific epitope (e.g. LCA antigen, CD68 macrophage differentiation clusters, etc.). The interaction between the antibody and the antigen can then be visualized directly or indirectly, by means of a reaction with a chromogen using reporter enzymes such as Horseradish Peroxidase (HRP) or Alkaline Phosphatase (AP).
For direct detection, the primary antibody (AbI) itself is labeled with HRP or AP enzymes. The preferred method of detection is the indirect technique (which was used in this study). It involves the use of an unlabeled primary antibody (the so called first-tier antibody) directed against the epitope/antigen of interest. Then, a "labeled" secondary antibody (the so called second-tier antibody) is used to recognize such an immune complex. A routine avidin-streptavidin complex (ABC) method was used to visualize the immune response. The ABC method is a three step detection method: The primary antibody binds to the antigen in the tissue. The resulting complex is then recognized by a biotinylated secondary antibody. Complexes formed by Avidin and Biotinylated Enzyme (ABC) can then attach to the latter. Since the avidin tetramer can bind four biotin, large ABC grids containing several reporter enzymes are formed, leading to strong signal amplification at the antigenic site.
Although antigen-antibody binding is characterized by high specificity, there is the possibility of non-specific interactions (e.g. through electrostatic interactions) between the test material and plasma proteins, including antibodies. In order to prevent these nonspecific reactions, before the actual immunohistochemical reaction is carried out, a so-called blocking is performed, which involves incubating the slides with immunologically inactive (containing no specific antibodies) serum from an animal species other than the one from which the antibodies were obtained.
The next step in the study involved microscopic evaluation of individual structures to localize lymphocytes and macrophages indicative of inflammation in the tissue. The foregoing analyses were performed using a microscope and a PreciPoint M8 scanner under 20X magnifications. A reference preparation from the palatine tonsil was used as a control.
The data obtained were statistically processed using statistica 13.0-Dell Inc. (2016) (Dell statistica data analysis software system, version 13. software.dell.com).
For statistical analysis of the results (comparisons of pigment depth, thickness of the dermis and epidermis depending on the donor site) used one-way analysis of variance according to a mathematical model:
yijl=m+ai+eijl
yij-The value of the studied feature (pigment depth, dermis thickness, epidermal thickness)
ai-The effect of the ith level of factor A (place of intake)
eijl-Random error
The significance of the correlation coefficient was verified at p ≤ 0.05.
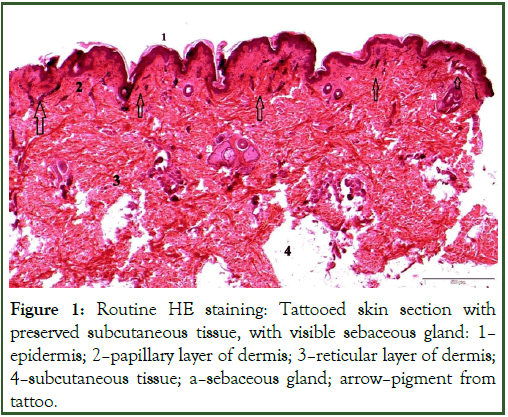
The HE staining technique used made it possible to visually analyze the structure of skin tissues-especially connective and epithelial tissue, and allowed us to determine how deep into the dermis the tattoo pigment penetrates (Figure 1).
In most of the observed preparations, the tattoo-derived pigment was localized under the epidermis, mainly in the papillary layer, and was very rarely visible in the reticular layer. Localization of the pigment at the blood vessels was often observed.
A significant relationship was found between the depth of embedded pigment and the thickness of the dermis and epidermis. Such correlations were observed in all tattoo locations: The deltoid, thoracic, abdominal and arm region. As the thickness of the epidermis and dermis increased, the depth of embedded pigment also increased.
The deepest pigment was deposited in a sample taken from a tattoo located on the forearm (1.2 mm), a depth that was not significantly different from a tattoo located on the abdomen (0.8 mm). The deltoid (0.1 mm) and thoracic (0.2 mm) areas had the shallowest pigment deposition (p ≤ 0.05 significance compared to abdomen, chest, forearm and deltoid area; p ≤ 0.05 significance compared to forearm and chest).
There is a significant relationship between the depth of the deposited pigment and the thickness of the dermis and epidermis. Such correlations were observed in all tattoo locations: The deltoid, thoracic, abdominal and arm region. As the thickness of the epidermis and dermis increased, the depth of embedded pigment also increased.
The depths of pigment penetration and retention, skin thickness and epidermal thickness were found to show statistically significant differences depending on the location of the tattoo. (- p ≤ 0.05 compared to abdomen and chest and forearm and deltoid area; *p ≤ 0.05 compared to forearm and chest).
Basic topographic HE and trichrome staining allowed observation of tattoo ink distribution in the tissue, distribution and thickness of collagen fiber layers, cell cytoarchitectonics and infiltrating cells. During the study, it was observed that some of the cells (macrophages) contain dye granules within them (macrophages), which would indicate the importance of phagocytosis in the removal of ink from the body. Free ink particles were found among collagen fibers (Figures 1 and 2), respectively.
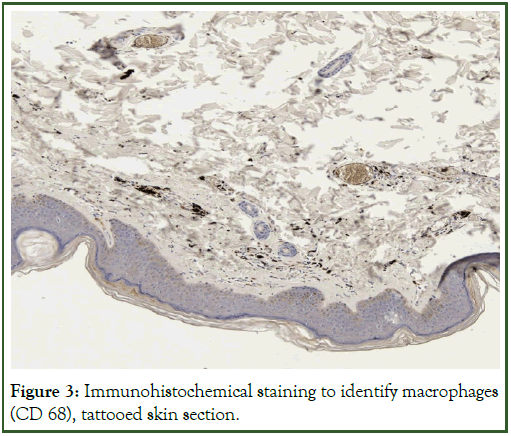
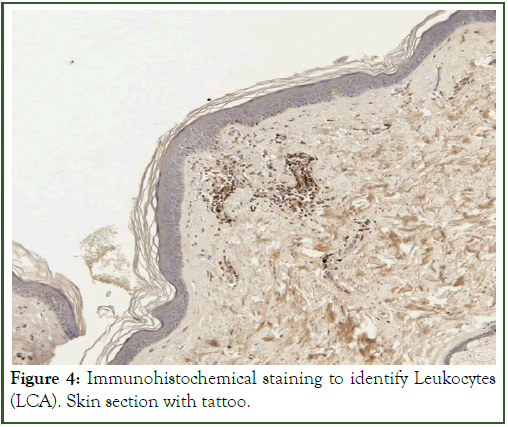
The immunohistochemical staining technique used indicated the presence of leukocytes and macrophages as infiltrative cells, while confirming the inflammation present around the dye papules (Figures 3 and 4), respectively. The study should be expanded, because chronic inflammation around the tattoo pigment can be associated with serious complications and even consequent diseases in distant organs, not only in the skin.
Figure 1: Routine HE staining: Tattooed skin section with preserved subcutaneous tissue, with visible sebaceous gland: 1-epidermis; 2-papillary layer of dermis; 3-reticular layer of dermis; 4-subcutaneous tissue; a-sebaceous gland; arrow-pigment from tattoo.
Figure 2: Trichrome staining: Skin section with tattoo; connective tissue-green, and cytoplasm and muscle fibers-red.
Figure 3: Immunohistochemical staining to identify macrophages (CD 68), tattooed skin section.
Figure 4: Immunohistochemical staining to identify Leukocytes (LCA). Skin section with tattoo.
Discussion
In view of reports, related to the harmfulness of tattooing, a study was conducted to evaluate the accumulation of tattoo dye lumps in skin cells (macrophages and fibroblasts), the cells responsible for normal skin function [14].
Macrophages were found to contain granules of tattoo dye, which would indicate the importance of phagocytosis in ink removal from the body. The immunohistochemical staining technique used further established that inflammation was present around the dye lumps, as indicated by the presence of lymphocytes, macrophages and neutrophils. Free ink particles were also found among collagen fibers.
Observations on the localization of pigment in skin cells were also described by Sleth, where tattoo ink is initially distributed fairly evenly in the skin, and was phagocytosed by fibroblasts, macrophages and mast cells during the healing process [15]. This was also demonstrated by a recent study by van der Bent, et al. wherein single biopsies showed pigment particle accumulation inside macrophages, as well as between collagen bundles [16].
The authors also described cases where ink particles and inflammatory infiltrates (lymphocyte infiltrates) had spread into the subcutaneous tissue. It is presumed that during the healing phase, an unknown fraction of the pigment is transported from the skin through the lymphatic system, as also documented in their study by Engel, et al. and later Schreiver, et al. [17].
Permanent tattoos are created by injecting ink containing solids through the epidermis into the dermis, where the pigment is usually deposited at the top of the skin, up to a third of its thickness. In some cases, injected tattoo ink can cause a skin reaction (allergy, inflammation or infection). Histopathological analysis of tissue taken from a typical tattoo reveals nodules of pigment located in the dermis and skin macrophages. The amount and distribution of pigment depends on factors such as: The depth of the needle insertion, the area of the body in which the tattoo is made and the age of the tattoo. Initially, the ink is distributed fairly evenly, then it is phagocytized by fibroblast cells, macrophages and mast cells and some of the pigment enters the lymphatic system.
According to the observations of Sepehri, particles of tattoo pigment are deposited in the skin and distributed to regional lymph nodes with the help of the circulatory system. According to the author, tattoo pigments are small particles and can be assumed to enter the bloodstream and be distributed to peripheral organs. Sepehri analyzed the movement of pigment particles into internal organs on an animal model. This author has identified tattoo pigment in the skin and lymph nodes, and both groups of tattooed mice showed deposits of tattoo pigment in Kupffer cells in the liver.
Our study analyzed the distribution of tattoo particles only in individual layers of skin (post-mortem static). It was found that the tattoo-derived pigment was localized under the epidermis in most of the analyzed preparations, mainly in the papillary layer, and was very rarely visible in the reticular layer of the skin. The accumulation of pigment near blood vessels is noteworthy, especially observed in older tattoos. The depth of pigment penetration depended on the location of the tattoo. Such correlations were observed in all tattoo locations: The deltoid, thoracic, abdominal and arm region. The pigment was most deeply deposited in a sample taken from a tattoo located on the forearm. The pigment was deposited most shallowly in the deltoid and thoracic area. Based on the observations that the authors of the publication made, tattoos located on the forearm were well-preserved compared to those located on the abdomen or forearm.
Tattoo permanence was analyzed in an animal model (mice) by Strand. She found that skin macrophages and fibroblasts contribute to tattoo stability in different ways, and will further advance the understanding of tattoo permanence. Our studies confirm the presence of pigment particles in fibroblasts and macrophages.
Latest research by Helen Strandt report that dermal macrophages play an important role in the storage and maintenance of pigment particles and influence their persistence. Strandt, et al. compared the cell number and particle load in dermal macrophages with dermal fibroblasts isolated from the skin of the tail of tattooed mice. A small number of macrophages contained a large number of pigment particles, while a large number of dermal fibroblasts contained only a few pigment particles.
In her research, Baranska showed that macrophages play an important role in the durability of a tattoo. Tattoo pigment particles contained in macrophages can undergo successive capture cycles without the tattoo disappearing. Therefore, consistent with dermal macrophage dynamics, the long-term durability of a tattoo likely depends on macrophage renewal rather than macrophage longevity. The study was conducted using an animal model (mouse). Moreover, regarding ink phagocytosis, there is evidence that ink phagocytosis is not proof of ink removal, because in fact macrophages may not break down and remove ink and therefore may release ink in the dermis [13].
Other authors emphasize the role of fibroblasts in preventing inflammatory processes in the skin, on which the tattoo was applied. For example, Fujita stresses the role of fibroblasts and points out that the uptake and long-term storage of ink particles by fibroblasts in the dermis and subcutaneous layer of the mice prevents skin inflammation [14].
Fujita found that ink particles were endocytosed by fibroblasts and macrophages in the dermis and subcutaneous tissue. The author believes that the fibroblasts that take up and store the ink molecules move more slowly, therefore the tattoos do not change significantly. He stresses that the long-term storage of ink particles by skin fibroblasts is a kind of non-inflammatory defense mechanism that protects the living organism from toxic tattoo lumps [14].
According to Sletht, after a few months, when the tattoo is finally healed, the ink particles are found only in the fibroblasts of the skin, trapped in the connective tissue network [15].
A study by Shinohara showed that the amount and distribution of pigment in the skin also depends on the age of the tattoo. Older tattoos have been reported to show a decrease in free pigment and its proliferation in perivascular macrophages. This may be due to exfoliation of the epidermis immediately after ink injection, transfer of some pigments to the lymphatic system, or even intradermal degradation under ultraviolet radiation, i.e., under the influence of light [3].
Some of the pigment is gradually assimilated by macrophages and is eventually deposited in the layers of the dermis during regeneration. This has also been evidenced by a recent study by Van der Bent, et al. who determined the depth of inflammation and pigment penetration, finding that inflammatory infiltrates and pigment particles were mainly confined to the upper and lower reticular layers of the dermis. In individual biopsies, ink particles and inflammatory infiltrates spread into the subcutaneous tissue. According to their study, pigment particles can be seen inside macrophages and also independently between collagen bundles in the upper and middle dermis [16]. Grant conducted studies on cultured fibroblasts. This author has studied the direct effect of ink pigment on cell viability. This resulted in finding an accumulation of tattoo particles in fibroblasts [17].
Conclusion
Based on the results we received and the publications we analyzed, it should be concluded that the research on tattooing, its components and its effects on the skin and the entire body, is most legitimate and must continue.
It is recommended to conduct further analysis on the depth of tattoo pigment penetration, with particular attention to the location and number of fibroblasts, macrophages and possible inflammatory reactions caused by the tattooing process.
Statement on the Conflict of Interest
The authors declare that they have no overt or potential conflicts of interest related to the publication of this article.
Author’s Contribution
All authors contributed to all of the following: The conception and design of the study, or acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be submitted.
References
- Engel E, Vasold R, Santarelli F, Maisch T, Gopee NV, Howard PC, et al. Tattooing of skin results in transportation and light-induced decomposition of tattoo pigments-A first quantification in vivo using a mouse model. Exp Dermatol. 2010;19(1):54-60.
[Crossref] [Google Scholar] [PubMed]
- Hogsberg T, Loschner K, Lof D, Serup J. Tattoo inks in general usage contain nanoparticles. Br J Dermatol. 2011;165(6):1210-1218.
[Crossref] [Google Scholar] [PubMed]
- Shinohara MM, Nguyen J, Gardner J, Rosenbach M, Elenitsas R. The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol. 2012;39(12):1110-1118.
[Crossref] [Google Scholar] [PubMed]
- Golab J, Jakobisiak M, Lasek W, Stoklosa T. Immunologia. Warszawa: Wydawnictwo Naukowe PWN. 2007:1-511.
- Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1.
[Crossref] [Google Scholar] [PubMed]
- Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10(5):520.
[Crossref] [Google Scholar] [PubMed]
- Hering H, Zoschke C, Konig F, Kuhn M, Luch A, Schreiver I. Phototoxic versus photoprotective effects of tattoo pigments in reconstructed human skin models: In vitro phototoxicity testing of tattoo pigments: 3D versus 2D. Toxicology. 2021;460:152872.
[Crossref] [Google Scholar] [PubMed]
- Hering H, Zoschke C, Kuhn M, Gadicherla AK, Weindl G, Luch A, et al. TatS: A novel in vitro tattooed human skin model for improved pigment toxicology research. Arch Toxicol. 2020;94(7):2423-2434.
[Crossref] [Google Scholar] [PubMed]
- Baumler W. Chemical hazard of tattoo colorants. Presse Med. 2020;49(4):104046.
[Crossref] [Google Scholar] [PubMed]
- Sepehri M, Sejersen T, Qvortrup K, Lerche CM, Serup J. Tattoo pigments are observed in the Kupffer cells of the liver indicating blood-borne distribution of tattoo ink. Dermatology. 2017;233(1):86-93.
[Crossref] [Google Scholar] [PubMed]
- Olszewska J, Charuta A, Paziewska A, Wawrzyniak A, Baj J, TeresiÅ?ski G, et al. The relation between the depth of pigment disposition and men’s skin thickness, the age and tattoo locations on the body. Histol Histopathol. 2023;38(5):503-511.
[Crossref] [Google Scholar] [PubMed]
- Strandt H, Voluzan O, Niedermair T, Ritter U, Thalhamer J, Malissen B, et al. Macrophages and fibroblasts differentially contribute to tattoo stability. Dermatology. 2021;237(2):296-302.
[Crossref] [Google Scholar] [PubMed]
- Baranska A, Shawket A, Jouve M, Baratin M, Malosse C, Voluzan O, et al. Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal. J Exp Med. 2018;215(4):1115-1133.
[Crossref] [Google Scholar] [PubMed]
- Fujita H, Nishii Y, Yamashita K, Kawamata S, Yoshikawa K. The uptake and long-term storage of India ink particles and latex beads by fibroblasts in the dermis and subcutis of mice, with special regard to the non-inflammatory defense reaction by fibroblasts. Arch Histol Cytol. 1988;51(3):285-294.
[Crossref] [Google Scholar] [PubMed]
- Sleth JC. Epidural anaesthesia and lumbar tattoo. Histology of tattoo: The missing link?. Ann Fr Anesth Reanim. 2007;26(3):266-267.
[Crossref] [Google Scholar] [PubMed]
- van der Bent S, Oyen E, Rustemeyer T, Jaspars L, Hoekzema R. Histopathology of red tattoo reactions. Am J Dermatopathol. 2021;43(5):331-337.
[Crossref] [Google Scholar] [PubMed]
- Grant CA, Twigg PC, Baker R, Tobin DJ. Tattoo ink nanoparticles in skin tissue and fibroblasts. Beilstein J Nanotechnol. 2015;6(1):1183-1191.
[Crossref] [Google Scholar] [PubMed]
Citation: Charuta A, Paziewska A, Luszczewska–Sierakowska I, Olszewska MGJ, Kosowska M, Wawrzyniak A (2025) Accumulation of Pigment Particles Used in Tattooing in Human Skin-Results of Histological Studies. J Dermatitis. 10:261.
Copyright: © 2025 Charuta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.