Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Study - (2023) Volume 9, Issue 2
A Young Male with Neurological Deficits: Diagnosis of Paroxysmal Nocturnal Hemoglobinuria
Tushar Menon1, Ameera C Mistry2*, Ryan Mcauliffe1 and Shahin Bhagwagar12Independent Researcher, International American University College of Medicine, Vieux Fort, Saint Lucia, USA
Received: 08-Mar-2023, Manuscript No. JTCOA-23-20081; Editor assigned: 10-Mar-2023, Pre QC No. JTCOA-23-20081 (PQ); Reviewed: 24-Mar-2023, QC No. JTCOA-23-20081; Revised: 31-Mar-2023, Manuscript No. JTCOA-23-20081 (R); Published: 10-Apr-2023, DOI: 10.35248/2572-9462.23.9.211
Abstract
Paroxysmal Nocturnal Hemoglobinuria is a rare acquired disorder of the blood, characterized by the destruction of red blood cells, blood clots, and impaired bone marrow function. The resulting symptoms of PNH can vary widely, but may include fatigue, weakness, shortness of breath, abdominal pain, and an increased risk of both venous and arterial thrombosis, as well as other serious complications. The diagnosis of PNH is made through laboratory testing, including flow cytometry and genetic testing. Although there is no cure for PNH, proper diagnosis and treatment can help manage symptoms and improve quality of life for those living with the disease. Discussed here is a case of a 22 year-old male with PNH that presented with an arterial stroke and symptoms of acute right-sided hemiparesis. A Computed Tomography angiography of head and neck showed an occlusion of the left middle cerebral artery at the M1 segment. Anticoagulant therapies was initiated and following MRI and CT scan confirmed the obstruction; while a hypercoagulable work-up came back positive for a PNH diagnosis. Treatment with eculizumab and a meningococcal vaccination was administered. The patient regained motor function with physical and occupational therapy.
Keywords
Paroxysmal nocturnal hemoglobinuria; PIG-A gene; Hemoglobinopathy; Hematopoietic stem cell disorder; Thrombosis; Hypercoagulable; Eculizumab
Abbreviations
PNH: Paroxysmal Nocturnal Hemoglobinuria; CTA: Computed Tomography Angiography; MCA: Middle Cerebral Artery; TPA: Tissue Plasminogen Activator; DVT: Deep Vein Thrombosis; TTE: Trans Thoracic Echocardiogram; TEE: Trans Esophageal Echocardiogram; RLE: Right Lower Extremity; GPI: Glycosylphosphatidylinositol; RBCs: Red Blood Cells; GPI-AP: Glycosylphosphatidylinositolanchored proteins: FCMI: Flow Cytometric Immunophenotyping; GCT: Gel Card Test; SLT: Sucrose Lysis Test; DOACs: Direct Oral Anticoagulants; LMWH: Low Molecular Weight Heparin
Introduction
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare disease, with an estimated prevalence of 1.5-2 cases per one million people. It is typically diagnosed in young adulthood, and affects both men and women equally. It is an acquired genetic disorder of the blood cells. It is caused by a mutation in the PIG-A gene, which leads to a deficiency in the complement regulatory proteins on the surface of red blood cells, white blood cells, and platelets [1]. The deficiency in these complement regulatory proteins causes the immune system to attack and destroy these blood cells, leading to a wide range of symptoms and complications.
The destruction of red blood cells leads to anemia, which can cause fatigue, weakness, and shortness of breath. Additionally, the breakdown of red blood cells releases hemoglobin into the bloodstream, which can damage to the lining of blood vessels. This damage leads to a variety of complications, such as thrombosis and pulmonary hypertension. The destruction of platelets can lead to bleeding and bruising while the destruction of white blood cells can lead to an increased risk of infections.
The name "Paroxysmal Nocturnal Hemoglobinuria" reflects the main symptoms of the disease: paroxysms, or sudden attacks, of hemoglobin in the urine that occur primarily at night. However, not all people with PNH experience this symptom, and the disease can cause a wide range of other symptoms and complications. Some of the most common symptoms of PNH include fatigue, weakness, shortness of breath, abdominal pain, and difficulty swallowing.
PNH can also increase the risk of thrombosis, or blood clots, and other serious complications, such as kidney failure and pulmonary hypertension [2].
PNH is a complex disease that can be challenging to diagnose and manage. It is typically treated with medications, such as eculizumab, that reduce the destruction of red blood cells and manage the underlying immune system dysfunction. Stem cell transplantation may also be considered in some cases. A hematologist or other specialist with experience in treating PNH can help guide diagnosis and treatment.
Case Presentation
A 22 year-old male presented to emergency department with acute onset right-sided hemiparesis and dysarthia on 01/02/2023. A Computed Tomography Angiography (CTA) of the head and neck showed occlusion of left Middle Cerebral Artery (MCA) at the M1 segment. Due to the short duration of symptoms, and no obvious contraindications, TPA was administered. Interventional neurology performed a cerebral angiogram with thrombectomy that the patient tolerated well. He was then admitted to the ICU post-TPA administration and surgical thrombectomy. The patient continued to have expressive aphasia the following day; however, right-sided hemiparesis had resolved.
Physical and occupational therapy, as well as speech-language pathology, evaluated the patient and recommended intensive inpatient therapy 3 hours a day, 5 times per week. A hypercoagulable panel and vascultitis panel were performed and results were pending. A lower extremity venous ultrasound showed no presence of a Deep Vein Thrombosis (DVT). Trans Thoracic Echocardiogram (TTE) and Trans Esophageal Echocardiogram (TEE) showed no presence of a patent foramen ovale, no valvular abnormalities, no valvular vegetation’s, and showed a preserved ejection fraction. A second MRI showed acute infarcts in right frontal, septal, and cerebellum. Rapid response was called on 01/05/2023 after the patient had undergone a TEE when a nurse reported he was unresponsive and developed right-sided facial droop. Patient received an immediate CT without contrast and a CTA, which revealed a new large vessel occlusion in the MCA. The patient underwent a second thrombectomy on 01/05/2023 (Figure 1).
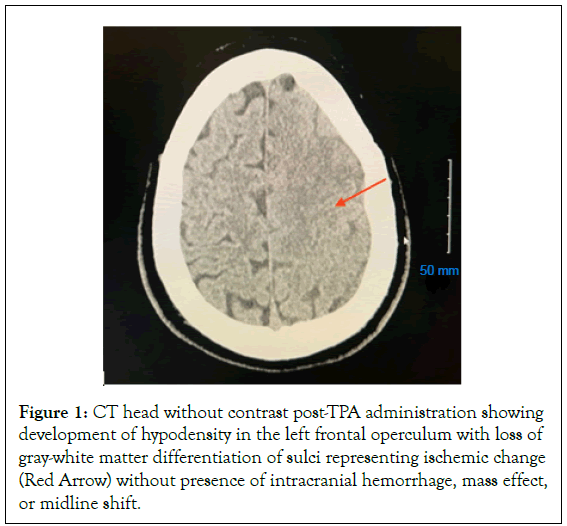
Figure 1: CT head without contrast post-TPA administration showing development of hypodensity in the left frontal operculum with loss of gray-white matter differentiation of sulci representing ischemic change (Red Arrow) without presence of intracranial hemorrhage, mass effect, or midline shift.
Following the second thrombectomy, he experienced a reemergence of right-side hemiparesis with significantly increased expressive aphasia, as well as newly developed confusion. After consultation with interventional neurology, the patient was placed on a heparin drip on 01/06/2023. Hypercoagulable work-up came back positive for a diagnosis of PNH. Patient remained on heparin drip. Hypercoagulable work-up came back positive for a diagnosis of paroxysmal nocturnal hemoglobinuria. Heme-oncologist was then consulted and recommended beginning treatment of eculizumab, ciprofloxacin for 14 days, and a meningococcal vaccination. Patient was transitioned from heparin to Eliquis (Figures 2 and 3).
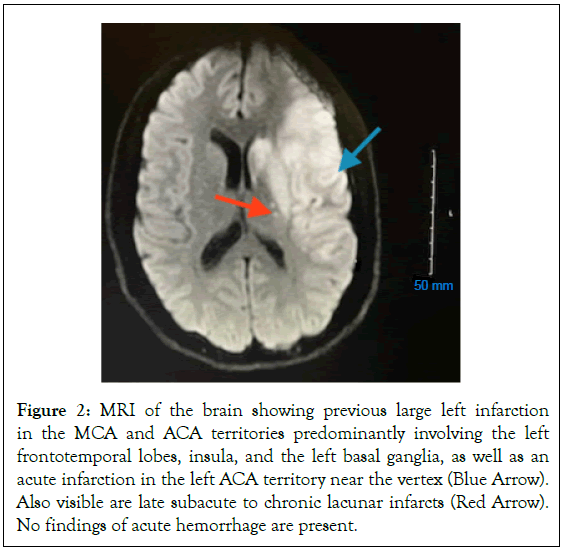
Figure 2: MRI of the brain showing previous large left infarction in the MCA and ACA territories predominantly involving the left frontotemporal lobes, insula, and the left basal ganglia, as well as an acute infarction in the left ACA territory near the vertex (Blue Arrow). Also visible are late subacute to chronic lacunar infarcts (Red Arrow). No findings of acute hemorrhage are present.
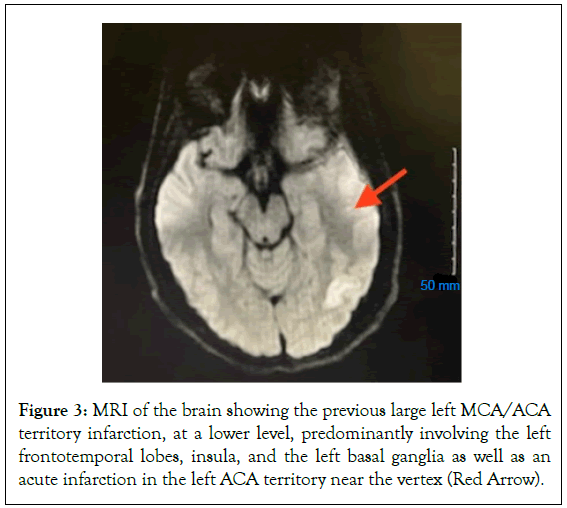
Figure 3: MRI of the brain showing the previous large left MCA/ACA territory infarction, at a lower level, predominantly involving the left frontotemporal lobes, insula, and the left basal ganglia as well as an acute infarction in the left ACA territory near the vertex (Red Arrow).
Patient regained motor function and showed signed of slowly improving dermatomes of the Right Lower Extremity (RLE). Left upper and lower extremities remained intact. Patient continued to have expressive aphasia, but showed improvement with the addition of words to his vocabulary overtime. With repeated treatment with physical therapy, he improved mobility by using a cardiac walker to take several steps with assistance. With the physical improvements overtime, it was recommended that the patient would benefit greatly from intensive inpatient rehabilitation services, including physical therapy, occupational therapy, and speech therapy. With the recommendations, the patent was well motivated and his family was supportive of the patient’s treatment recommendations and current progress. He will require continued follow-up with specialists including neurosurgery, neurology and hematologyoncology. Patient was scheduled for second infusion of eculizumab on 01/20/2023.
Results and Discussion
Paroxysmal Nocturnal Hemoglobinuria is a rare acquired hematopoietic stem cell disorder that results in hemolysis, or the destruction of Red Blood Cells (RBCs), as well as thrombosis, or the formation of blood clots. The underlying pathophysiology of PNH is due to a mutation in the PIG-A gene, which is located on the X chromosome and encodes for a protein required for the synthesis of Glycosylphosphatidylinositol (GPI) anchors. GPI anchors are important for attaching various proteins to the cell surface, particularly RBCs, including complement regulatory proteins such as CD55 and CD59 [3]. The lack of complement regulatory proteins on the surface of RBCs also leads to the activation of platelets and endothelial cells, which can promote the formation of blood clots in the arterial and venous systems. Overall, the pathophysiology of PNH involves a complex interplay between the immune system, the complement system, and blood cells, leading to the destruction of these cells and the development of a range of symptoms and complications.
Recent ICCS guidelines recommend using blood obtained peripherally over bone marrow samples to test for PNH. Bone marrow samples are not ideal for routine clinical laboratory testing due to immature myeloid cells often lack uniform expression of “GPI-AP” [4]. Additionally, in MDS, the altered expression of CD16 on granulocytes are difficult to interpret in the presence of smaller PNH clones and can affect diagnostic results [4]. Prior to 1990, PNH was diagnosed using complement-based tests, which has been replaced with the gold standard diagnostic test of flow cytometry [5]. The transition in testing is due to the increased sensitivity of flow cytometry in detecting small clones, especially those not affected by blood transfusions [5]. Gel card test utilizes the antigen-antibody reaction, Ham test and SLT test utilizes the activated complement protein-mediated cell lysis of PNH cells [4]. Flow cytometry utilizes monoclonal antibodies tagged with fluorochromes that bind specifically to “GPI-AP” on peripheral blood cells and bind directly to GPI-anchors [4].
In a study by Gupta et al, 50 patients were evaluated using four available diagnostic testing techniques used in clinical practice, including: Flow Cytometric Immunophenotyping (FCMI) evaluating CD55 and 59, PNH Gel Card Test (GCT), Ham test, and Sucrose Lysis Test (SLT) [6]. It was found FCMI was the most sensitive and can quantify and delineate PNH cells with differential expression of GPI-anchored proteins and detected a PNH clone in 28% of patients, compared to 26% by GCT and 20% by SLT and the Ham test [6]. The GCT and SLT tests showed 100% specificity and sensitivity was 92.8% and 71.1%, respectively; with GCT test correlating with type III cells, compared to CD59 negative type III cells via SLT test. Both these tests were similar in their sensitivity to detect type II cells [6].
Clinically, the GCT test is a useful screening tool, as it is fairly sensitive and easy to interpret, as well as easy to perform [6]. GCT test is not quantitative and does not pick up smaller clones, and may not detect PNH cells after a blood transfusion or during hemolytic episodes [4]. The Ham and SLT tests are inexpensive, but require a lot of time by a skilled technician; so routine testing in a clinic is not ideal. The two tests are also not quantitative and their results are compromised by blood transfusions and during hemolytic episodes. The test can also give false positives in congenital dyserythropoietic anemia type II, megaloblastic anemia, and autoimmune hemolytic anemia [4]. Flow cytometry is the most sensitive, and assesses the clone size and delineation of types I, II, and II cells and is the “gold standard” [4].
The risk of thrombosis in those affected with PNH is increased compared to the general population. The loss of GPI-anchored proteins can lead to a range of clinical manifestations, including arterial and venous thrombosis; however, the exact mechanism by which PNH increases the risk of thrombosis is not fully understood. It is thought that arterial thrombosis can occur due to the presence of platelet aggregates and micro-particles, as well as the activation of the coagulation system; which can lead to the formation of clots in the blood vessels [7]. Arterial thrombosis in PNH is more commonly associated with the cerebrovascular system, resulting in strokes; but can also affect other organs, such as the kidneys, heart, and gastrointestinal tract. Venous thrombosis in PNH is thought to be caused by a combination of factors, including platelet activation, endothelial damage, and activation of the coagulation system [8]. The most common sites of venous thrombosis in PNH are the hepatic and portal veins, leading to portal hypertension and potentially life-threatening complications such as Budd-Chiari syndrome [2]. Individuals with PNH much more commonly present with venous thrombosis (80%-85% of the time) compared to arterial thrombosis (15%-20% of the time) Patients may present with symptoms of both arterial and venous thrombosis, such as chest pain, shortness of breath, and leg swelling, or sudden onset of severe headache.
Management of acute thrombotic events in PNH includes immediate use of ecluzumab and full dose anti-coagulation. Complement inhibition with eculizumab, a monoclonal antibody that targets the complement protein C5, has been shown to reduce the risk of thrombotic events in patients with PNH by blocking the formation of the terminal complement complex; which is thought to be involved in platelet activation and endothelial damage [9]. It is important that all patients on eculizumab be vaccinated against meningitis and placed on penicillin prophylaxis due to increased susceptibility to neisseria infections, particularly Neisseria meningitides, due to blockage of the terminal complement complex [9]. Anticoagulation therapy is typically initiated with full dose heparin or Low Molecular Weight Heparin (LMWH). The use of DOACs in the management of acute thrombosis in PNH has not been well studied; and as of right now is not recommended in current standard guidelines [9].
There is also little evidence on whether long term anti-coagulation should be continued once the acute thrombotic episode has resolved; however, current guidelines recommend continuing long-term anticoagulation [9]. With thrombotic events being the leading cause of morbidity and mortality in PNH, primary prophylaxis with full-dose oral anticoagulation has shown to reduce the risk of such events [10]. Around 29%-44% of PNH patients present with at least one thrombotic event [10]. Although oral anticoagulation does reduce the deleterious results of thrombotic events and poor outcomes, compliment inhibitors have become the mainstay therapy, reducing thrombotic risks by up to 80%; and thromboprophylaxis has virtually stopped in developed countries [10]. Clonal size >50% in granulocytes correlates with an increased in thrombotic risk and is hypothesized to be associated with no depletion, lack of “GPI-AP” to cause defective fibrinolysis and loss of inhibition of tissue factor [4]. The larger size of clonal cells is a stronger predictive factor for both increased thrombotic events without anticoagulation therapy, and complications on such therapy [11]. In a retrospective study with a group of 39 patients on primary prophylaxis anticoagulation, two warfarin-associated hemorrhages and one death occurred [11]. The patients that experienced the complications, both had erratic platelet counts and increased granulocyte clone sized, and the events occurred in their first year of treatments—the period when most hemorrhagic complications occur [11].
In addition to the previously mentioned therapies, it is also important for individuals with PNH to manage other risk factors for thrombosis, such as smoking, hypertension, and diabetes. Close monitoring by healthcare providers is essential for the management of reducing thrombotic risks in individuals with PNH.
The treatment of PNH is focused on controlling the symptoms and possible complications of the disease and improving the quality of life of the patient. There is no curative treatment for the disease. There are several treatment options available for PNH, including:
Eculizumab
The most common treatment for PNH is eculizumab—a monoclonal antibody. It blocks the activity of the complement system, which is overactive in PNH. Eculizumab is given intravenously every two weeks and has been shown to reduce the risk of hemolysis, blood clots, and the need for blood transfusions.
Stem cell transplant
In some cases, a stem cell transplant may be recommended for younger, healthier individuals. The goal of a stem cell transplant is to replace the defective bone marrow with healthy bone marrow, which produces normal blood cells that are not affected by the PIG-A mutation. This is a complex procedure that carries many risks of complications; it is generally reserved for younger patients with severe disease who have failed other treatments [12].
Red blood cell transfusions
Individuals with severe anemia may require blood transfusions to replace the RBCs that are destroyed in patients with PNH. Transfusions may be given on an as-needed basis, or on a regimented schedule, depending on the severity of the anemia.
Iron chelation therapy
Individuals who receive frequent blood transfusions may develop excess iron in their body, which can lead to hemochromatosis and eventual organ damage. Iron chelation therapy treatment helps to remove excess iron from the patient’s body.
Anticoagulants
Individuals with PNH are at increased risk of developing blood clots, so anticoagulants are recommended to reduce the risk of clots and the associated complications.
Investigational therapies
There are several investigational therapies for PNH, including gene therapy and complement inhibitors. These therapies are still in development and are not yet widely available or recommended as standard therapy.
Supportive care
Supportive care measures such as pain management, physical therapy, and antibiotics to treat infections may also be necessary to manage the symptoms and complications of PNH.
Overall, the choice of treatment depends on the severity of the disease, the risk or presence of complications, the patient's overall health, the individual patient's medical history and preferences, and the potential risks and benefits of each treatment option. With multiple ways to approach treatment of PNH, it is crucial to work with healthcare providers experienced in managing PNH to determine the best treatment approach for each individual patient.
Conclusion
After initial diagnostic work up of common hyper coagulation abnormalities, other risk factors such as IVC anomalies must be considered as a possible differential diagnosis in the adolescent patient. Despite its rarity, thrombosis secondary to IVC anomalies is a well-defined cause of bilateral lower extremity DVTs, especially in young adults (<30 years old). The diagnosis of IVCA poses a clinical challenge and requires detailed imaging studies. In this case, the patient required extensive pharmacomechanical therapy including acoustic pulse wave thrombolysis and Zelante AngioJet technology. The patient must adhere to lifelong anticoagulation with avoidance and careful consideration of risk factors such as smoking, estrogen containing oral contraceptives, and pregnancy to reduce risk of future thrombotic events.
Conflict of Interest
All authors agree for the publication. For all authors, there is no competing interests to declare.
References
- Ware RE, Rosse WF, Howard TA. Mutations within the Piga gene in patients with paroxysmal nocturnal hemoglobinuria. Blood. 1994;83(9):2418-2422.
- Malato A, Saccullo G, Coco LL, Mancuso S, Santoro M, Martino S, et al. Thrombotic complications in paroxysmal nocturnal haemoglobinuria: A literature review. Blood Transfus. 2012;10(4):428-435.
- Risitano AM, Rotoli B. Paroxysmal nocturnal hemoglobinuria: Pathophysiology, natural history and treatment options in the era of biological agents. Biol Targets Ther. 2008;2(2):205-222.
- Manivannan P, Ahuja A, Pati HP. Diagnosis of paroxysmal nocturnal hemoglobinuria: Recent advances. Indian J Hematol Blood Transfus. 2017;33(4):453-462.
- Madkaikar M, Gupta M, Jijina F, Ghosh K. Paroxysmal nocturnal haemoglobinuria: Diagnostic tests, advantages, & limitations. Eur J Haematol. 2009;83(6):503-511.
- Gupta R, Pandey P, Choudhry R, Kashyap R, Mehrotra M, Naseem S, et al. A prospective comparison of four techniques for diagnosis of paroxysmal nocturnal hemoglobinuria. Int J Lab Hematol. 2007;29(2):119-126.
- Hills A. Thrombosis in paroxysmal nocturnal Haemoglobinuria: Proposed mechanism of thrombosis in paroxysmal nocturnal haemoglobinuria. Blood. 2013;121(25):4985-4996.
- Van Bijnen ST, Van Heerde WL, Muus P. Mechanisms and clinical implications of thrombosis in paroxysmal nocturnal hemoglobinuria. J Thromb Haemost. 2012;10(1):1-10.
- Griffin M, Munir T. Management of thrombosis in paroxysmal nocturnal hemoglobinuria: A clinician’s guide. Ther Adv Hematol. 2017;8(3):119-126.
- Chavez EA, Rangel-Patiño J, Tuna-Aguilar EJ, Garcés-Martínez A, Demichelis R. Total oral anticoagulation for classic PNH as primary prophylaxis strategy in a complement inhibitor restricted scenario: A retrospective analysis. Blood. 2021;138:1118.
- Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in Paroxysmal Nocturnal Hemoglobinuria (PNH). Blood. 2003;102(10):3587-3591.
- Brodsky RA. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2010;95(6):855-856.
Citation: Menon T, Mistry AC, Mcauliffe R, Bhagwagar S (2023) A Young Male with Neurological Deficits: Diagnosis of Paroxysmal Nocturnal Hemoglobinuria. J Thrombo Cir. 9:211.
Copyright: © 2023 Menon T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
