Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2020) Volume 5, Issue 5
A Case of Severe Thyroid Eye Disease Treated with Tocilizumab
Aysel Mehmet1*, Eirini Kanella Panagiotopoulou1, Aristeidis Konstantinidis1, Charalampos Papagoras2, Panagiotis Skendros2, Doukas Dardabounis1, Athanasia Maria Mikropoulou1 and Georgios Labiris12Department of Internal Medicine, University Hospital of Alexandroupolis, Alexandroupoli, Greece
Received: 05-Oct-2020 Published: 27-Oct-2020, DOI: 10.35248/2684-1622.20.5.146
Abstract
This is a case report describing a patient with severe thyroid eye disease complicated with dysthyroid optic neuropathy that was unresponsive to intravenous steroids and orbital radiotherapy but responded well to intravenous tocilizumab.
Keywords
Thyroid eye disease; Tocilizumab; Dysthyroid; Optic neuropathy
Introduction
Thyroid Eye Disease (TED) is an inflammatory disorder of the orbit secondary to Graves’ disease. Although the inflammatory phase is self-limiting it can severely affect the vision. Sight threatening TED is rare. The overall incidence of TED is 0.42/10,000/year with women and the 40-60 age group being mostly targeted by the disease [1]. Only about 2% of patients with TED have complications that threaten the vision primarily due to optic neuropathy and/or corneal exposure.
Cessation of smoking is the most important lifestyle factor that positively influences TED; while systemic steroids is the first line medication in the case of moderate-to-severe and sight threatening TED [1]. However poor or no response to steroids is occasionally encountered [2]. In these cases, steroid administration could be attempted again; or proceed to cyclosporine or rituximab administration and/or orbital radiotherapy. Tocilizumab is a recombinant antihuman monoclonal antibody of the immunoglobulin G1 subclass directed against the interleukin‐6 receptor (IL‐6R) that has been used in TED patients with variable efficacy. Several theories have been proposed regarding Tocilizumab’s potential beneficial impact in TED. Among them, an overall modification of the immunomodulatory function, remodeling of the extracellular matrix, and reduction of the inflammatory response [3].
Within this context, we present a case with sight-threatening TED with minimal response to steroids and orbital radiotherapy, which responded well to tocilizumab treatment.
Case Description
A 60-year-old man, heavy smoker (40 cigarettes/day), was referred to our outpatients’ service by his doctor in February 2019 due to sight-threatening TED, which manifested with gradual reduction of his visual acuity. He was diagnosed with Graves’ hyperthyroidism 14 years earlier, and ever since he was under regular systemic treatment with carbimazole. The patient had also had I-131 treatment in July 2018.
On the initial examination his Best Corrected Visual Acuities (BCVA) were 0.4 logMAR in the right eye (OD) and 0.3 logMAR in the left eye (OS) and the Clinical Activity Score (CAS) was 5/7. The exophthalmometry measurements were 32 mm OD and 30 mm Hg OS. The intraocular pressures (measured with Goldmann Applanation Tonometry-GAT IOPs) were 30 mmHg in both eyes in the primary gaze and 35 mmHg in the upgaze. The right upper eyelid was retracted 2 mm above the limbus and the left was 0.5 mm above the limbus. Both lower lids were retracted 1.5 mm below the limbus (Figure 1 above). The patient was hypemetropic (+3 sph dpt spherical equivalent) and the angles were moderately closed in the superior quadrants and moderately open in the inferior quadrants and gave a plateau iris configuration on identation gonioscopy. Fundoscopy showed early disc swelling in the OD and increased retinal nerve fiber thickness bilaterally (Figure 2). Patient was advised to quit smoking and a fixed combination of dorsolamidetimolol (FCDT) bid was immediately started. Automated perimetry showed defects bilaterally (Figure 3a).
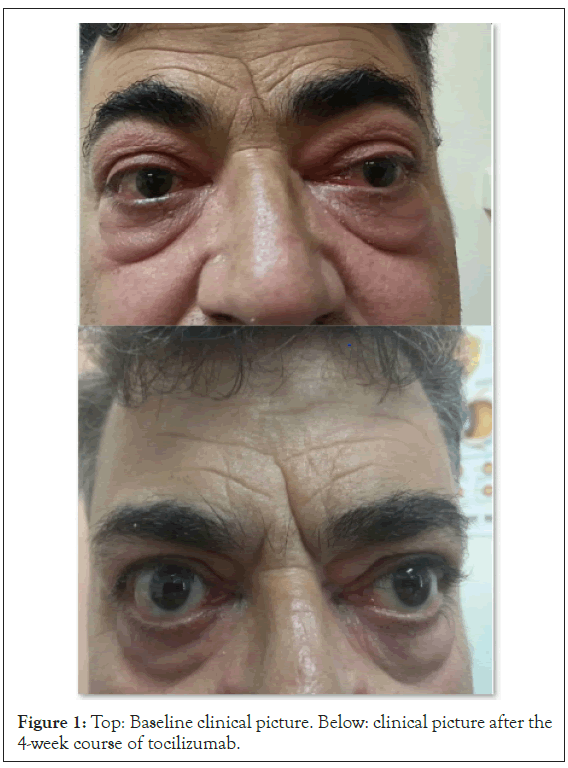
Figure 1: Top: Baseline clinical picture. Below: clinical picture after the 4-week course of tocilizumab.
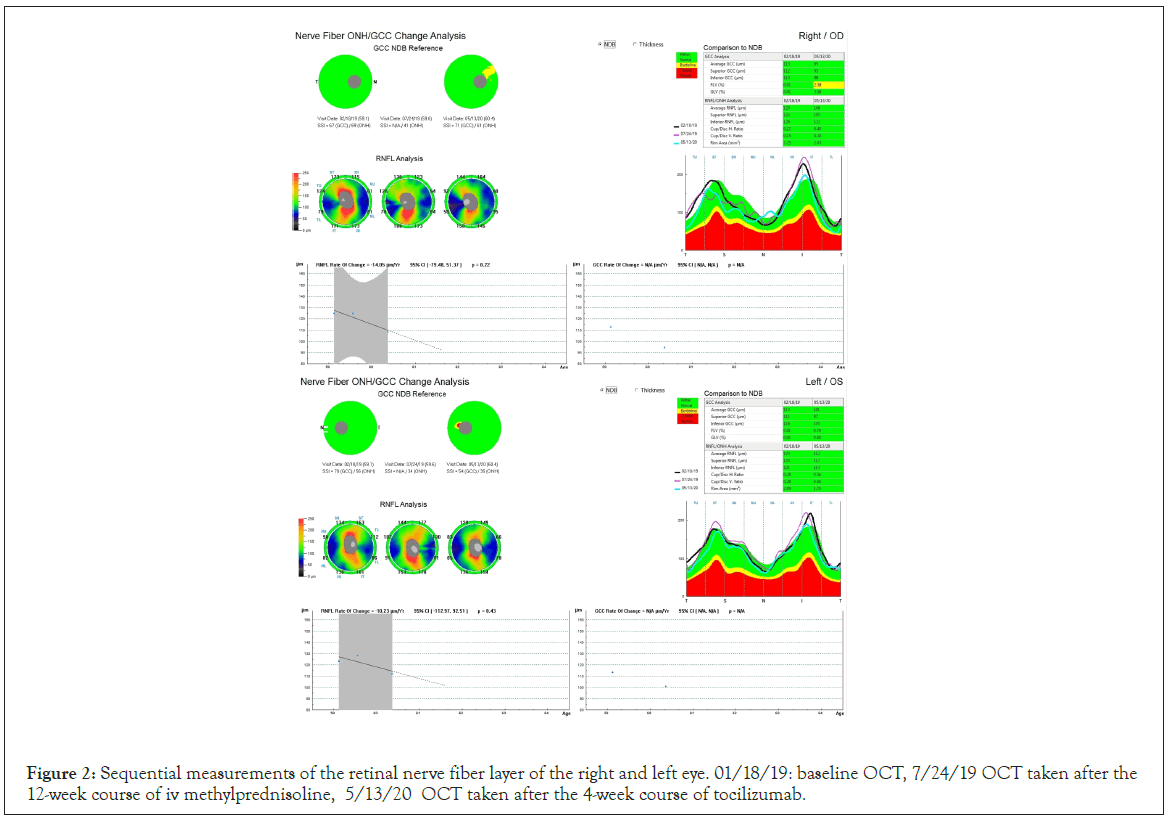
Figure 2: Sequential measurements of the retinal nerve fiber layer of the right and left eye. 01/18/19: baseline OCT, 7/24/19 OCT taken after the 12-week course of iv methylprednisoline, 5/13/20 OCT taken after the 4-week course of tocilizumab.
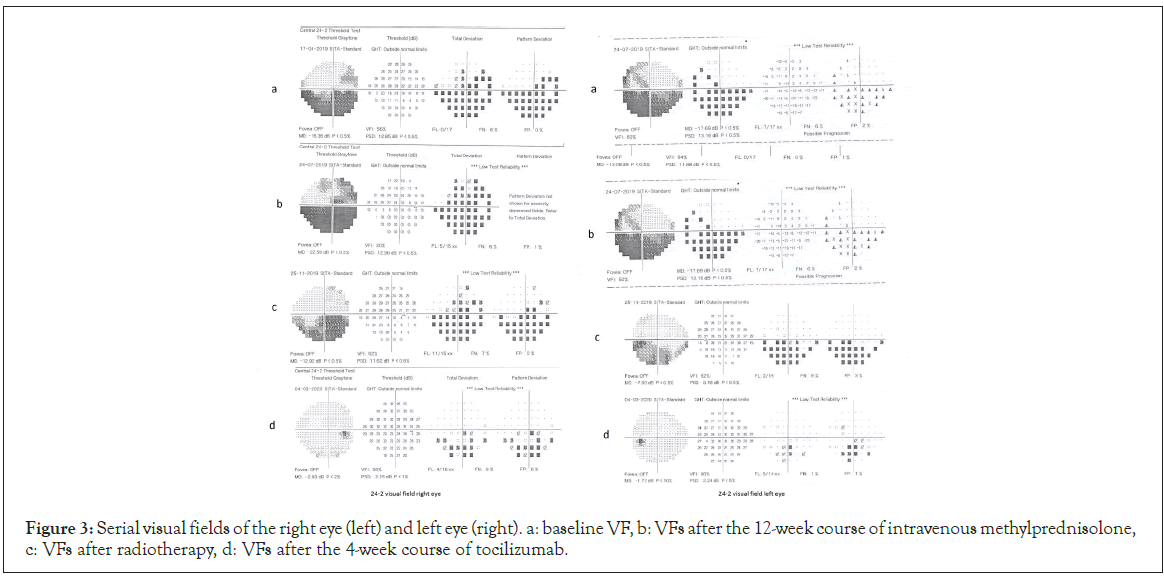
Figure 3: Serial visual fields of the right eye (left) and left eye (right). a: baseline VF, b: VFs after the 12-week course of intravenous methylprednisolone, c: VFs after radiotherapy, d: VFs after the 4-week course of tocilizumab.
In order to address the increased intraorbital pressure, the patient was scheduled immediately for a 3-day course of intravenous methylprednisolone 1000 mg/day. However, neither BCVA, nor exophthalmos was improved. Orbital decompression was suggested, but was rejected by the patient who was against any surgical intervention. As a second option, a 12-week course of intravenous methylprednisolone was proposed (500 mg/week for the first six weeks and then 250 mg/week for the following six weeks) as per EUGOGO recommendations [1] aiming to achieve a prolonged anti-inflammatory effect. At the end of the course the CAS score was 8/10 with no clinical improvement. The patient complained that his eyesight was worse and his BCVA was 0.5 logMAR OD and 0.4 logMAR OS. GAT-IOPs were 27 mmHg in both eyes despite the FCDT with deterioration of his visual fields (Figure 3b). The patient consented to orbital radiotherapy and had 10 sessions with a cumulative dose of 20Gy. Despite the treatment there was no improvement in BCVA, GAT-IOPs or visual fields (Figure 3c). One month after the orbital radiotherapy he underwent thyroidectomy.
As the postoperative period was uneventful and in order to address the persisting sight-threatening situation we decided to start the patient on intravenous tocilizumab (RoACTEMRA, Roche International Ltd, Walwyn Garden City, UK), initially one injection every 4 weeks at a dose of 8 mg/ml. After the fourth session the patient had a marked improvement of his BCVA (0.2 logMAR OD, 0.0 logMAR OS), reduction of the IOP (20 mmHg bilaterally in primary position, 23 mmHg in upgaze), slight improvement of the lid retraction (the right upper eyelid was 1 mm above the limbus, the left upper eyelid 0.5 mm below the limbus but both lower lids remained 1.5 mm below the limbus), the range of ocular movements was extended and the visual fields were also improved (Figure 3d). The exophthalmometry measurements remained the same (Figure 1 below). The retinal nerve fiber thickness was also reduced bilaterally (Figure 2). The patient had only managed to reduce smoking to 20 cigarettes/day. He was on a dietary supplement of selenium, topical artificial tears and the FCDT. Three months the course of tocilizumab all patient’s indices remain stable.
Results and Discussion
Thyroid eye disease can potentially cause blindness in a small minority of patients. The mainstay of treatment for the moderateto- severe disease is a 12 week course of intravenous steroids and for the sight threatening form a 3-day course of high-dose intravenous steroids and/or orbital decompression [1].
Intravenous steroids have been associated with recurrences and poor response as well as 6, 5% morbidity and 0.5% mortality rate [4]. For this reason other modalities have been tried such as cyclosporine, azathioprine, mycophenolatemofetil, methotrexate along with specific immunosuppresants (rituximab, adalimumab, etanercept, infliximub) [5]. These treatments are reserved for the moderate-to-severe TED. In the case of sight threatening TED more radical measures are required.
In our case, sight-threatening TED was manifested with reduction of visual acuity, uncontrolled intraocular pressure and deterioration of visual fields in a man who responded poorly to both a 3-day high dosage treatment with intravenous methylprednisolone and to a 12-week course of the same steroid as suggested by EUGOGO. We decided to proceed with rituximab as orbital decompression was rejected by the patient and gradual deterioration of the visual fields and high GAT-IOPs were recorded. Literature suggests that tociclizumab in moderate-to-severe TED reduces the soft tissue swelling, Clinical Activity Score and exophthalmos but this improvement does not translate into significant clinical improvement from the patients’ point of view [6,7].
Our patient had very congested orbits with the soft tissues impinging on the optic nerves. We also felt that the high IOPs were due to poor aqueous outflow secondary to episcleral venous congestion. The patient’s response was impressive which reflected on the improvement of the eyelid and conjunctival swelling and redness, the improvement of the ocular motility, the reduction of the IOP (presumably due to the decrease of the episcleral venous drainage) and the ameliorated perimetric findings secondary to the reduction of the soft tissues bulk. Exophthalmometry readings did not change although the upper and lower lid retraction was minimally improved compared to the baseline measurements.
We believe that tocilizumab can have a positive impact on the aforementioned aspects of TED. However it is very unlikely that it could be used in the case of exposure keratopathy as it does not reduce eyelid apertures [6]. In the same manner dysthyroid optic neuropathy which can cause quick and profound loss of vision should not be treated with tocilizumab as its effects take days to weeks to develop. It should, though. Be used in cases were the first line medication fails to offer adequate alleviation of signs and symptoms of moderate-to-severe TED.
Research has shown that thyroidectomy may improve the signs of TED but this process takes months to develop [8,9]. In the case of our patient we did not have the time to wait for the possible beneficial effects of thyroidectomy. For this reason we started the patient on the tocilizumab treatment 6 weeks after the thyroidectomy. We believe that the improvement of symptoms was due to the tocilizumab treatment as it is was already observed from the second intravenous infusion and the time course by that time was too short for the effects of the thyroidectomy to manifest.
As far as it concerns side effects, tocilizumab can cause hepatitis, opportunistic infections or flare ups of latent infections as it interferes with the immune response and development or exacerbation of cancer [6]. Our patient developed a moderate thrombocytopenia which recovered after 3 weeks without intervention.
To the best of our knowledge this is the first case reported where tocilizumab was used in a patient with severe TED. Although these cases require treatment that evoke a quick response we felt the rate of the development of the optic neuropathy gave us the luxury of time to use a medication that takes longer to act and indeed the clinical response supported our decision. We would like though to stress that tocilizumab (as other immunoregulatory medication) is not a substitute for the recommended management of severe TED and its main indication is moderate-to-severe disease.
Conclusion
In the present article we describe a case of sight threatening thyroid eye disease which was treated with tocilizumab. This IL-6 inhibitor plays a key role in reducing the swelling of the soft tissues in moderate-to-severe TED. Our patient benefited from this treatment as the extraocular muscles compressed the optic nerve causing symptoms and signs of dysthyroid optic neuropathy. The reduction of the soft tissue swelling led to an improvement of the optic nerve function that was maintained at the last follow up eight months after the last intravenous dose of tocilizumab.
Authors Statement of Consent
The authors have obtained written consent by the patient stating that the patient agrees to have the pictures attached to this paper published.
REFERENCES
- Perros P, Hegedüs L, Bartalena L, Marcocci C, Kahaly GJ, Baldeschi L, et al. Graves’ orbitopathy as a rare disease in Europe: A European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. 2017;12(1):1-6.
- Bartalena L. What to do for moderate‐to‐severe and active graves orbitopathy if glucocorticoids fail? Clin Endocrinol. 2010;73(2):149-152.
- Azzam SH, Kang S, Salvi M, Ezra DG. Tocilizumab for thyroid eye disease. Cochrane Database Syst Rev. 2018.
- Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 european thyroid association/european group on graves' orbitopathy guidelines for the management of graves' orbitopathy. Eur Thyroid J. 2016;5(1):9-26.
- Strianese D, Rossi F. Interruption of autoimmunity for thyroid eye disease: B-cell and T-cell strategy. Eye. 2019;33(2):191-199.
- Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves Orbitopathy: A randomized clinical trial. Am J Ophthalmol. 2018;195:181-190.
- Maldiney T, Deschasse C, Bielefeld P. Tocilizumab for the management of corticosteroid-resistant mild to severe Graves’ ophthalmopathy: A report of three cases. Ocul Immunol Inflamm. 2018.
- Erdoğan MF, Demir Ö, Ersoy RÜ, Gül K, Aydoğan Bİ, Üç ZA, et al. Comparison of early total thyroidectomy with antithyroid treatment in patients with Moderate-Severe Graves' Orbitopathy: A randomized prospective trial. Eur Thyroid J. 2016;5(2):106-111.
- Weber KJ, Solorzano CC, Lee JK, Gaffud MJ, Prinz RA. Thyroidectomy remains an effective treatment option for Graves’ disease. Am J Surg. 2006;191(3):400-405.
Citation: Mehmet A, Panagiotopoulou EK, Konstantinidis A, Papagoras C, Skendros P, Dardabounis D, et al. (2020) A Case of Severe Thyroid Eye Disease Treated with Tocilizumab. J Eye Dis Disord. 5:146.
Copyright: © 2020 Mehmet A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
