Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 5
Which COVID-19 Vaccine is Efficiently Most Cost-Effective and Why?
Rabnawaz Khan*Received: 01-Sep-2021 Published: 22-Oct-2021, DOI: 10.35248/2157-7560.21.12.464
Abstract
This study presents the dynamic boost of the COVID-19 vaccines. The intensive approach is indicating how the effective COVID-19 vaccine promotes and distributes into various official channels. The Cost-Effectiveness Analysis (CEA) approach is used with Quality-Adjusted Life Years (QALYs). The key findings are revealing the length of life improves the quality of life of the middle family. The QALYs show that Utility Value (UV) by health and the value of statistics in countries in the term of coexistent causation of vaccines. The Pfizer (BNT162b2) and Moderna (mRNA- 1273) have instantly created favourable and significant effects probably on many patients, comparatively SARSCoV- 2, AstraZeneca (AZD1222), Russia’s Sputnik (AstraZeneca), and Sinopharm Sinovac Biotech. The opportunity cost provides a valuable benefit in the future.
Keywords
COVID-19; Vaccines; Cost-Effective Analysis (CFA); Health; Quality-Adjusted Life Years (QALYs)
Introduction
Healthcare and its measurement have inevitably to remain an economic activity, invest money, time, and sources for secure human life with various infected and unmeasured diseases. However, the unnecessary consequences of health measures improve human life, and medical institutions exchange the value of life worth with others because of profit, investment, and market penetration medicated products [1]. As a global health priority, it’s time to conceive about human life and as a nation to identify ways of considering the medicated problems because of the COVID-19 pandemic and applying for the quality improvement and measurement of human life in various dimensions. Health priorities play a vital role in articulating and effect the health system that is diverting toward Universal Health Care (UHC) [2,3].
The Food and Drug Administration (FDA) has avoided the Emergency Use Authorization (EUA). In this process, the chloroquine and hydroxychloroquine medication employing for the diagnosing purposes of COVID-19 patients. The FDA recognized these medicines it is not efficient drugs for treatment. In the 550-Drug Development Program (DDP), the FDA has examined 350 trials for unknown gender. At last, after a long time, SARS-CoV-2 (novel coronavirus) has been introduced, and around 80% of people recover from COVID-19 diseases without specialized medical treatment. According to the World Health Organization (WHO), 1 to 5 patients develop foster illness and have face breathing problems. After the invention of this alternative medicine (SARS-CoV-2), it is thus amenable to the extensive frame of economic analysis is understanding the worth of this medicine and its demand. In the first phase, it’s a challenge and risky to lunch in private sectors because of the market penetration with fixation of prices and supplies new and active investor interest.
The Cost-Effectiveness Analysis (CEA) approach is using to examine as an evaluation tool where the output of the goods (medicines) produced by a project does not count in the monetary term. It shows the interaction of health and pharmaceutical industries, where the benefits are qualitative rather than qualitative. The CEA approach to identify the efficient analysis of SARS-CoV-2 medicine is that of a project or investment [4-6]. In this acute condition, when all over the world is assuming massive risk because of the COVID-19 pandemic so we should not assess them for their monetary value factor should equally consider the quantitative approach. The cost of the investment divided by the outcome of the selected investment can compute the Cost-Effectiveness Ratio. The economic analysis procedure compares the relative cost of the project and the result of the CEA technique. As nature of evaluation, this technique is a mixed approached of quantitative and qualitative. It is more suitable for the service-related health sector, and this analysis approach does not consider the opportunity costs where typical investors’ concern about more benefits or business misses.
Figure 1 shows the linear change in total death from January 23 to December 21, 2020. It revealed a modification in the total percentage that 47% reduced by 1% during this period.
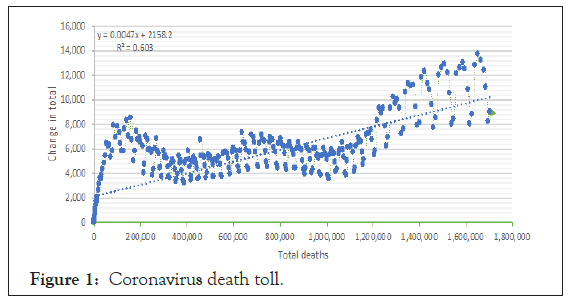
Figure 1: Coronavirus death toll.
The COVID-19 vaccine prevention is perhaps the best hope for the sound world and for ending the pandemic. The U.S Food and Drug Administration (FDA) begin authorizing an emergency after that critical situation of deaths. Examine the benefits of COVID-19 vaccines, how they work, side effects, and infection prevention steps. World Health Organization (WHO) has confirmed 444,437 recent cases on December 29, 2020. January 31, 2020: Contemporary evidence showed that people who are affected with the unique coronavirus (2019-nCoV) could turn into infectious before that symptoms were prominent neither the CDC (Centers for Disease Control and Prevention) nor WHO (World Health Organization) have changed their instructions considering the treatment [7,8].
On 16-Nov-2020, Johanson and Johnso (J&J) has launched the second phase of the COVID-19 vaccine with the cooperation of the UK National Institute for Health Research (NIHR) and enrolled around 30,000 members in the US, Germany, UK, South Africa, Spain, Philippines, and Colombia. Phase 3 ensemble trial on 1 and 2 dose regimen during September and November and inspected the results of 90,000 participants across the world to avert SARSCoV- 2-mediated COVID-19 [9].
However, the practical recognition of COVID-19 patients with an occupational attribute for compensation remains an enormous challenge for the unified world. In most countries, the number of related COVID-19 patients identified as positive symptoms and it much lower than the total estimated burden of COVID-19 patients and their causes.
In this study, we analyze the COVID-19 vaccines by the CEA method is approached with Quality-Adjusted Life Years (QALYs). The CEA approach estimates the annual numbers of COVID-19 patients, aggregate losses of QALYs, and health measurement costs that could adjust by improving the lifestyle in a secure environment. CEA method analyzes 213 countries and assessed which vaccine is more cost-effective and why? However, the QALY remains an attempt to combine two components of health by length and quality of life i.e., mortality. We indicate extra months of life of patients (in years) when considering treatment for COVID-19 patients. In this study, the health of the COVID-19 patients analyzes by partial healthy, healthy, and perfectly healthy conditions. The utility value of the QALYs assigned to treatment based on COVID-19 patients and their expected results examine by SARS-CoV-2 (GBS-price IPO), Pfizer (BNT162b2), and mRNA-1273 (Moderna’s). We analyze and determine a more effective and effective solution to vaccines. This paper comprises a review of scholarly materials on the vaccines, methodological approaches, findings, and a discussion. We conclude by making recommendations based on our outcomes.
Materials and Methods
The study intends to examine the magnitudes and connection of the COVID 19 vaccine and infected confirmed cases of COVID 19. It contains 213 dominant territories from Europe, the USA, Asia, South Africa, and Oceania. The interaction of the COVID 19 vaccine analyzes by cost-effectiveness analysis (CEA) and Quality- Adjusted Life Years (QALYs).
The CEA is a type of evaluation that measures the differences in cost and benefits in minimum intervention with effectiveness measures.The use of the signal logical unit to value health gain. However, measurement units are involved in the number of lives saved, the number of out-patient appointments a day in the hospital, or the measurement of drug usage. In this study, we confirm how safe a number can live. The problem is it is one dimension, which is only of a diverse inveterate number of patients. The reason is that we don’t know about the quality of life of these patients. We need them to live longer and also the number of lives saved, not the quality of lives [10]. The extensive economic evaluation is limited benefits side to consideration of life expects and age of deaths, the only outcome of treatment. The allocation of outcome health resources is unlikely to reflect the patient’s health order needs and quality of live post-treatments. In this study, the CEA is calculating based on the Value of Statistics (VSL). The value of cost is considering the government donations or contributions. The life in expected years is supposed to calculate on the confirmed number of patients. However, the expected life cost is centered on different utility values of life in partial, utility, and other utility. The prescribed vaccines SARS-CoV-2 (GBS-price IPO), Pfizer (BNT162b2), and mRNA- 1273 (Moderna’s) is practice for the analysis of QALYs.
In this study, QALYs represent the measured value of confirming cases of COVID-19 patients and their benefit of health outcomes with prescribe COVID-19 vaccines. The perspective of health is distinct as a function of the length of life (mortality) and quality of life (morbidity), but in this case, we estimate only the mortality of life. QALYs carry out an idea about the expected life, how many extra years or months of life a realistic quality of person might gain, the results of different treatment by utility value [11,12]. The expected life of patients is computing based on partial healthy (0.25 years), healthy (0.50 years), and perfect healthy (1 year). The calculation of QALYs indicates the quality of life between 1=perfect healthy and 0=dead [13]. Also, 0.25 QALYs representing a particular patient achieve a partial healthy and fit status only one quarter of a year. The essential function of QALYs indications is to quantify the effectiveness of a modern treatment (SARS-CoV-2, Pfizer, and mRNA-1273) compare to the current treatment for this pandemic situation. It also evaluates the cost-effectiveness of the different vaccines, and this is the cost of prescribing a vaccine to provide a year of the best quality of life available. Also, we ignore the altered life stages and do not consider the specific age of the people group. It fact that generally younger have many times more QALYs than older [14].
Cost-Effective Analysis (CEA) and Quality-Adjusted Life Years (QALYs) techniques
The Cost-Effective Analysis (CEA) technique used on COVID 19 confirmed patients' Value of Statistics (VSL). The VSL shows three different levels of contribution of money in groups of countries. Groups are classifying the number of confirmed cases. There are five groups (GP1 to GP5) are analyzed in 213 countries (Table 1). The application of this method is indicating the length of individual patient life. Also, it represents that how QALYs can boost the participation and donation of the government. In this pandemic situation, the opportunity cost of living is more valuable, and it can provide the maximum out to the individual patients and community.
| Groups | Cases | Value of statistics life ($) million | Continents | Total Countries | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VSL-1 | VSL-2 | VSL-3 | Europe | America | Asia | South | Oceania | |||
| Africa | ||||||||||
| GP-1 | <10 million | 25 | 50 | 100 | 7 | 5 | 2 | 0 | 0 | 14 |
| GP-2 | less than 10 million | 2.5 | 5 | 10 | 23 | 12 | 18 | 5 | 0 | 58 |
| GP-3 | less than 1 million | 0.15 | 0.25 | 1 | 14 | 2 | 11 | 23 | 2 | 52 |
| GP-4 | less than 10000 | 0.025 | 0.05 | 0.1 | 6 | 13 | 4 | 22 | 2 | 47 |
| GP-5 | less than 1000 | 0.0025 | 0.005 | 0.01 | 5 | 16 | 7 | 7 | 7 | 42 |
Table 1: Groups distribution.
Data collection
The paper dataset typically contains from created 1st to 14th December 2020. The number of confirmed cases and death of patients was carefully analyzed in five separate continents and groups. There are 213 (55 Europe, 48 USA, 42 Asia, 57 South Africa, and 11 Oceania) countries eagerly examined in GP1 to GP 5 [15]. Understated of the European Centre for Disease Prevention and control measured results show the highest prevention impact of the current vaccine. And the most inflated number of confirmed cases recorded in Europe (Russia, France, UK, Italy, Spain, Germany, and Poland), the USA (America, Brazil, Argentina, Colombia, and Mexico), and two independent countries of Asia (Iran and India).
Procedures
In the first phase, the continent distributes according to the confirmed number of COVID-19 patients. And the highest number of confirmed cases registered in Russia, the USA, and India. As per QALYs, the expected years of patients are estimated by 0.25 to 1 year. The distribution of the year determined three scenarios. According to the first scenario, imagine a person (confirmed case) is fully healthy but has a risk of unexpected death caused by this infected virus (COVID-19). The newly introduce medicine will execute patients more life. And his life considers as one year (1*100/100=1) in excellent health condition. The medicine dose increases a year of life at 100% of the expected quality of life. It would be QALYs of one (Perfect healthy). They expect the live line of the patient based on QALY between 0 and 1 [16]. And the death has indicated with 0 QALY. In the second scenario, the quality of life of an infected person will be only 50% based on the fully healthy person.
The new medicine might give him extra to life but not fully restore him to complete perfect health. The new vaccine will gain (0.5*50/100=0.25) life in a healthy situation. Conditionally, the new vaccine doze increases a year of life by 50% of the expected quality of life. Therefore, the QALY would be 0.25. In the third scenario, the expected life will increase by partial (0.25 years). The quality of life will be only 25%, and the current vaccine might give him extra life with QALY (0.25*25/100=0.0625). As per the expected estimation of QALYs, in the first scenario, the vaccine MD-3 (Mrna1273 Moderna’s) is influential on the infected health and provides the maximum probability of his life in perfect health comparatively from the second and third scenarios.
Furthermore, it might be possible to calculate the expected life of the confirmed number of patients by (0.25,0.50, and 1) years. And if the expected life will increase in a situation of perfect healthy (10 or more than ten years) condition. So, the QALYs would be at maximum level with 100% experience of the fully healthy situation. And the patient will endure lives (5 to 10) years. Also, with the intervention of (Pfizer BNT162b2) vaccine patient expected 75% healthy. Therefore, the QALY would be 7.5 (10 years*0.75). The partial healthy patient might endure lives (0 to 5) years. Furthermore, with the SARS-CoV-2 vaccine, the QALY would be 1.25 (5 years*0.25).
Estimation of QALYs by utility values of vaccine
The second phase is based on the utility value of life with current vaccines some as following:
SARS-CoV-2 (GBS-price IPO): Graciously according to GBS Inc, Antibody Biosensor (SARS-CoV-2) manufacture with the coordination of Harvard University in the Department of Wyss Institute. They sufficiently indicate the first modern Biosensor Platform technology with a dedicated nanomaterial coating. Moreover, a saliva-based test for the SARC-CoV-2 begun in New York (NY) in August 2020. Noninvasive SARS-CoV-2 antibody testing castoff estimates the mild symptoms and apparent prevalence of the rare SARS-CoV-2 infection [17]. They unanimously declared that more than 23 million COVI-19 patients had registered, and only 50 to 80% of COVID-19 cases didn’t diagnose because of lack of vaccination in August 2020.
In November 2020, there were 212 COVID-19 vaccines introduced in clinical (48 vaccines) and principle (164 vaccines) evaluation by World Health Organization (WHO). Of these, only 11 vaccines can have reached Phase III trials, and six vaccines early tested in Russia and China have not completed the third trial [18-20]. Saliva-based glucose introduces and fixed the GBS price at $17 (23 December 2020). It is evolving saliva-based (sb) test for SARSCoV- 2 with diabetes management in China. It raised $22 million (m) by proposing 1.3 (m) units within the range of $16 to $18. As for managing the unexpected situation of COVID-19, the company offered 0.2 (m) more units than predicted.
Pfizer (BNT162b2): After the 2nd and 3rd clinical trials Pfizer- BioNTech, the vaccine has introduced. And two shots (21 days apart) to preventing laboratory-confirmed and manage COVID-19 illness in people without any other indication of infection. The 95% effective patients result of the Pfizer vaccine have increased. Therefore, in the international market, the vaccine demand raised because it works in average conditions and provided maximum results [21]. On 11 December 2020, the Emergency Use Authorization (EUA) permitted the announcement of the Pfizer-BioNTech vaccine at $19.50 per dose in the USA and other countries [22,23].
mRNA-1273 (Moderna’s): On 16th November 2020, Moderna has reported 94.5% effectiveness trial results from 30,000 participants in the 3rd phase. According to FDA, the initial price of the vaccine was introduced between $25 to $37. And, there are 2 (shots 28 days apart) needed for each infected patient. On 23rd November 2020, according to Moderna, the vaccine price adjusted between $32 to $37 per dose. The European Union was reportedly conferring an agreement to keep the per-dose price under $25 [24]. The Pfizer and BioNTech vaccine BNT162b2 more than 90 effectually, and no federal funding considering for manufacturing. It needs to be diluted by the pharmacist. Initially, 50 million doses suggest being manufacture by the end of 2020 and another 1.3 billion next year. Also, comparatively the Moderna vaccine mRNA-1273 was 94.5% effective in preliminary trials and developed in collaboration with the U.S. government. And it does not require dilution. Total 20 million doses suggest being available by the end of 2020 and up to 1 billion more next year [25].
Validation incidences of work-related by estimating the QALYs
QALYs estimation is typically based confirmed number of likely patients, model years, and the utility value. In this study, the QALYs are computing for each content, where the number of patients individually multiple with years and then utility value. The expected QALYs value (QA-1, QA-2, and QA-3) value is sufficiently indicating the individual country and their confirmed patients. The moral value of QALYs distributed on the economic basis of utility value, where the three-different vaccination (MD-1, MD-2, and MD-3) shows the intervention of effective vaccine and their profound effects on particular patients. The MD-3 value is more effective comparatively than MD-2 and MD-1. The rare case of different prices of vaccines shows the organizational QALYs' effectiveness. It indicated the divine intervention of vaccines on variant patients. Additionally, where the quality of life ignores because of the indifferent contents a different number of likely patients have been carefully scrutinized.
Difference of QALYs
Potential QALYs difference confirms the operational effectiveness of different vaccines. The estimated results of QA-3 and QA-1 indicate the difference of vaccine effects based on diverse contents. Plus, the anticipated results are representing the quality of the vaccine. Besides, an effective vaccine gives more life to the infected patients under ordinary circumstances. Figure 2 shows the diverse contents QALYs with a liner line of leading QA-3 cause of highly effective results differentiate the excellent quality of life is computing in the rare case of the mRNA-1273. The maximum number of likely patients might naturally increase QALYs in perfect health conditions.
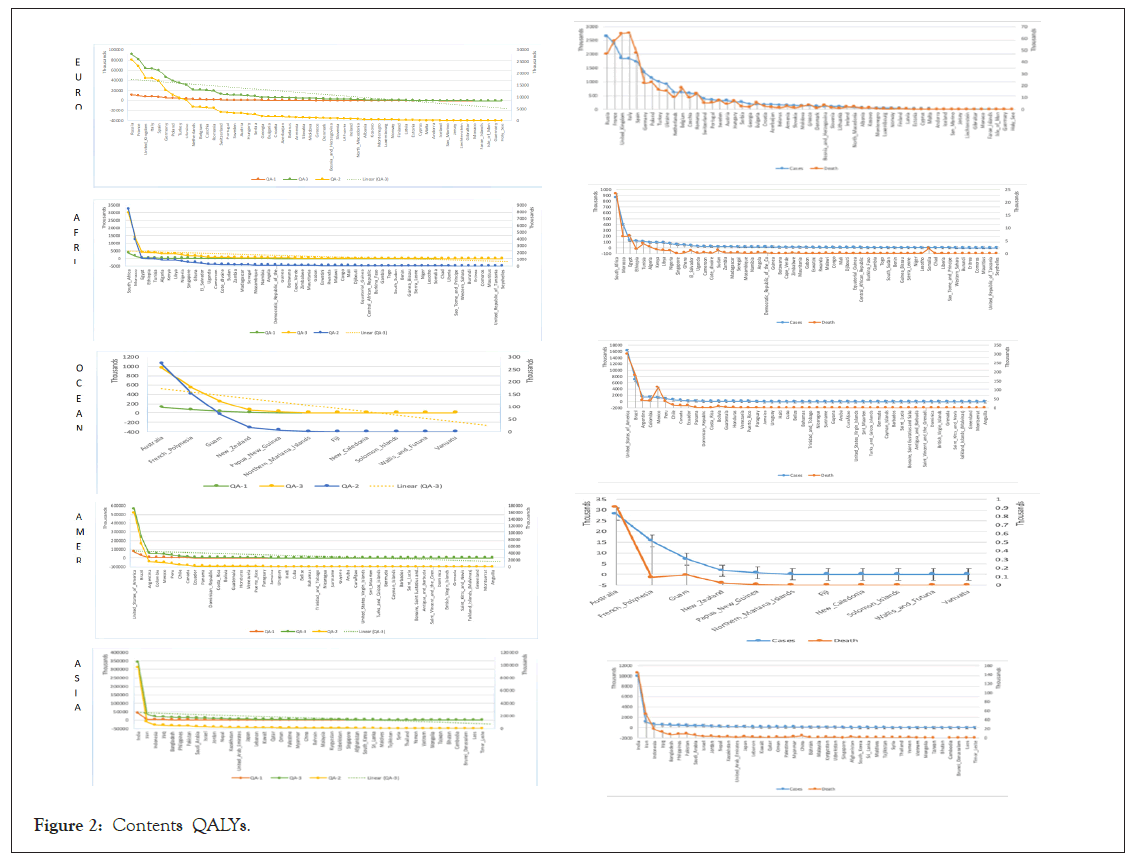
Figure 2: Contents QALYs.
MD-3 vaccine (mRNA-1273-Moderna’s) is highly effective. The efficiency rate of this vaccine is 94.5% as competitively Pfizer- BioNTech, Adenovirus-AstraZeneca (AZD1222), ENSEMBLE 2 (Johnson & Johnson), Russia’s Sputnik V Vaccine (AstraZeneca), and Sinovac Biotech (Table 2).
| Vaccines | Date | Type | Developer | Countries | Efficiency rate (about) | Doses | Price per/dose |
|---|---|---|---|---|---|---|---|
| Pfizer-BioNTech | December 11, 2020 | mRNA | BioNTech | Germany | 95% | 2 | $19.50 |
| Moderna’s mRNA-1273 | December 18, 2020 | NIAID | USA | 95% | $25-$37 | ||
| AstraZeneca (AZD1222) | January 12, 2021 | Adenovirus-based | University of Oxford | Europe and USA | 70% | $25-$37 | |
| ENSEMBLE 2 (Johnson & Johnson) | April, 2021 | USA | USA | 72% (USA) 66%(Overall) and 85% (severe disease) | 1 | $10 | |
| Russia’s Sputnik V Vaccine (AstraZeneca) | March or April, 2021 | GRIEM | Russia | 91.40% | 2 | $10 | |
| Sinopharm Sinovac Biotech | January 13, 2021 | SARS-CoV-2 | Biopharmaceutical | China | 50.38% | 2 | $50.38 to $91.25 |
Table 2: Description of vaccines.
Value of statistics
According to Table 1 value of statistics is computed scientifically based on VSL donations or outstanding contributions. The contribution value of VSL base on the considerable number of confirmed patient cases in the groups (GP-1 to GP-2) between 25 to 100 million (USD). And the individual government participation is efficiently computed by the confirmed number of patients in different contents.
Results
For the QALYs model, the confirmed number of cases differentiates into five groups. Including the different contents. And in the GP-1, there is a total of 17 (7 Europe, 5 America, and 2 Asia) countries carefully examined individually based on their contents. The highest death ratio is recorded in Italy (0.034), Colombia (0.027), and Iran (0.047) based on the population in the contents of Europe, America, and Asia. As per accurate QALYs estimation, the value of statistics indicated that each patient reimburses cash 13.55 (VSL-1), 27.11(VSL-2), and 54.23(VSL-3) under the utility value of MD1 to MD-3. We efficiently computed the average values VSL- 1(14.596), VSL-2(29.192), and VSL-3(58.384) of 7 Europe; VSL- 1(11.876), VSL-2(23.752), and VSL-3(47.504) of 5 America; VSL- 1(12.543), VSL-2(25.087), and VSL-3(50.174) of 2 Asian countries in the GP-1 for estimation of vaccines donation.
Supplementary Figure 1 shows that all three above vaccines effectiveness after two doses, the reimbursement of vaccination cost will be 39% (34/VSL-1),69.5%(39/VSL-2), and 78.60%(69/ VSL-3), and the remaining amount the patient will recover by self. Likewise, this confirmed number of patient claims at 0.51%, 0.89% (in the case of MD-1 and MD-2) in Colombia. And 0.663% (in the case of MD-1) in Iran from the government or donations agencies.
Furthermore, the outstanding contribution of a charitable donation shows the Value of Statistics (VSL) for two effective doses. There are 213 independent countries individually representing their donation in a considerable percentage the Germany, Poland, Colombia, Mexico, and Iran in the GP-1. Second, Slovakia, Moldova, Greece, Denmark, Bosnia, Egypt, Ethiopia, Tunisia, Guatemala, Honduras, Venezuela, Puerto_Rico, Qatar, Oman, Palestine, and Myanmar in the GP-2. Third, Malta, Guinea, Botswana, Cape_Verde, Zimbabwe, Mauritania, Jamaica, Maldives, and Tajikistan in the GP-3; Gibraltar, Sao_Tome, Sint_Maarten,and Vietnam in the GP-4. Fourth, Holy_See, Saint_Vincent, Dominica, British_Virgin_Island, Grenada, Saint_Kitts, Falkland Islands, Greenland, Montserrat, Anguilla, Northern_Mariana_ Island, Fiji, New_Caledonia, Solomon_Islands, Wallis_and Futuna, Vanuatu, Laos, and Timor_Leste in GP-5. Many patients unincluded in the donation list because of the least number of confirmed patients registered in the different hospitals. In this case, each country's government individually patriciate without any extra funding for each patient. The x-axis indicates the number of countries and the Y-axis on % of donations of each country patient.
January, 11 (2021) U.S. Food and Drug Administration (FDA) has sanctioned to use COVID-19 vaccine funded by Pfizer and Moderna [26]. The Operation Warp Speed (OWS) have purchased millions of vaccine doses from supplier, and it controlled by Health and Human Services (HHS) and the Department of Defense (DOD). Also, OWS instigating a National Vaccine Program (NVP) by coronavirus supplemental appropriation acts (FY2020, FY2021). In particular, $30 billion (FY2020) and $22.945 billion (FY2021) are reserved for the Public Health and Services Emergency Fund (PHSSEF) for development, manufacturing, and purchase from the supplier until September. 30, 2024.
Asian Development Bank (ADB) has launched $9 billion for the Asia Vaccine Access Facility (APVAX. Additionally, sustenance investment activities to build vaccines [27,28]. One of the leading companies (ANKARA) has secured about $500 million in funds for the development, manufacturing, and distribution of Sino Biopharmaceutical. Initially, the company has manufactured 300 million doses of Sinovac. Besides, China has donated a vaccine to Pakistan on February 1st, 2021, to prevent seriously confirmed COVID-19 cases. Hence, Pakistan has already lost more than 11 thousand live cause of this outbreak [29].
Supplementary Figure 2 shows the confirmed number of COVID-19 patients (less than 10 million) in 58 (23 Europe, 5 South Africa, 12 America, and 18 Asia) countries based on their contents. The highest death ratio was recorded in Bosnia (Europe), Tunisia (South Africa), Ecuador (America), and Myanmar (Asia) with 0.032%, 0.034%, 0.068%, and 0.020% based on the confirmed number of COVID-19 patients. QALYs estimation indicated the value of years (partial health, healthy, and perfectly healthy) and the utility value used in all the groups. The value of statistics representing that each patient will be reimbursed donations from the funding agencies for vaccines. We computed the average values of one dose VSL- 1(11.476), VSL-2(22.953), and VSL-3(45.906) of 23 Europe; VSL- 1(14.716), VSL-2(29.433), and VSL-3(58.866) of 5 south Africa; VSL-1(14.602), VSL-2(29.204), and VSL-3(58.408) of 12 America; VSL-1(11.891), VSL-2(23.781), and VSL-3(47.563) of 18 Asian countries in the GP-2 as for estimation of vaccine donation (Table 3). Same as above, vaccination cost computed (Two dose price/VSL) estimation of the reimbursement (Supplementary Figure 1).
| Groups | Continents (No of Countries) | Average % (value of statistics of two doses) | ||
|---|---|---|---|---|
| VSL-1 | VSL-2 | VSL-3 | ||
| GP-1 | Europe (7) | 0.429 | 0.749 | 0.846 |
| USA (5) | 0.349 | 0.609 | 0.688 | |
| Asia (2) | 0.369 | 0.643 | 0.727 | |
| GP-2 | Europe (23) | 0.338 | 0.589 | 0.665 |
| South Africa (5) | 0.433 | 0.755 | 0.853 | |
| USA (12) | 0.429 | 0.749 | 0.846 | |
| Asia (18) | 0.35 | 0.61 | 0.689 | |
| GP-3 | Europe (14) | 0.141 | 0.204 | 0.462 |
| South Africa (23) | 0.215 | 0.312 | 0.705 | |
| USA (2) | 0.212 | 0.308 | 0.696 | |
| Oceania (2) | 0.22 | 0.32 | 0.722 | |
| Asia (11) | 0.122 | 0.178 | 0.401 | |
| GP-4 | Europe (6) | 0.366 | 0.637 | 0.72 |
| South Africa (22) | 0.243 | 0.424 | 0.48 | |
| USA (13) | 0.174 | 0.304 | 0.344 | |
| Oceania (2) | 0.263 | 0.459 | 0.518 | |
| Asia (4) | 0.283 | 0.494 | 0.558 | |
| GP-5 | Europe (5) | 0.706 | - | - |
| South Africa (7) | 0.157 | 0.273 | 0.309 | |
| USA (16) | - | - | - | |
| Oceania (7) | - | - | - | |
| Asia (7) | 0.743 | - | - | |
Table 3: Average value of statistics (two-dose).
Supplementary Figure 3 shows the confirmed number of COVID-19 patients (less than one hundred thousand) in 52 (14 Europe, 23 South Africa, 2 America, 2 Oceania, and 11 Asia) countries based on their contents. The highest death ratio recorded in Ireland (Europe), EI_Salvadao (South Africa), Jamaica (America), Australia (Oceania), and Kyrgyzstan (Asia) with 0.027%, 0.028%, 0.023%, 0.032%, and 0.016% based on confirm number of COVID-19 patients. We computed the average values of one dose VSL-1(4.780), VSL-2(7.966), and VSL-3(31.866) of 14 Europe; VSL-1(7.299), VSL-2(12.165), and VSL-3(48.662) of 23 south Africa; VSL-1(7.206), VSL-2(12.014), and VSL-3(48.041) of 2 America; VSL-1(7.477), VSL-2(12.462), and VSL-3(49.851) of 2 Oceania; VSL-1(4.155), VSL-2(6.925), and VSL-3(27.701) of 11 Asian countries in the GP-3 as for estimation of vaccine donation. Same as above cost of the vaccine was computed (Two dose price/ VSL) for calculating the reimbursement.
Supplementary Figure 4 shows the confirmed number of likely COVID-19 patients (less than ten thousand) in 47 (Six Europe, 22 South Africa, 13 America, two Oceania, and four Asia) countries based on their contents. The highest death ratio is recorded in San Marino (Europe), Somalia (South Africa), the Bahamas (America), Guam (Oceania), and Yemen (Asia) with 0.026%, 1%, 0.021%, 0.016%, and 0.290% based on confirming the number of COVID-19 patients. We efficiently computed the average values of one effective dose VSL-1(12.427), VSL-2(24.855), and VSL- 3(49.711) of six Europe: VSL-1(8.274), VSL-2(16.548), and VSL- 3(33.097) of 22 South Africa; VSL-1(5.926), VSL-2(11.852), and VSL-3(23.705) of two America; VSL-1(8.943), VSL-2(17.886), and VSL-3(35.771) of two Oceania; VSL-1(9.631), VSL-2(19.262), and VSL-3(38.525) of four Asian countries in the GP-4 as for accurate estimation of vaccine donation.
Supplementary Figure 5 shows the confirmed number of COVID-19 patients (less than one thousand) in 42 (5 Europe, 7 South Africa, 16 America, 7 Oceania, and 7 Asia) countries based on their contents. The highest death ratio recorded in Isle_ of_Man (Europe), United_Republic_of_Tanzan (South Africa), Antigua_and_Barbuda (America), Fiji (Oceania), and Taiwan (Asia) with 0.067%,0.041%, 0.027%,0.043%, and 0.009% based on confirming several COVID-19 patients. We computed the average values of one dose VSL-1(24.007), VSL-2(48.015), and VSL-3(96.030) of 5 Europe; VSL-1(5.332), VSL-2(10.664), and VSL-3(21.328) of 7 south Africa; VSL-1(61.741) of 16 America; VSL-1(25.265), and VSL-2(50.532) of 7 Asian countries in the GP-5 as for estimation of vaccine donation.
Discussion
The estimated results of cost-effectiveness analysis (CEA) and Quality-Adjusted Life Years (QALYs) show the confirmed number of COVID-19 patients in five continents (213 countries) from the period of 31st December 2019 to 14th December 2020. The indicated technique of this study represents mRNA-1273 (Moderna) effective vaccine is more effective and efficient. SARSCoV- 2 (GBS-price IPO) and Pfizer (BNT162b2) are weak, therefore not strongly affected on different COVID-19 patients. Based on expected (0.25,0.5 and 1) year and utility value (17,19.5 and 34.5) dollars QALYs and CEA estimated. Considering the above analysis, we make the following discussion.
The COVID-19 pandemic is an economic revolution in the modern history of medical science. We accurately detect this pandemic first in Wuhan, China. Because of the imprecise or economic uncertainty instantly surrounding its spread, the SARSCoV- 2 virus rapidly spread to other parts of the world. It promptly declared that the possible outbreak of a pandemic from the World Health Organization (WHO) in March 2020. Over 375 Chinese cities reported COVID-19 confirmed cases, but about 79% of infection cases across that period didn’t heighten [30,31]. The possible spread of the infectious virus in China was intense because of its untimely detection [32]. This silent transmission blew out instantly and typically spread within the independent country China implemented strict measures to control the COVID-19 in February 2020 [32-34].
After 16th November 2020, Moderna effectively reported a 94.5% effective rate shows the high effectual demand in the international market for all funding agencies and potential donors. Therefore, the COVID-19 vaccine program could face similar challenges to those faced by prior vaccines. USA, Turkey, Slovenia, Andorra, and Monaco show the highest number of confirmed patients. Their accurate QALYs estimation represents 43.58% (QA-1/QA-2) and 28.26% (QA-2/QA-3) in the group (GP-1 to GP-5). Therefore, the above estimation properly representing that mRNA-1273 is 56.41% and 71.73% effective from GBS-price IPO and BNT162b2.
As per daily news SABAH [33] Dec 30, Turkey approved Sinovac (developed by China) vaccine under the consideration of Health Ministry Laboratories (HML) and received emergency approval. Jan 14, 2021, Turkey unanimously approved Sinovac and paving the way for the rollout of Turkey’s vaccination (Ankara’s) program with coordination of health care [34]. After the first shots (Sinovac) to the health minister (Fahrettin Koca), the Turkey medicine and medical agency approved and announced to deliver the concerned department. Also, three million (Sinovac) doses are already received. Turkey is scheduled to collect a total of 50 million doses [35]. Brazil, Turkey, and Indonesia have examined an effective Sinovac vaccine that is 65% to 91% effective at protection against COVID-19. Global health has already declared that if the vaccine is at least 50% would use in an emergency. Before 4.5 (million) doses were announced to procure by Pfizer (BioNTech) initially and then 30 million more but although it was ongoing. On Feb 8, 2021, Peru received the first consignment of Sinovac Biotech. The next consignment tentatively scheduled arrives on Feb 13, [36]. Furthermore, Indonesia has also received 3 million doses of the effective Sinovac Biotech vaccine. We accurately estimated that the Sinovac Biotech vaccine was rarely effective from Pfizer and mRNA-1273. Therefore, the QALYs estimation results show indecisive results. The mRNA-1273, GBS-price IPO, and BNT162b2 are more effective than Sinovac. So, the QALYs (Years) is sufficiently indicated that COVD-19 patients included in partial health condition and their live estimation around or less than 0.25 years without the standard of quality of life.
Shows that Slovenia approved mRNA-1273 and typically receives a total of 2 million doses at the end of 2021, which is enough for 1.1 million confirmed patients [37]. Besides, 1.4 million doses of the effective AstraZeneca vaccine have been registered and approved by Medicines Agency. And on Feb 1, 2021, they received 9200 and 245000 doses in the first quarter. The European Commission produces actions and accelerates vaccination campaigns, intending to vaccinate 70% of the adult population to prevent this pandemic [38]. After Jan 28, 2021, at least 22 countries accurately reported and sustenance administrating policies to manage the COVID-19 pandemic from Moderna. Jan 30, 2021, Algeria start the COVID-19 vaccination program with coordination of Russian shot (Sputnik-V). The first batch of 0.5 million had promptly ordered for prevention [39]. In Australia, Pfizer/BioNTech has been registered and approved by Therapeutic Good Administration (TGA) and invested $363 million to support R&D and provide an effective vaccine for the spread of COVID-19 [40]. A Chinese company (Sinopharm) has similarly developed Sinovac work as Pfizer and Moderna. Dec 30, 2020, trials of the effective vaccine showed that it correctly was 79% effective-lower than Pfizer and Moderna. January 2021, several Asian (Singapore, Malaysia, Philippines, Pakistan, Indonesia, Turkey, Brazil, Chile, UAE, and Bahrain) have approved Sinovac and gradual rollout with different vaccination programs [41]. In the diplomacy race of vaccine China dearly wins and pledge to set aside $2bn for African continents. While the offer to the Caribbean and Latin American a $1bn loan to buy vaccines [42,43]. In this economic situation, the accurate estimation of QALYs has indicated that partial healthy condition of likely patients without the moral standard of quality of modern life but at least reduce the death rate. However, the excellent health condition of potential COVID-19 patients with the first year after two effective doses of the mRNA-1273.
The EU has approved only Pfizer and Moderna vaccines for preventing this outbreak so far, Andorra implementing the Pfizer vaccine and employing it for the vaccination campaign. Alyssa McMurtry was representing that Spain will deliver 30,000 doses of the Pfizer vaccine [44]. Also, France has agreed to supply Moderna and AstraZeneca to Andorra for the prevention and control of this epidemic situation [45]. Spain will receive 4.5 million doses end of this march. And t’s enough for 2.3 million people. With the coordination of the Council of Europe Development Bank (CEB), Andorra purchases COVID-19 vaccines. In Sep 2020, CEB approved Public Financing Facility (PFF) of €12 million to Andorra. It aimed to prevent and fight against COVID-19. In Jan 2021, Andorra announced a loan utilized for the COVID-19 vaccination program [46]. The African Centers for Disease Control and Prevention (CDC) commended the financial gap with African-Import Bank (AIB) in retrieving the COVID-19 vaccine in South Africa. Africa Medical Supplies Platform (AMSP) will play a significant role and secure 270 million COVID-19 doses (Pfizer) with African Vaccine Acquisition Task Team (AVATT) [47]. In Oceania countries, Guam administered 12,637 vaccine doses. And 1,760 people have been fully vaccinated (Pfizer) with the department of public health and social services [48]. Also, China delivered 150,000 COVID-19 doses to Syria. As for the fight against the COVID-19 pandemic with the coordination of the health ministry [49]. The finding of this study clearly shown that QALYs (Years) is in a healthy condition with 0.5 years after two doses of Pfizer. The CEB funding would be adjusted and used for the impost of VSL-2 and VSL-3 for the preclusion of the COVID-19 pandemic.
Monaco vaccination center approved Pfizer under the governing bodies and declared vaccination process extend to the entire population with opposed groups [50]. The first COVID-19 vaccine took place on Dec 31, 2020. According to Dr. Katherine O’Brien (WHO-director of immunization), AstraZeneca is the effective vaccine for the South African variant [51]. And 90% of new COVID-19 cases vaccinate by one million doses of AstraZeneca (Oxford) [52]. China has decided to provide vaccines to Papua New Guinea (PNG) and strains to overcome the pandemic situation [53]. The Australian governing bodies also have committed to giving $500 million to PNG to support health security and recovery of the economy for this pandemic [54]. Mongolia human drug council approved Pfizer, AstraZeneca, and Moderna vaccines. They also declared that to provide COVID-19 vaccine to every entire population with coordination of World Health Organization (WHO). The finding of this study clearly shown that QALYs (Years) are in a healthy and excellent healthy condition with 0.5 to 1 years. And dynamic strategic policies, donations of different governments, and donor agencies extent to the VLS of human life.
It can provide excellent health perceptive to infected patients. As the mRNA-1273 vaccine flattered the frontrunners of the COVID-19 pandemic, challenges on the formulation of this vaccine and immovability became graciously ostensible for the unified world in highly affected areas of all continents [55]. It has been accepted from the WHO for the emergency use listing and accordingly fulfills WHO’s criteria for Strategic Advisor Group of Experts (SAGE) consideration [35]. Therefore, the QALYs estimation shows a more effective technique by expected year, and quality of life will be ignoring. According to Jackson et al., Moderna is a precise important vaccine, delivered the likely essential of T-cell immunity to vaccination against COVID-19 and produce cytokines (T-Cell Responses), state that anti-COVID vaccine.
Conclusion
This study investigates Cost-Effective Analysis (CEA) of effective COVID-19 vaccine quality-adjusted life years (QALYs) in five continents (213 countries). To examined SARS-CoV-2 (GBS-price IPO), Pfizer (BNT162b2), and Moderna (mRNA-1273) vaccine effects in separate continents typically based on the confirmed number of patients. We gainfully employ modern QALYs techniques. Because three COVID-19 vaccine prices Value of Statistics (VLS) computed individually, this paper uses level data of the European Centre for Disease Prevention and Control, discovered continents, countries, confirmed cases, and deaths cases. Employing the CEA technique, we also analyzed the vaccine’s operational effectiveness for the continents, the price of the vaccine, and availability by different health ministries and funding agencies.
The consistent results of QALYs show that Utility Value (UV) by partial healthy (0.25), healthy (0.5), and perfect healthy (1) based on years in 213 countries (Five continents). Additionally, the Value of Statistics (VLS) in countries in coexistent causation of vaccines. This finding shows that Pfizer (BNT162b2) and Moderna (mRNA- 1273) have caused beneficial and significant effects probably on many likely patients, comparatively SARS-CoV-2 (GBS-price IPO), AstraZeneca (AZD1222), Russia’s Sputnik (AstraZeneca), and Sinopharm Sinovac Biotech. The EU, American, South Africa, Oceania, and Asia have heartily approved Pfizer and Moderna vaccines for preventing this outbreak so far, most countries also implementing the AstraZeneca (AZD1222), ENSEMBLE 2, Russia’s Sputnik (AstraZeneca), and Sinopharm Sinovac Biotech vaccine and employing it as for the vaccination campaign. The accurate QALYs estimation typically shows the UV and expected life of likely COVID-19 patients. Plus, we presented CEA analysis by the value of statistics (VLS) based on donation, and prices of vaccines reduce the flow of this possible outbreak in continents. The direct result from QALYs amply provides empirical evidence that the potential COVID-19 patient’s social life is more valuable equaled to the price of the COVID-19 vaccine. The excellent quality of life precious is between 1 and 0, so each country should invariably respond to properly implement effective strategies for maximum protection against COVID-19 with the most magnificent possibility. To be specific, Pfizer and Moderna obtain a 95% effective rate, while other vaccines (AstraZeneca, ENSEMBLE 2, AstraZeneca, and Sinovac) typically have 50.38% to 70% significant results against COVID-19. This finding voluntarily contributes to the unique (Effective COVID-19 vaccine) results of the QALYs from the previous results. We base it on the technical commission immunization system of each continent. The COVID-19 vaccine could confront similar challenges to those faced by prior vaccination programs.
WHO program to lower the stress of COVID-19, it necessary to consider individual states? The MOH programs subsidize to reduce this pandemic with vaccines and adhere to the regulatory requirements under health projects. In this respect, the European and Americans should analyze and monitor other countries’ pandemic situations and check the sources of vaccines to make sure their efforts are productive in a healthy sense. WHO Strategic Advisory Group of Experts (SAGE), European Centre for Disease Prevention and Control (ECDC), U.S. Department of Health & Human Services (HHS), African Centers for Disease Control and Prevention (CDC), and China National Biotec Group (CNBG) are the primary market driver for COVID-19 production and distribution with coordination of funding bodies. Each continent should implement and develop supplemental policies or vaccinated programs to improve and expand the COVID-19 vaccine to comply with the increasing demand for a healthy and secure life. Additionally, the WHO policies and tactical strategies can develop a more dynamic structure for vaccination. Provisional/ federal governments, regional health organizations, funding, donor agencies, and local communities to foster practical strategies for reducing and preventing this COVID-19 outbreak.
REFERENCES
- Pruckner GJ, Schneeweis N, Schober T, Zweimüller M. Birth order, parental health investment, and health in childhood health care utilization. J Health Econ. 2021;76:102426.
- Mensah Abrampah N, Syed SB, Hirschhorn LR, Nambiar B, Iqbal U, Garcia-Elorrio E, et al. Quality improvement and emerging global health priorities. Int J Qual Health Care. 2018; 30(suppl_1):5-9
- Qian M, Chou SY, Lai EK. Confirmatory bias in health decisions: Evidence from the MMR-autism controversy. J Health Econ. 2020;70:102284.
- New SARS-CoV-2 test is a simple, cost-effective, and efficient alternative for SARS-CoV-2 testing.
- Moorlag SJ, van Deuren RC, van Werkhoven CH, Jaeger M, Debisarun P, Taks E, et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: a retrospective cohort study. Down Syndrome and COVID-19: A Perfect Storm?Espinosa JM. Cell Rep Med. 2020; 1(5):100073
- Moderbacher CR, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996-1012.
- COVID-19 (coronavirus disease 2019) January 2020 updates
- Dai L, Zheng T, Xu K, Han Y, Xu L, Huang E, et al. universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722-33.
- J&J launched Phase 3 trial for 2-dose COVID-19 vaccine
- Goldman L, Weinstein MC, Goldman PA, Williams LW. Cost-effectiveness of HMG-CoA reductase inhibition for primary and secondary prevention of coronary heart disease. JAMA. 1991;265(9):1145-51
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21(5):402-408.
- Liu J, Chen Y, Cao H, Zhang A. Burden of typical diseases attributed to the sources of PM2. 5-bound toxic metals in Beijing: An integrated approach to source apportionment and QALYs. Environ Int. 2019 ;131:105041.
- Pliskin JS, Shepard DS, Weinstein MC. Utility functions for life years and health status. Operation Res. 1980;28(1):206-224.
- Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1(1):1-8.
- European Centre for Disease Prevention and Control. 2020.
- Chen J, Xu C, Song M. Determinants for decoupling economic growth from carbon dioxide emissions in China. Regional Environ Change. 2020;20(1):1-2.
- Pisanic N, Randad PR, Kruczynski K, Manabe YC, Thomas DL, Pekosz A, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol. 2020;59(1):e02204-e02220.
- Chakraborty S, Mallajosyula V, Tato CM, Tan GS, Wang TT. SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand. Adv Drug Deliv Rev. 2021.
- DRAFT Landscape of COVID-19 Candidate Vaccines. 2021.
- Danielson WA, Lasorsa DL. Perceptions of social change: 100 years of front-page content in The New York Times and The Los Angeles Times. In Text Analysis for the Social Sciences. 2020: 103-116.
- Terry M. Updated comparing COVID-19 vaccines: Timelines, types and prices. 2021.
- Latkin CA, Dayton L, Yi G, Konstantopoulos A, Boodram B. Trust in a COVID-19 vaccine in the US: A social-ecological perspective. Soc Sci Med (1982). 2021;270: 113684.
- Cao S. COVID-19 vaccine prices revealed from Pfizer, Moderna, and Astrazeneca. 2020.
- Weintraub K. Moderna's candidate COVID-19 vaccine looks to protect 94.5% of those who get it, trial shows. 2020.
- Sekar K. Funding for COVID-19 Vaccines: An Overview. 2021.
- ADB announces $9 billion COVID-19 vaccine initiative for developing Asia. 2020.
- China’s sinovac gets $500M funding for COVID-19 vaccine.
- Huaxia. China-donated COVID-19 vaccines arrive in Pakistan. 2021.
- Olubiyi OO, Olagunju M, Keutmann M, Loschwitz J, Strodel B. High throughput virtual screening to discover inhibitors of the main protease of the coronavirus SARS-CoV-2. Molecules. 2020;25(14): 3193.
- Haridy R. Study finds COVID-19 spread in China fuelled by “stealth transmission”. 2020.
- Countries where COVID-19 has spread. 2021.
- Turkey exceeds 2 million COVID-19 vaccinations Daily Sabah. 2021.
- Turkey approves China-based sinovac vaccine’s emergency use.
- Sevinclidir P. Turkey’s COVID vaccination plan depends entirely on a Chinese vaccine with mixed trial result. CBS News. 2021.
- Peru receive first batch of Chinese COVID-19 vaccine. 2021.
- Ralev R. Slovenia to get 2 mln doses of Pfizer, Moderna vaccines in 2021-PM.
- Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU/EEA. Technical Report. 2021.
- Algeria starts COVID-19 vaccination drive with Russian shots. 2021.
- About COVID-19 vaccines. 2021.
- Khan R. Cost-Effectiveness Analysis (CEA) of Preventive COVID-19 Vaccines by Quality-Adjusted Life Years (QALYs). 2021.
- Tan Y. Covid: What do we know about China’s coronavirus vaccines?. BBC News. Retrieved. 2020.
- Motta M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc Sci Med. 2021;272:113642.
- Doorley RM, Berke A, Noyman A, Alonso LA, Ribo J, Arroyo V, et al. Mobility and COVID-19 in Andorra: Country-scale analysis of high-resolution mobility patterns and infection spread. medRxiv. 2021.
- Spain to send COVID-19 vaccine to Andorra. 2021.
- Funding vaccinations. Fighting the virus. 2021.
- Huaxia. Roundup: Africa CDC commends African Export-Import Bank for financing COVID-19 vaccine. Xinhuanet. 2021.
- Staff P. Covid-19 vaccine doses given on Guam the Guam Post daily. 2021.
- China to send 150,000 doses of COVID-19 Vaccine to Syria. 2021.
- Errami N. Monaco:vaccination centre, appoitnment, where and how to get vaccinated? Monaco Tribune. 2021.
- Chereau A. Results:there weeks after the start of vaccination in Monaco, where are we? Monaco Tribune. 2021.
- Huaxia. China to assist Papua New Guinea with vaccine: FM. Xinhua Net. 2021.
- Australia providing $144 million to help Papua New Guinea procure and deliver safe and effective COVID-19 vaccine. 2021.
- Mongolia registers three coronavirus vaccine. 2021.
- Crommelin DJ, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997-1001.
- Oronsky B, Gruber HE, Reiners W, Reid TR. A short discussion about the SARS-CoV-2 mRNA-1273 vaccine. Int J Infect Dis. 2021;104:532-533.
Citation: Khan R (2021) Which COVID-19 Vaccine is Efficiently Most Cost-Effective and Why? J Vaccines Vaccin. 12:464.
Copyright: © 2021 Khan R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

