Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2022) Volume 13, Issue 2
Vaccine Epidemiology, Evaluation, and Constraints of Vaccine Effectiveness: A Review
Habtamu Endale*, Saliman Aliye, Haben Fesseha and Mesfin MathewosReceived: 01-Mar-2022, Manuscript No. JVV-22-16075; Editor assigned: 03-Mar-2022, Pre QC No. JVV -22-16075 (PQ); Reviewed: 24-Mar-2022, QC No. JVV-22-16075; Revised: 31-Mar-2022, Manuscript No. JVV-22-16075 (R); Published: 07-Apr-2022, DOI: 10.35248/2157-7560.22.13. 476
Abstract
Vaccines are all biological substances produced from living things, administered to trigger the host’s body defense system to develop immunity against a specific pathogen from which they are produced. They are produced either from the whole organism or parts of it. There are several types of vaccines like live virulent, live attenuated, inactivated (killed), subunit, toxoid, sero-vaccine, and autogenous vaccine. Vaccines work by stimulating either humoral or cellmediated immunity or both to differentiate. Even though vaccination is the powerful and cost-effective weapon of disease prevention and control of infectious and diseases, there are factors those, hinder its effectiveness (constraints of vaccine effectiveness). These factors are technical constraints, pathogen-related constraints, vaccine related factors, host-related and environmental and management-related constraints. While planning a vaccination regimen, it is important to test the potency of the vaccine, whether it much with circulating serotype, availability of cold chain and skilled manpower, the status of the target group, whether condition. Vaccine epidemiology, the study of vaccine interactions and impacts on the epidemiology of vaccine-preventable diseases also has an impact on vaccine effectiveness. it includes basic reproductive number, the force of infection, herd immunity, and epidemiologic shift. Some review papers mostly deal with constraints of specific vaccines and species of animals and with a specific constraint of the vaccine. However, the papers which review all common constraints of vaccine are limited. Therefore, this review paper is to address the most common constraints on the effectiveness of vaccines in all animal species and to highlight on evaluation of vaccine effectiveness and epidemiology.
Keywords
Constraints; Effectiveness; Failure; Vaccine
Abbrevations
APCs: Antigen Presenting Cells; BCG: Bacillus of Calmette and Guerin; CDV: Canine Distemper Virus; FeLV: Feline Leukemia Virus; Hib: Haemophilus influenzae-b; HPA: Highly Pathogenic Avian Influenza; IIV: Inactivated Influenza Vaccine; LAIV: Live Attenuated Influenza Vaccine.
Introduction
The phrases vaccine and vaccination are derived from Variolae vaccinae (cowpox), a name coined by Edward Jenner (who invented the first vaccine as well as the concept of vaccines) to describe cowpox. In 1798, he coined the expression to describe the protective effect of cowpox against smallpox in his Inquiry into the Variolae vaccinae known as the Cowpox [1]. To honor Jenner, Louis Pasteur proposed in 1881 that the words be extended to include the new defensive inoculations that were being developed at the time [2].
Because it was originated from a virus that affects cows (Latin: Vacca ‘cow'), the immunization was given the name vaccination [3]. Edward Jenner's discovery of vaccinology in 1796 was the first scientific-methodical examination of cowpox vaccination, but numerous variants in technique were used in Central Asia, China, and Turkey by utilizing dried pus from smallpox skin lesions. Jenner's cowpox vaccination process was introduced into Japan in the Edo Period, and practitioners of herbal traditional medicine, studying western modern medicine, intended to employ Jenner's cowpox vaccine as a prophylactic procedure for smallpox [4].
Vaccines are crucial in the prevention and control of the disease. Infectious illness mortality and morbidity have been steadily decreasing thanks to vaccines. In many countries, vaccination is an important part of commercial disease management programs since it is the most cost-effective way of preventing and controlling the spread of economically important diseases including Marek's disease, Newcastle disease, FMD, Rabies, and others [5].
Vaccines can include the complete disease-causing microbe or just a portion of it. They can be made from living organisms that have been weakened, usually through growing under less-than-ideal conditions (also known as attenuation), or via genetic modification that reduces their potential to cause disease. Others come from inactivated complete organisms or components of the disease-causing organism, such as particular proteins and polysaccharides, or nucleic acids. Inactivated toxins from toxin-producing bacteria and the linking (conjugation) of polysaccharides to proteins (which improves the efficiency of polysaccharide vaccinations in children) are other sources [6].
According to Day et al [7] there are several vaccine formulations available in the field of veterinary medicine, used for immunization of both infectious and non-infectious diseases. These are live virulent, live attenuated, heterologous, killed, subunit, marker vaccines, and naked DNA vaccines, and recombinant organisms.
Veterinary vaccinations are believed to be available for around 400 diseases affecting mammals, birds, and fish, as well as farm animals, pets, and wildlife [8]. When used in conjunction with other control methods like quarantine and movement control, culling, improved producer and trader knowledge, improved sanitation and husbandry, improved biosecurity (both bio exclusion and biocontainment), and improved food safety, important diseases of the animals can be eradicated by vaccination [9].
The major goal of livestock vaccinations, on the other hand, is to boost total production for primary producers, and the cost-benefit analysis that results from vaccination is the industry's bottom line. The fundamental purpose of zoonotic or food-borne infection vaccination is to reduce or eliminate consumer risk and, in some situations, to improve individual animal productivity. Vaccination of wildlife is usually only addressed for infections that can be transmitted to humans (zoonotic illnesses), though animal welfare is becoming more important [10].
"Ideal" immunity not only defends against clinical disease (morbidity and mortality), but it would also prevent infectious organisms from infecting, replicating, spreading, or progressing. Some vaccines are capable of providing this level of protection. Some, on the other hand, may just reduce morbidity and/or death without resulting in sterilization [11].
Vaccine failure and protection are two sides of the same coin [12]. Infield conditions, vaccine effectiveness is defined as a reduction among risk in vaccinated persons compared to similarly exposed unvaccinated persons It is determined not only by the vaccine's initial (intrinsic) quality as supplied by the manufacturer, but also by extrinsic factors including vaccine storage and distribution, vaccine match, vaccination schedule, and, indirectly, vaccine coverage [13].
According to Crowkraft et al [12], vaccine effectiveness and vaccine efficacy, are two distinct concepts that are frequently confused in the literature. Vaccine efficacy is the proportional reduction in infection in a vaccinated group compared to an unvaccinated group under optimal conditions such as a randomized controlled trial, whereas vaccine effectiveness is the proportional reduction in infection in a real-world immunization program delivered with normal storage and administration processes to an unselected population.
A vaccine's ability to prevent disease is dependent on its potency and proper administration to people who are capable of responding to it [14, 15]. Vaccine failure occurs when an individual is fully vaccinated but contracts an infection or sickness. It is either primary (infection or disease in a fully vaccinated individual who failed to mount an immune response to the vaccine) or secondary (infection or disease in a fully vaccinated individual who mounted a normal immune response to the vaccine (which may or may not have been measured) but whose immunity has since waned) [12,15]. Some review papers mostly deal with constraints of specific vaccines and species of animals and with a specific constraint. However, the papers which review all common constraints of vaccine are limited. Therefore, this review aims to highlight vaccine epidemiology, evaluation, and the most common constraints on the effectiveness of vaccines in veterinary service.
Epidemiology of Vaccine
The study of vaccination interactions and their effects on the epidemiology of vaccination-preventable diseases is known as vaccine epidemiology. Illness burden and immunization coverage are linked using epidemiological ideas such as detecting disease trends based on geographical, management, and sex disparities. When should the mass vaccination campaign take place? What age group should mass campaigns be aimed at? Where should immunization programs concentrate their efforts? What causes epidemics in the first place? Why do some youngsters appear to be immune to illness while not having gotten any vaccinations? Understanding vaccine epidemiology has the potential to save more lives and improve people's health around the world. Vaccine epidemiology is crucial for a variety of reasons, including expanding vaccination advantages to new populations and selecting vaccine target groups [16].
Basic reproductive ratio
The basic reproduction ratio (R0) for every infectious disease is a measure of a pathogen's transmissibility, representing the average number of new infections caused by an infectious person in a community of completely naive individuals. For R0 > 1, the number of infected people is expected to rise, but for R0 1, transmission is likely to stop. The basic reproduction number is a key notion in infectious disease epidemiology, showing a disease's risk of spreading epidemics [17]. The estimated number of hosts infected after one generation of the parasite by a single infectious person who was introduced into an otherwise naive population is known as R0 [18]. The epidemic threshold parameter aids health providers in calculating coverage (the degree of vaccination coverage/portion of the population vaccinated) and guiding them through the vaccination strategy planning process [19,20].
Force of infection
The "power or rate of infection" determines the likelihood of becoming infected. The number of infectious individuals present, the rate at which they come into touch with one another, and the infectiousness of those persons are all factors that go into determining the magnitude of infection. Because transmission is a dynamic process, the force of transmission will alter over time [16,21]. To make informed vaccine policy decisions for vaccine-preventable diseases, a thorough understanding of disease burden and transmission patterns will be necessary [22]. As long as the transmission rate is larger than the removal rate, infected people reproduce at a higher rate than those who recover (basic reproduction number). This has an impact on vaccine effectiveness.
Herd immunity and herd effect
Herd immunity is defined as "the proportion of subjects in a population who have immunity." This distinction is made between herd immunity and the "herd effect," or the indirect defense observed in the unimmunized section of a population in which a high proportion is immunized. It refers to the reduction in illness or sickness among the unvaccinated population as a result of immunizing a subset of the population" [23]. When a high number of animals in a group or community are resistant to an agent, the chances of diseased and susceptible individuals interacting are lowered [18]. This population-level impact is frequently discussed in the context of vaccination programs, which aim to develop herd immunity in animals that cannot be vaccinated, such as the very young and immune compromised [24].
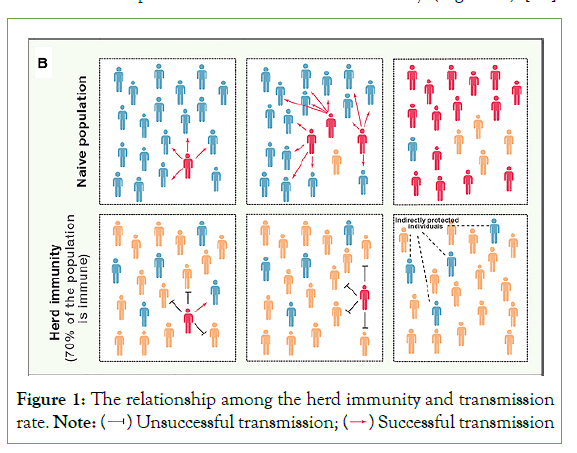
Selective vaccination of groups that are critical in transmission can delay transmission in general populations or lower incidence in population segments that are at risk of serious infection consequences. The potential for “transmission-blocking vaccines” is an especially intriguing example of using vaccines to limit transmission [25]. For certain disease life cycles to propagate efficiently, a certain portion of the population must be vulnerable. If the proportion of the population that is resistant (through vaccination or spontaneous infection) approaches the threshold, the pathogen's occurrence will drop. This is known as the 'herd immunity threshold,' and it emerges because a fraction of the vulnerable population receives 'indirect protection' from the immunization of those in their immediate vicinity. Individual immunity has a significant impact on pathogen evolution and host health. Individual immunity has an impact on disease transmission dynamics and the efficacy of vaccination campaigns for entire host populations, which is important to note [26]. The infectious agent's transmissibility, the essence of the vaccine's immunity, the pattern of mixing and infection transmission in populations, and, most importantly, the vaccine's and immunity's distribution throughout the population all influence the size of the indirect impact of vaccine-derived immunity [25] (Figure 1).
Figure 1: The relationship among the herd immunity and transmission rate.
The herd immunity threshold is calculated by the formula 1–1/R0, where, R0, is the basic reproduction number. For instance, if R0 is 3 the herd immunity threshold is 0.667, which means 67% of the population is needed to stop disease spread. As a result, the more communicable a pathogen is, the higher its R0 and the bigger the fraction of the population that must be immune to prevent long-term transmission [24].
Epidemiologic shift or transition
A transformation in the pattern of disease in a community is referred to as an "epidemiological shift" or "transition." The individual characteristics of the disease are affected by changes in the age of occurrence and severity of diseases, as observed in populations with incomplete immunization coverage or vaccine coverage for specific age groups alone [27-28,16]. It's especially important in diseases like pneumococcal illness, where numerous serotypes are linked to the disease, and vaccines targeting a single serotype can contribute to the creation of new serotypes. An epidemiological shift or transfer could have an impact on the immunization program's benefits. This highlights the significance of regularly monitoring epidemiological improvements in immunization programs and taking corrective action when necessary [16].
Evaluation of Vaccine Effectiveness
Vaccines affect both people and populations. Susceptibility [29], Infectiousness (VEi), and illness progression (biological or individual level effect) are all influenced by vaccines. The "population-level impacts" of vaccination are dictated by vaccine coverage and distribution, as well as how well different groups blend. The biological and behavioural effects of the vaccine may be to blame for these side effects. The effect of both the vaccinated and unvaccinated populations determines the overall effects of vaccination programs on public health [16].
There are at least three forms of vaccine consequences at the population level as a result of this: The immediate outcome is a reduction in the probability of developing the disease by comparing vaccinated and non-vaccinated animals from a population that has the same immunization programs aimed at eliminating program-specific effects from the same population [30]. Indirect influence: The influence of extended immunization on animals that do not get the vaccination at the community level. Vaccination of one animal can prevent infection of another, either by preventing the first animal from being infected and infectious for the second or by making the pathogen less communicable even if the first animal developed the disease. The former of these mechanisms is known as the contagion effect, while the second is known as the infectiousness effect [16].
According to Lahariya and Weinberg et al. [16,31], observational research in the field of a vaccination program or experiments performed under standard program conditions is used to assess vaccine efficacy. It is based on internal or individual variables, such as the existence of maternal antibodies at a level that can neutralize the vaccine, maturity of the immune system of the vaccine recipient, and the antigen load and strain of pathogen in the vaccine; for example, the effectiveness of the measles vaccine is based on the presence of high level of maternal antibodies, the maturity of the immune system of the vaccine recipient, and the antigen load and strain of the virus in the vaccine [32]. The mathematical expression of the ability of vaccine protect from infection was proposed by Greenwood and Yule in 1915 for inactivated whole-cell cholera and typhoid vaccines and used nearly 100 years ago [33].
VE (%) = (RU-RV)/RU ×100
RU = the or attack rate in unimmunized animals and
RV = the rate of disease occurrence or attack rate in vaccinated animals
According to Thomas et al. [21], vaccine effectiveness is the cumulative result of the reduction in the expected clinical events that might be associated with the disease. Observational studies like Cohort studies, case-control study and screening are used to assess vaccine effectiveness. Retrospective case-control analysis is the most commonly used study design to assess a vaccine's effectiveness and the odds ratio obtained in the study (Retrospective case-control analysis) is used in the formula to deduct vaccine effectiveness, as follows:
Vaccine effectiveness = (1-OR) × 100
Where OR = Odds Ratio
Constraints of Vaccine Effectiveness
Vaccines are typically very effective but permanent and complete protection from infection is rare. Problems with either client education or compliance with good animal management practices are one of the causes of vaccine failure to protect from disease. Vaccine effectiveness is determined by several factors. It is constrained by the vaccine, pathogen, host, environment, and vaccination technique-related factors [34].
Technical constraints
Failure of selection of appropriate vaccine and using the expired vaccine: It is important to assess the various options in terms of purity, safety, potency, subtype, thermo-tolerance, ease of use, labels and instruction sheets in local languages, availability, registration status, and vaccine ability to provoke an immune response that can be differentiated from that generated in response to infection with field strains [35]. It is also important to know the prototype of the pathogen circulating in the area to which the vaccine regimen to be started in the case of pathogens having more than one serotype with no cross-reactivity among the serotypes [36].
For example, the period 2013/2014 to 2015/2016, in the United Kingdom, like the USA was dominated by circulation of A(H1N1) pdm09, followed by drifted A (H3N2) and A (H1N1) pdm09, respectively, together with Influenza-B each season. During the three seasons in the UK pediatric program, the overall live attenuated influenza vaccine (LAIV) was effective 53.1% (significant), for both laboratory-confirmed and influenza B infection in the UK was but the effectiveness for inactivated influenza vaccine (IIV) was 31.5% (non-significant). The live attenuated influenza vaccine (LAIV) effectiveness was significant vaccine effectiveness against both laboratory-confirmed A(H3N2) and influenza B infection but effectiveness against A(H1N1) pdm09 was moderate although non-significant. Conversely, at the same season, IIV showed an insignificant effect against influenza B or A(H3N2) but a significant effect (100%) against A(H1N1) pdm09 [37]. Botha [38] and Heininger et al. [39] showed that the shelf life of the vaccine has an effect on its effectiveness and vaccine loss potency before expiry date with mishandling so, vaccines are best when used before the expiry date provided by the manufacturer along with proper storage and transportation and ineffective when used after expiration.
Defect in vaccination technique and route
The technique used to vaccinate the victims greatly influences both the cost and the effectiveness of the vaccination programs [35].
Handling: Mishandling of vaccine in either a storage or application were speculated to be the actual cause of vaccine failure especially live vaccines, which result in killed or destroyed vaccine (e.g., live cell-mediated Marek’s disease vaccines) [40-41].
Diluent used: Water sanitizers destroy live vaccines when given in drinking water if not removed before the vaccine is added [42].
Associations: Vaccination with unrecommended live virus vaccines that have the same target tissue affect the immune response for individual vaccines by overwhelming the function of the immune system [43].
Route: The route of vaccination is the major problem in mass vaccination and when vaccinators do not deliver the vaccine to the appropriate vaccination site shown by manufacturers of the vaccine (drinking water and aerosol) in the former their uniformity and the subsequent result are lower than individual administration[44, 45].
For instance, Giri et al. [46] showed the variation in protective efficacy of BCG (vaccine against tuberculosis) when administered by different route by evaluating the bacterial burden in the lungs and spleen of mice by inoculating with Mycobacterium tuberculosis H37Rv after BCG vaccinating through intranasal (I.N) and subcutaneous (S.C.) routes. The mice developed significant immune responses at the local level (mediastinal lymph nodes, cervical lymph nodes, and lung) with intranasal BCG vaccination than S.C BCG immunization. Further, there was a significantly higher reduction in bacterial load in the lungs of mice with I.N vaccination than S.C. BCG vaccination, whereas, the bacilli load in the spleen was comparable in both the groups. Hence, intranasal vaccination of BCG is the preferable route for pulmonary tuberculosis. Also, in fishes, oral vaccination results in a smaller immune response, in which the vaccine is degraded in hindgut and lymph nodes and a small amount of vaccine absorbed and in immersion if the temperature of the water is low, diffusion of vaccine reduced leading to lower dose absorption [47].
Problem in vaccine conservation and distribution
Vaccine instability is frequently a major issue during clinical development (from lab to clinic) and commercial distribution (production to the patient) [48]. On exposure to temperatures outside of the recommended narrow range,2°C-8°C recommended by WHO vaccines lose potency faster, while the exposure to heat is bad for almost all vaccines, exposure to freezing is equally damaging for the freeze-sensitive vaccines such as diphtheria, Hepatitis B, pertussis, tetanus toxoid, liquid Haemophilus influenzae-b [41], and killed poliovirus making them inactive. On exposure to freezing temperatures, the adjuvants in these vaccines clump together, which adversely affect the immunogenic potency [49].
Heat sensitivity is common in live attenuated vaccines during long-term storage in the solid-state and during short-term storage preparatory to injection. As a result, the vaccine cold chain must be carefully maintained for these vaccinations. Inactivated and subunit vaccines, on the other hand, are more stable and are usually created as liquids, but they might be frozen sensitive and lose effectiveness during storage and delivery [48]. Cold chain requirements are a significant financial and logistical burden, particularly in developing countries where refrigeration and electricity are limited. As a result, nearly half of all global immunizations are lost, resulting in the use of inefficient, sub-therapeutic doses [50].
Missing annual revaccination or booster dose
Booster doses of vaccine are frequently recommended by vaccine producers after an initial course. Most of the time, this advice is based on their own duration of immunity tests, which show that animals given a primary round of immunization are immune when challenged for 12 or sometimes 24 months, primarily with inactivated vaccines [51,52]. For example, observation shows that antibody levels persist for three years after vaccination against the canine virus, therefore vaccination is not required before three years, but revaccination (booster dose) is required after that. The excessive time between the first and second (booster) doses reduces the secondary antibody response, as well as the length and quality of the resulting immunity [53]. The first dose primes the host's immunity, which is then completely enhanced by the booster dose. As a result, vaccination failure occurs when a booster dose is missing [54]. For example, because small, young fishes' immunity is underdeveloped, vaccination by immersion does not provide long-lasting protection, hence revaccination is required [55].
Wrong timing of vaccination
Mainly during winter seasons, vaccination of animals in the hot hour of the day when animals feel stressed, this, in turn, affects the function of the immune system of the animals, so does not respond efficiently, instead, vaccine may result in disease and subsequent vaccine failure. Therefore, the regimen must be in the morning and later time of the cold hours when animals feel comfortable [56, 54].
Insufficient vaccination coverage and inadequate dosage
It is mandatory to vaccinate as many animals as possible within a herd or population. This is related to the concept of 'herd immunity,' in which a particular degree of vaccine protection (usually reported as 75% of a human population) lessens the risk of disease endemics. Infectious illnesses may re-emerge if vaccine coverage in a population falls below this level [57]. Even though the vaccine efficacy is very high, its effectiveness is affected by vaccination coverage. Van Boven et al. [58] shows that the overall incidence rate is substantially lower in the vaccine group than in the placebo group, hence there is an inverse link between vaccine coverage and effectiveness. With great vaccination coverage and low transmission, the incidence rate of placebo becomes very low [59].
If an optimal dose is not injected into animals, the vaccine does not produce a fruitful result, overdosage leads to detrimental reaction (mainly live vaccines, require fewer dose) and under dosage (mainly killed vaccines, need higher dose) contain low antigen thus, does not stimulate the immune system and both culminate in vaccine ineffectiveness. The use of chlorine-containing water for vaccination, presence of antimicrobials in the water used and use of vaccine beyond the number of animals allowed by the manufacturer are among factors resulting in suboptimal dosage [54]. It is common in poultry vaccination in mass vaccination through drinking water, in which all birds do not take the optimal amount of the vaccine and spray in which the temperature and humidity of the room, water used, and the size of the particle affects the amount of vaccine absorbed [60].
Insufficient time between vaccination and exposure
Vaccination does not confer instantaneous immunity. The body of an animal takes days to weeks if not longer, to respond to a vaccine. It can take up to two weeks following the second immunization in a series for some vaccinations to provide effective immunity. If an animal is exposed to a disease before a vaccine has had time to stimulate the body's immune system, the animal is susceptible to the disease [53].
Pathogen related constraints
Antigenic shift and drift and pathogen evolution: Antigenic shift is associated with major changes and generally occurs through horizontal gene transfer when a single host co-infected with more than one strain of a single pathogen, while, antigenic drift refers to relatively minor changes in surface structures that occur through point mutations both results in antigenically novel pathogen [61]. The virus that has undergone antigenic shift remains prone to antigenic drift. Due to genetic mismatch between the vaccine strain and the infective strain, an antigenically drifted or shifted strain can result in reduced vaccine effectiveness (though not all drifted strains evade vaccine-induced immunity in the population), as demonstrated by numerous studies documenting vaccine effectiveness against drift variants and well-matched viral strains [62-64].
In-host evolution of pathogens can provide severe hurdles to vaccine development, as seen in the human immunodeficiency virus. As observed in the situations of influenza and dengue fever, several diseases have complex patterns of strain evolution and recurrence in which previous immunity influences vaccine response [65]. Prior immunological responses to vaccination or infection might diminish the immunological response to later vaccinations (a process known as "original antigenic sin" or "antigenic seniority") [66, 12].
Vaccine related factors
Vaccine quality and degree of attenuation: The efficacy of vaccines is influenced by their quality (e.g., potency). Vaccines normally function better in the lab than in the field because the settings are cleaner, and the animals used in research are frequently devoid of specific pathogens and have not been exposed to other immunosuppressive substances [39]. There is a need to develop ways to directly test the antigenic content of vaccines and to create guidelines for the minimal amount of antigen in a vaccine so that vaccine batches can be evaluated without challenge trials [67]. Normally, virulent live organisms are not utilized as vaccines because they have been attenuated. The pathogen loses its virulence not just to cause disease but also to prompt the immune system to respond to the vaccine if the attenuation degree is very high [53].
Vaccine serotype
In the case of pathogens with more than one serotype (e.g. infectious bronchitis virus), vaccine strain does not protect from all field strains of the same pathogen and the protection level of vaccine is low when it is highly attenuated and/ or the pathogen is highly virulent [45]. Some serotypes are more common in one place than others. Any area's disease-causing agents and serotypes are critical for vaccine development. The foreign vaccine may not contain serotypes same as the field strain and disease outbreaks can occur if local vaccine antigens are not used, so vaccines must comprise all probable locally circulating serotypes of the infectious agent [68,54].
Direct exposure to sunlight
It has been established that vaccines are transported in the same manner as other drugs and that direct sunlight emits UV radiation that is lethal to live vaccines. This causes antigens in vaccine vials to die or be destroyed, reducing the number of viral antigens (antigen load), and ultimately rendering the vaccine ineffective [69].
Using polyvalent vaccines
Polyvalent vaccines are those protecting against several infectious diseases, as such, they are all-in-one vaccines. These vaccines culminate in vaccine failure by causing immunosuppression which may occur as a result of antigen overload or of one antigen component of the vaccine preventing the immune system from responding to another component which is termed vaccine interference [71]. When utilizing multi pathogen or multivalent vaccinations, the various components interact with one another, resulting in an inappropriate immune response. This can include antagonistic or synergistic effects, antigenic competition, and/or epitope suppression. Overburdening the immune system is another term [72].
Adjuvant used in vaccine
To elicit an appropriate immune response, all non-living vaccines need an adjuvant [73]. It's also been observed that the adjuvant in non-living vaccines can create an allergic reaction in the patient and prevent the immune system from producing a response to the vaccine [74, 75].
Host related factors
Maternal antibody: During the perinatal stage, new born animals receive immunoglobulins from their mothers. For a few weeks, the mother's antibodies circulate in the blood of the infant. The maternal antibodies are too low to provide disease protection yet too high to allow vaccination to act for a period of days to weeks. This period is known as the susceptibility window [53]. When animals are vaccinated (especially with live vaccine) in the first two weeks of the age when antibody found at a high level in the body, the vaccine is neutralized and result in vaccine failure [45].
Most maternal antibodies have a decay half-life of 16 to 28 days; during this time, antibody levels often decay to a level that is still high enough to prevent vaccine responses but not strong enough to withstand a field infection, allowing infecting organisms to take advantage of a window of opportunity [76]. Due to factors like the amount of antibody in maternal colostrum and the amount of colostrum consumed and absorbed by individual young animals, the timing and duration of the 'window of sensitivity can vary greatly between individual animals and even across animals within the same litter. As a result, individual animals from the same litter had different responses to immunization. For instance, one animal in a litter may have a ‘window of vulnerability between ten and twelve weeks of age, whereas another, who had less colostrum or of lower quality colostrum, may lose maternal protection early and have a ‘window of susceptibility between six and eight weeks of age [57].
Immune status of the target group animals
Considering or checking the health status of the animal before vaccination is highly important. In sick and ill animals, vaccination may not be effective; instead, a vaccine reaction may ensue, resulting in more stress and a higher rate of morbidity and mortality. Furthermore, any other illness condition could lead to vaccination failure. When animals become morbid due to the same disease for which vaccination was administered, vaccine failure occurs because antibodies created against the pathogenic agent neutralize the vaccine antigen, causing a reaction in the animals' bodies, and immunization may worsen the disease's condition [54].
Animals' specific and non-specific immune responses are negatively affected by secondary immunodeficiency associated with various concurrent viral and parasite illnesses. Immunosuppression can be the cause or result of any endo-parasitosis. Various variables, such as the animal's surroundings, stress, nutrition, chemotherapy, surgery, or long-term antibiotic treatment, cause leukogram disruption and subsequent immunosuppression, resulting in the animal's failure to respond to immunization.
Genetic factor
Vaccine responses vary depending on the species or commercial hybrids [77, 45]. The immune system is in charge of surveying, identifying, and responding to an exposure. Recognition of foreign and hazard signals stems from the ability of antigen-presenting cells (APCs) to expose specific pathogen-derived peptide-binding groove, and the genetic constitution of the individual determine it. Thus there is great genetic variation among the population resulting in varied responses to vaccination (failures) [78]. Overall, genetic factors play important roles in regulating responses to vaccines, and identification of the genes involved in the responses will likely help in effective vaccine development [79].
Environmental microbiota
Increasing data suggest that early life exposure to microbial flora promotes immune system expansion; consequently, the microbiological environment into which a newborn is born has a significant impact on immunological development and, as a result, subsequent ability to make appropriate responses [80]. Studies showed that environmental exposure influences the animal body microbiota, and this affects vaccine immune responses. For example, gut microbiota influence the growth and differentiation of gut epithelial cells and play a pivotal role in nutritive, metabolic, immunological, and protective functions [58].
Average age of the animals to be vaccinated
With aging, both the immunological and endocrine systems undergo significant changes, including a decrease in the ability to mount suitable antibodies, reducing vaccine efficacy. Innate immune cells' functional capability deteriorates. Dendritic cell phagocytic capability is reduced, which affects antigen presentation and adaptive immune system activation [81]. Aging is linked to deterioration in immunological capabilities, leading to immunosenescence, as a result of changes such as a drop in the B and T cell repertoire, as well as a drop in the naive cell pool, while memory and terminally differentiated T effector cells of limited diversity rise. As a result, the vaccine's antigenicity is low, and its efficiency is reduced [82].
Vaccine effectiveness is affected by the age at which the animal receives the vaccine. The age at which the last vaccination was given to the children before they became ill was found to be a major risk factor for immunization failure. For example, there was a considerable negative connection between vaccination failure and age at last immunization previous to sickness. The later a puppy gets this last immunization, the smaller the chance of the canine parvovirus vaccine failing. This supports the concept that final vaccination in puppies under 16 weeks of age predisposes to vaccination failure, and that all canine parvovirus vaccines should be given to puppies at least 16 weeks of age, especially in outbreak settings [34].
Environmental and management factors
Uncomfortable microenvironment: Stress is a susceptible homeostatic condition that is influenced by management and environmental factors. Cold stress, heat stress, high humidity, and a dusty environment are among environmental stressors. Factors resulting from mismanagement like shipping stress, intensive farming, high stocking density, overcrowding, decreased ventilation, poor litter conditions (like very wet or extremely dry litter), inappetence, lack of water, bad sanitary conditions, fever, and so on. These all cause stress in animals resulting in immune suppression and subsequent vaccine failure in the herd [54].Nutrition
Malnutrition is linked to innate and adaptive immune dysfunctions and is thus a primary factor reducing vaccination efficiency and effectiveness [83]. Nutrition affects the immune system and its responses to vaccines. Inadequate macronutrients or certain micronutrients, including zinc, selenium, iron, and antioxidant vitamins, are critical for immunological response and vaccine effectiveness. Antioxidants and cofactors are involved in cytokine regulation. For instance, malnutrition impairs immune responses to yellow fever, smallpox, tuberculosis, and polio vaccines [79]. The effects of malnutrition on immune status is given in (Table 1).
| Nutrient Deficiency | Immune Status |
|---|---|
| Vitamin A | ↓ IgA and IgG serum levels, ↑ inflammatory cytokine levels, lymphopenia, ↓ T-cell responses, especially TH2, ↓ mucosal barriers function, ↓ phagocytic and NK cell functions |
| Vitamin C | ↓ Phagocyte function, ↑ infection risk |
| Vitamin E | ↓ T-cell function, ↓ antioxidant defense, ↑ PGE2 production, ↑ the virulence of pathogens |
| Zinc | ↓ Th1 cytokines and thymic hormone activity, ↑ glucocorticoid production, ↑ cellular immune responses to pathogens, lymphopenia, thymic atrophy, altered T-cell subsets |
| Iron | ↓ T-cell immune response and IL-2 production, ↑ IgG levels, ↓ phagocytic activity, ↓ cytokine response, risk of parasitic and opportunistic Candida species infections |
| Selenium | ↓ Infection virulence and progression, ↓ antioxidant defense |
| Copper | Lymphopenia, ↓ IL-2 response |
Table 1: Effects of malnutrition on immune status.
Climatic factors
Fluctuation in elements of climate (temperature, rainfall, humidity, and soil moisture) affects both living and non-living creatures. When it became unfavorable, climatic change leads to stress which in turn affects the host`s immune system resulting in a defect in the function of immune cells and systems with subsequently reduced response to vaccine or vaccine failure [54].
Conclusion
The vaccine is an immune-stimulant chemical that is used for disease control and prevention strategies. Yet, its effectiveness is constrained by several factors that emanate from different sources, like technical factors which consist failure of selection of appropriate vaccine, defect in vaccination technique and route, antigenic shift and drift of the pathogen, vaccine conservation, and distribution problem, missing annual revaccination or booster dose, wrong timing of vaccination time, inadequate dosage, use of the expired vaccine, insufficient vaccine coverage and insufficient time between vaccination and exposure. Vaccine epidemiology has a role in increasing vaccine effectiveness through address the constraints.
REFERENCES
- Baxby D. Edward Jenner's unpublished cowpox inquiry and the Royal Society: Everard Home's report to Sir Joseph Banks. Med. Hist. 1999;43(1):108-110.
[Crossref] [Google Scholar] [Pub Med]
- Hussain S. Immunization and vaccination. Psychiatry of Pandemics. 2019:153-177.
- Lombard M, Pastoret PP, Moulin AM. A brief history of vaccines and vaccination. Rev. sci. tech. - Off. int. épizoot. 2007;26(1):29-48.
[Crossref] [Google Scholar] [Pub Med]
- Nakayama T. Vaccine chronicle in Japan. J. Infect. Chemother. 2013;19(5):787-798.
- Collett SR, Smith JA, Boulianne M, Owen RL, Gingerich E, Singer RS, et al. Principles of disease prevention, diagnosis, and control. Diseases of poultry. 2020; 13:1-78.
- Clem AS. Fundamentals of vaccine immunology. J. Glob. Infect. Dis.2011;3(1):73.
[Crossref] [Google Scholar] [Pub Med]
- Day MJ, Schultz RD. Veterinary immunology. CRC Press; 2010.
- Knight-Jones TJ, Edmond K, Gubbins S, Paton DJ. Veterinary and human vaccine evaluation methods. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1784):20132839.
[Crossref] [Google Scholar] [Pub Med]
- Capua I, Alexander DJ. The challenge of avian influenza to the veterinary community. Avian Pathol. 2006;35(3):189-205.
[Crossref] [Google Scholar] [Pub Med]
- Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev.2007;20(3):489-510.
[Crossref] [Google Scholar] [Pub Med]
- McVey S, Shi J. Vaccines in veterinary medicine: a brief review of history and technology. Vet Clin North Am Small Anim Pract. 2010;40(3):381-392.
[Crossref] [Google Scholar] [Pub Med]
- Crowcroft NS, Klein NP. A framework for research on vaccine effectiveness. Vaccine. 2018;36(48):7286-7293.
[Crossref] [Google Scholar] [Pub Med]
- Plotkin SA, Plotkin SA. Correlates of vaccine-induced immunity. Clin. Infect. Dis.2008;47(3):401-409.
- Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J. Immunol. 2005;175(2):633-639.
[Crossref] [Google Scholar] [Pub Med]
- Klein NP, Bartlett J, Fireman B, Marks MA, Hansen J, Lewis E, Aukes L, Saddier P. Long-term effectiveness of zoster vaccine live for postherpetic neuralgia prevention. Vaccine. 2019;37(36):5422-5427.
[Crossref] [Google Scholar] [Pub Med]
- Lahariya C. Vaccine epidemiology: A review. Journal of Family Medicine and Primary Care. 2016;5(1):7.
[Crossref] [Google Scholar] [Pub Med]
- Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020.
[Crossref] [Google Scholar] [Pub Med]
- Smith DR. Herd immunity. Vet. Clin. N. Am. - Food Anim. Pract. 2019;35(3):593-604.
[Crossref]
- Ke R, Romero-Severson E, Sanche S, Hengartner N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J Theor Biol. 2021;517:110621.
- Nikbakht R, Baneshi MR, Bahrampour A. Estimation of the basic reproduction number and vaccination coverage of influenza in the United States (2017-18). Journal of Research in Health Sciences. 2018;18(4):e00427.
- Thomas M, Sahu D, Raj Y, Pandey A. A probability model for estimating the force of transmission of HIV infection and its application. Am J Math Stat. 2014;4(3):171-177.
- Prayitno A, Taurel AF, Nealon J, Satari HI, Karyanti MR, Sekartini R, et al. Dengue seroprevalence and force of primary infection in a representative population of urban dwelling Indonesian children. PLoS Negl Trop Dis.2017;11(6):e0005621.
[Crossref] [Google Scholar] [Pub Med]
- John TJ, Samuel R. Herd immunity and herd effect: new insights and definitions. Eur. J. Epidemiol.2000;16(7):601-606. [Crossref]
- Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020 19;52(5):737-741.
- Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin. Infect. Dis. 2011;52(7):911-916.
[Crossref] [Google Scholar] [Pub Med]
- Metcalf CJE, Ferrari M, Graham AL, Grenfell BT. Understanding herd immunity. Trends Immunol. 2015. 36: 753-755.
- Banerjee A. Outbreaks of rubella indicate epidemiological shift in age. Indian Pediatr. 2015 ;52(2):169.
[Crossref] [Google Scholar] [Pub Med]
- Gioula G, Fylaktou A, Exindari M, Atmatzidis G, Chatzidimitriou D, Melidou A, et al. Rubella immunity and vaccination coverage of the population of northern Greece in 2006. Euro Surveill. 2007;12(11):9-10.
[Crossref] [Google Scholar] [Pub Med]
- Davesne D, Meunier FJ, Schmitt AD, Friedman M, Otero O, Benson RB. The phylogenetic origin and evolution of acellular bone in teleost fishes: insights into osteocyte function in bone metabolism. Biol. 2019;94(4):1338-1363.
[Crossref] [Google Scholar] [Pub Med]
- Doherty M, Buchy P, Standaert B, Giaquinto C, Prado-Cohrs D. Vaccine impact: Benefits for human health. Vaccine. 2016;34(52):6707-6714.
[Crossref] [Google Scholar] [Pub Med]
- Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201(11):1607-1610.
[Crossref] [Google Scholar] [Pub Med]
- Decker MD. Principles of pediatric combination vaccines and practical issues related to use in clinical practice. Pediatr Infect Dis J.. 2001;20(11):S10-18.
[Crossref] [Google Scholar] [Pub Med]
- Greenwood M, Yule GU. The statistics of anti-typhoid and anti-cholera inoculations, and the interpretation of such statistics in general.
[Crossref] [Google Scholar] [Pub Med]
- Altman KD, Kelman M, Ward MP. Are vaccine strain, type or administration protocol risk factors for canine parvovirus vaccine failure?. Vet. Microbiol.2017;210:8-16.
[Crossref] [Google Scholar] [Pub Med]
- Alders RG, Bagnol B, Young MP, Ahlers C, Brum E, Rushton J. Challenges and constraints to vaccination in developing countries. Developments in biologicals. 2007;130(8):73-82.
- Bari FD, Parida S, Tekleghiorghis T, Dekker A, Sangula A, Reeve R, et al. Genetic and antigenic characterisation of serotype A FMD viruses from East Africa to select new vaccine strains. Vaccine.2014;32(44):5794-5800.
[Crossref] [Google Scholar] [Pub Med]
- Rondy M, Kissling E, Emborg HD, Gherasim A, Pebody R, Trebbien R, et al. Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies. Euro Surveill.. 2018;23(9):18-00086.
[Crossref] [Google Scholar] [Pub Med]
- Botha W. Discard expired vaccines. Ubisi Mail. 2006;2(1):21.
- Heininger U, Bachtiar NS, Bahri P, Dana A, Dodoo A, Gidudu J, et al. The concept of vaccination failure. Vaccine. 2012;30(7):1265-1268.
[Crossref] [Google Scholar] [Pub Med]
- Arsalan A, Naqvi SB, Habib S, Hussain M, Shakeel O. Storage of vaccines in different health centers and pharmacies at Karachi, Pakistan: The handling errors. Pak. J. Pharm. Sci.. 2019;32(5).
- Hibbs BF, Miller E, Shi J, Smith K, Lewis P, Shimabukuro TT. Safety of vaccines that have been kept outside of recommended temperatures: reports to the vaccine adverse event reporting system (VAERS), 2008–2012. Vaccine. 2018;36(4):553-558.
[Crossref] [Google Scholar] [Pub Med]
- World Health Organization. WHO guidance note: vaccine diluents: the proper handling and use of vaccine diluents. World Health Organization. 2015.
- Halsey NA. Safety of combination vaccines: perception versus reality. Pediatr Infect Dis J. 2001;20(11):S40-S44.
[Crossref] [Google Scholar] [Pub Med]
- Fulton RM, Schrader DL, Will M. Effect of route of vaccination on the prevention of infectious laryngotracheitis in commercial egg-laying chickens. Avian Dis. 2000:8-16.
[Crossref] [Google Scholar] [Pub Med]
- Marangon S, Busani L. The use of vaccination in poultry production. Rev. sci. tech. - Off. int. épizoot. 2007;26(1):265.
- Giri PK, Verma I, Khuller GK. Protective efficacy of intranasal vaccination with Mycobacterium bovis BCG against airway Mycobacterium tuberculosis challenge in mice. Journal of Infection. 2006;53(5):350-356.
[Crossref] [Google Scholar] [Pub Med]
- Usman HR, Kristensen S, Rahbar MH, Vermund SH, Kirby RS, Chamot E. Determinants of diphtheria-tetanus-pertussis third dose (dtp3) completion among children visiting immunization centers for the first dose (dtp1) in rural pakistan: a cohort study. Randomized Controlled Trial of Low Cost Interventions to Reduce Childhood Immunization Dropouts in Pakistan. 2009:39.
- Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biol. 2014 ;42(5):237-259.
[Crossref] [Google Scholar] [Pub Med]
- Das MK, Arora NK, Mathew T, Vyas B, Sindhu M, Yadav A. Temperature integrity and exposure to freezing temperature during vaccine transfer under the universal immunization program in Three States of India. Indian J. Public Health. 2019;63(2):139.
[Crossref] [Google Scholar] [Pub Med]
- Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, et al. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc. Natl. Acad. Sci. 2012;109(30):11981-11986.
[Crossref] [Google Scholar] [Pub Med]
- Bertran K, Lee DH, Criado MF, Balzli CL, Killmaster LF, Kapczynski DR, et al. Maternal antibody inhibition of recombinant Newcastle disease virus vectored vaccine in a primary or booster avian influenza vaccination program of broiler chickens. Vaccine. 2018;36(43):6361-6372.
[Crossref] [Google Scholar] [Pub Med]
- Voysey M, Kelly DF, Fanshawe TR, Sadarangani M, O’Brien KL, Perera R, et al. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr.. 2017;171(7):637-646.
[Crossref] [Google Scholar] [Pub Med]
- Rashid A, Rasheed K, Akhtar M. Factors influencing vaccine efficacy: a general review. J Anim Plant Sci. 2009;19:22-25.
- Sharif A, Ahmad T. Preventing vaccine failure in poultry flocks. Immunization-Vaccine Adjuvant Delivery System and Strategies. 2018:80-94.
- Wali A, Balkhi MU. Fish vaccination and therapeutics. Int. Multidiscip. Res. J. 2016;3(4):55-60.
- Bagley CV. Vaccination program for beef calves. AH/Beef. 2001;40:1
- McVey S, Shi J. Vaccines in veterinary medicine: a brief review of history and technology. Vet Clin North Am Small Anim Pract.2010;40(3):381-392.
[Crossref] [Google Scholar] [Pub Med]
- Van Boven M, Kretzschmar M, Wallinga J, O'Neill PD, Wichmann O, Hahné S. Estimation of measles vaccine efficacy and critical vaccination coverage in a highly vaccinated population. J R Soc Interface. 2010; 7:1537-1544.
[Crossref] [Google Scholar] [Pub Med]
- Ali M, Emch M, Von Seidlein L, Yunus M, Sack DA, Rao M, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366(9479):44-49.
[Crossref] [Google Scholar] [Pub Med]
- Talebi A, Pourbakhsh SA, Dorostkar K. Effects of vaccination routes against IB on performance and immune responses of broiler chickens. Int. J. Poult. Sci.. 2005;4(10):795-798.
- Tenforde MW, Kondor RJ, Chung JR, Zimmerman RK, Nowalk MP, Jackson ML, et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis.2021;73(11):e4244-250.
[Crossref] [Google Scholar] [Pub Med]
- Boni MF. Vaccination and antigenic drift in influenza. Vaccine. 2008;26:C8-14.
[Crossref] [Google Scholar] [Pub Med]
- Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39-40):6852-6862.
[Crossref] [Google Scholar] [Pub Med]
- Harrison LH, Jolley KA, Shutt KA, Marsh JW, O’Leary M, Sanza LT, et al. Antigenic shift and increased incidence of meningococcal disease. J Infect Dis. 2006;193(9):1266-1274.
[Crossref] [Google Scholar] [Pub Med]
- Henry C, Palm AK, Krammer F, Wilson PC. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol. 2018;39(1):70-79.
[Crossref] [Google Scholar] [Pub Med]
- Arevalo CP, Le Sage V, Bolton MJ, Eilola T, Jones JE, Kormuth KA, et al. Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proceedings of the National Academy of Sciences. 2020;117(29):17221-17227.
[Crossref] [Google Scholar] [Pub Med]
- Spackman E, Pantin-Jackwood MJ. Practical aspects of vaccination of poultry against avian influenza virus. Vet J. 2014; 202:408-415.
[Crossref] [Google Scholar] [Pub Med]
- Nongo NN, Bosh J. Poultry vaccine handling and administration in makurdi–a preliminary investigation. Proceeding of the Nigeria Veterinary Medical Association held at NVRI, Vom 20th-24th November. 2004.
- Bishop Y. The veterinary formulary. Pharmaceutical Press; 2000.
- Yohannes B. Assessment on vaccine cold chains management: in the case of public health facilities at n/s/lafto sub city, adis ababa.
- Rock AH. Veterinary pharmacology: a practical guide for the veterinary nurse. Elsevier Health Sciences; 2007.
- Lauer KB, Borrow R, Blanchard TJ. Multivalent and multipathogen viral vector vaccines. Clin. Vaccine Immunol. 2017;24(1):e00298.
[Crossref] [Google Scholar] [Pub Med]
- Gerdts V. Adjuvants for veterinary vaccines--types and modes of action. Berl. Munch. Tierarztl. Wochenschr.2015;128(11-12):456-463.
- Christensen D. Vaccine adjuvants: Why and how. Hum Vaccin Immunother. 2016;12(10):2709-2711.
[Crossref] [Google Scholar] [Pub Med]
- Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J. Vet. Intern. Med. 2003;17(3):273-281.
[Crossref] [Google Scholar] [Pub Med]
- Chase CC, Hurley DJ, Reber AJ. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. North Am. Food Anim. 2008;24(1):87-104.
[Crossref] [Google Scholar] [Pub Med]
- Kampmann B, Jones CE. Factors influencing innate immunity and vaccine responses in infancy. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671):20140148. [Crossref] [Google Scholar] [Pub Med]
- Castiblanco J, Anaya JM. Genetics and Vaccinology. Vaccines and Autoimmunity. 2015:65-78.
[Crossref] [Google Scholar] [Pub Med]
- Ganji KS, Mohammad-Zadeh I, Mohammadnia-Afrouzi M, Ebrahimpour S, Shahbazi M. Factors affecting immune responses to vaccines. Gazzetta Medica Ital. Arch. per le Sci. Mediche. 2018; 177(5):219.
- Inman CF, Haverson K, Konstantinov SR, Jones PH, Harris C, Smidt H, et al. Rearing environment affects development of the immune system in neonates. Clin. Exp. Immunol. 2010;160(3):431-439.
[Crossref] [Google Scholar] [Pub Med]
- Giefing‐Kröll C, Berger P, Lepperdinger G, Grubeck‐Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging cell. 2015;14(3):309-321.
[Crossref] [Google Scholar] [Pub Med]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis.. 2000;31(2):578-585.
[Crossref] [Google Scholar] [Pub Med]
- Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens. 2017;6(4):65.
[Crossref] [Google Scholar] [Pub Med]
Citation: Endale H, Aliye S, Fesseha H, Mathewos M (2022) Vaccine Epidemiology, Evaluation, and Constraints of Vaccine Effectiveness: A Review. J Vaccines Vaccin. 13:476.
Copyright: © 2022 Endale H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.