Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 11, Issue 3
Use the Best of MNPS-IHSP Nanoparticles with Coating of Ampicillin Antibiotic, as Bactericidal Properties
Mansour Binandeh*, Farokh Karimi and Sadegh RostamniaReceived: 07-Mar-2019 Published: 30-Apr-2019
Abstract
In this research, we have tried to maximize the potential of Fe3O4 magnetic nanoparticles coated by SiO2 and immobilized with imidazoline hetropolymer (MNPS-IHSP) to stabilize and release the drug molecule called ampicillin (amp). In this purpose, nanoparticles of 50 nm in size were investigated under a tool such as; FT-IR, SEM, EDX detection and its structure in-vitro conditions. MNPS-IHSP nanoparticles is a specific and suitable nanocomposite that it have a mission as well as stabilizing the amp in the presence of silica coating (electrostatic bonding), that it called MNPS-IHSPA, and the results indicate a stabilization with a higher percentage of 85. It was beneficial to the silica coating. This measurement was performed by a UV-V is spectrophotometer analysis. Ultimately, in-vitro conditions, these magnetic nanoparticles that were migrating with ampicillin was tested on growing bacteria and resulted in the bacterial killing, indicating the antibacterial properties of magnetic nanoparticles. Ultimately, the project tells us that it can also be useful for destroying pathogenic bacteria living cells in vivo conditions.
Keywords
Magnetic nanoparticles; Antibacterial property; Silica-coated; SH-m-polymer; EDX analysis
Introduction
Use of magnetic grains that goes back to ancient Greece and Rome, but as a research system of medical and medical research in the 1980s, suggested that the use of particles in the future would be beneficial for human health. Based on nanotechnology, a range of diagnostic and therapeutic programs are improved in diseases such as heart and neurological diseases. Magnetic nanoparticles are used for targeted delivery of drug therapy agents and the frequency of drug transfer between the ligand and the receptor are by a magnet that involves intense or specific magnetic absorption due to high performance of Intelligence control of the agents in the desired and very important parameters is. Therefore, the magnetic carrier allows the access to this action [1].
TEOS-MPTES (3-mercaptopropyl triethoxy silane), SH-polymer
Magnetite synthesized in two stages to perform chemical reactions are modified, the first step in the coating of silica on the surface of magnetite and placement of functional groups on the second step of the reaction the group functionalized by silanol (tetraethyl orthosilicate or TEOS) levels of magnetite compounds to a simple and convenient method is to magnetite and silica-mercapto modified to be covered, in an aqueous solution of sodium silicate (from rice husk ash as a precursor made) and triethoxysilane 3-mercaptopropyl (as a source of a group (-SH)). In addition, the (mercapto-silica) on the properties and stability of magnetite with a variety of solvents were done. Similar results of the reduction in the size of magnetic nanoparticles, and magnetic energy also will be reduced [2]. Today, trying to find ionic liquid as a catalyst that continues with an active level of various reactions. The compounds in recent years have revolutionized the Centre for Research and Technology. These compounds are organic chemicals that act as a green solvent and play a very important role in reducing the use of hazardous substances that are toxic to the environment. One of the most important ionizing imidazole fluid contains an organic molecule (imidazole ion compounds with the formula C3H4N2), which is a cation. Cations and anions are used to provide a variety of ionic liquids that can be applied to a wide range of ionic fluids with specific physiochemical properties. Common anions, including F-, Br-, Cl- [2].
Use of magnetic nanoparticles has anti-bacterial properties
Magnetic nanoparticles are in use in important biological fields [3,4], including the isolation and detection of bio marker (cells, proteins, DNA, nucleic acids, concentrations, microbes, etc.).
For almost half a century magnetite magnetic nanoparticles have been used as an option in vitro. Magnetic nanoparticles have a broadly applicable surface that has high chemical activity. The size and surface of nanoparticles play an important role in their use. Thus, a particular way that microbial resistance increases to healthy antibiotics protected by magnetic nanoparticles reduces the amount of magnetic nanoparticles with certain chemical and physical agents that are considered by scientists, and some methods advanced instruments for controlling the size of magnetic nanoparticles based on proper design and optimal performance and control with extensive use in the industry, as an antibacterial in physics-biology. Active antibiotics [5,6] are due to the small particle size and the active level it has, that they can interact with nano powders, viruses, mice, and bacteria (there used is model) [3]. In this project, we offer nano scale magnet for Fe3O4@SiO2/ SH nanoparticles with a unique structure at a scale of 50-100 nm. The surface of nanoparticles has a high performance to absorb biological molecules. Thus, the absorption of ampicillin (amp) antibiotics reaches a complete stable level on its surface. The complete mechanism for this biochemical reaction is shown in Figure 1.
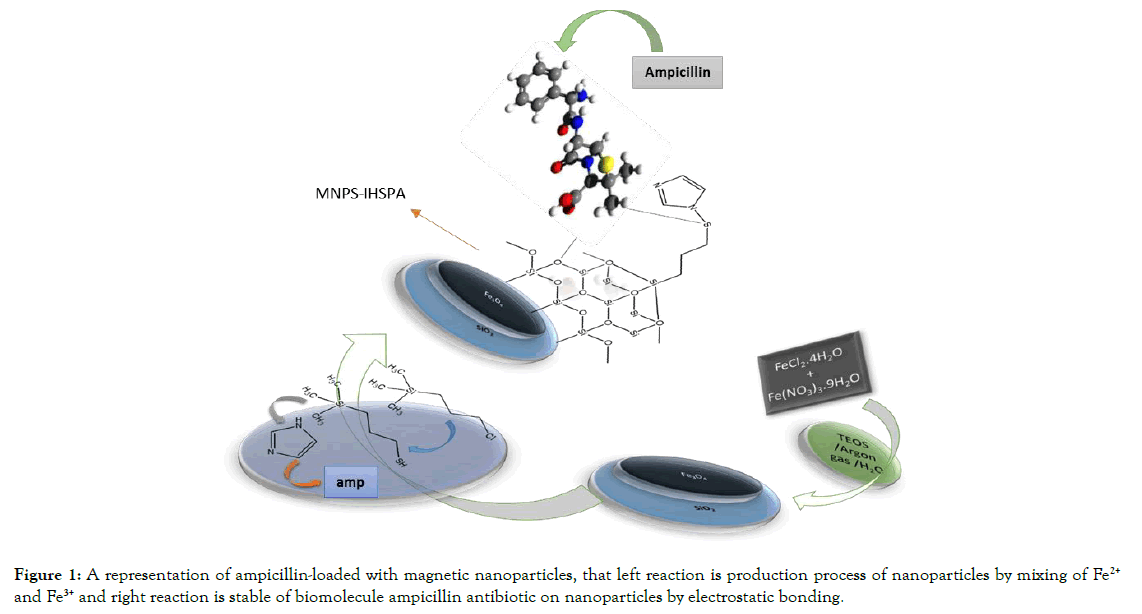
Figure 1. A representation of ampicillin-loaded with magnetic nanoparticles, that left reaction is production process of nanoparticles by mixing of Fe2+ and Fe3+ and right reaction is stable of biomolecule ampicillin antibiotic on nanoparticles by electrostatic bonding.
Materials and Methods
Materials
All solvents and chemicals are purchased from commercial Suppliers. The structure of materials was provided by TEM and SEM (Philips CM-200), University of the Shahid Mohaghegh Ardabili. Materials such as; (FeCl2.4H2O), (Fe (NO3)3.9H2O) and (NaOH) were purchased from Merck (Germany). And, TEOS (tetraethyl-orthosilicate), Hydrazine (34% by weight aqueous solution, as reducer), imidazole (C3N2H4, molar mass 68.077 g/ mol), (3-choloropropyl) triethoxysilane or CPTES, (molecular weight 198.72, 97% purity), (3-mercaptopropyl) triethoxysilane or MPTES, (molecular weight 196.34 g/mol, 95% purity), Na-amp salt (<98% pure; molecular weight 371.4 g/mole), were purchased from Sigma-Aldrich (USA). Bacteria’s cultured (University of Maragheh, Iran) is model. Double deionized water was used.
Synthesis of silica-coated with Fe3O4 magnetic nanoparticles with NH2 linker
Chemical Co-precipitation also one of the best and most current method of synthesis of magnetic nanoparticles. Thus, the sample salts containing iron (+) with a ratio of 1/2 (75 mM FeCl2.4H2O and 150 mM Fe (NO3)3.9H2O) were dissolved in double distilled water. The reaction temperature was 25°C and the high-intensity rotation was negative under nitrogen gas. After 180 minutes, for preventing the additional oxidation of nanoparticles, 4 ml of TEOS was materials. Finally, the yellow and brown product was synthesis [3].
After synthesizing Fe3O4@SiO2 magnetic nanoparticles weigh out 100 mg of Fe3O4@SiO2 magnetic nanoparticles and dissolve in distilled water 2 ml to completely double dissolve in water. After complete dissolution of the Fe3O4@SiO2 sample, place in reflex conditions at 100°C, and the amount 200 μl (CPTES) linker is added to the solution. Then 24 hours, 200 μl of (MPTES) was added to the solution. On another 24 hr, 200 μl of N-methyl imidazole was added to remove chloride from the first linker. Finally, we wash the magnetic nanoparticles Fe3O4@SiO2/SH prepared by distilled water twice ionized and placed in the oven. Finally, magnetic nanoparticles containing a yellowish-brown amine agent group was prepared Figure 1.
Results
Synthesis and characterization of magnetic nanoparticle coated with silica
Chemical co-precipitation was used to prepare magnetic nanoparticles (Fe3O4) with metal oxide of Fe2+/Fe3+ [7-9]. After the synthesis of magnetic nanoparticles, silica particles (SiO2) were used in addition to prevent the oxidation of magnetic nanoparticles by air, and as a coating for increasing the functional level of nanoparticles for chemical-biomedical reactions. The size of nanoparticles should be made at a scale of x<50 nm to be useful for use in biomedical fields. SEM analysis is used to stabilize the structure of magnetic nanoparticles. The structure of the magnetic nanoparticles coated with silica by an analytical SEM (Figure 2), in the exactly, is the size of a nanoparticle that is 50-100 nm.
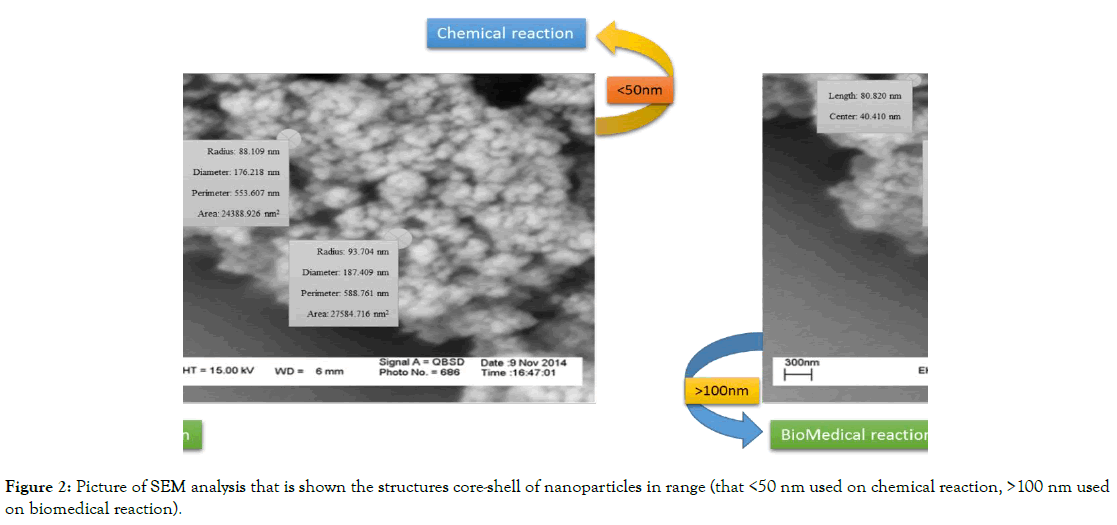
Figure 2. Picture of SEM analysis that is shown the structures core-shell of nanoparticles in range (that <50 nm used on chemical reaction, >100 nm used on biomedical reaction).
The FT-IR spectrum of Fe3O4@SiO2/SH (MNPS-IHSP)
Analysis FT-IR, the analysis to show the operating groups, and also established a covalent bond between functional groups. It values in terms of frequency from 500 to 4000 cm-1 are based on. The FTIR spectra of as-prepared hollow magnetite micro spheres (Figure 3a) and SiO2@hollow magnetite micro spheres (Figure 3b) were characterized by a high absorption band at 802 cm-1 imputed to the typical band of Fe3O4, equivalent to the stretching vibration modes of FeO [10]. Afterward coating by a silica layer, a new band appeared at about 1,122 cm-1 (Figure 3b) being determined to stretch of Si-O-Si bands on the surface of the SiO2@hollow magnetite micro spheres. By comparing the two images (Figure 3a and b), it can be concluded that the FeO bond peak gradually was sharped that represented the crystallization of the nanoparticles. Thus, the structure of magnetite magnetic nanoparticles coated with silica layer that in addition to preventing additional oxidation and increase the reactivity of nanoparticles, magnetic nanoparticles can also cause regular crystalline structure. Results for identifying the structure of magnetic nanoparticles by functional groups shown in (Figure 3). Courier 694 and 879 cm-1 of the group (-SH) is (Figure 3c). Courier 1,122 cm-1 relating to silica coated on surface of magnetic nanoparticles of magnetite is that even after the added linker group (-SH) value has not changed, but peaks more regularly obtained indicated the stable structure and orderly nanoparticles, and the magnetic nano composite structure of magnetite bed of silica after having been preserved, that (Figure 3) is again evidence of the claim.
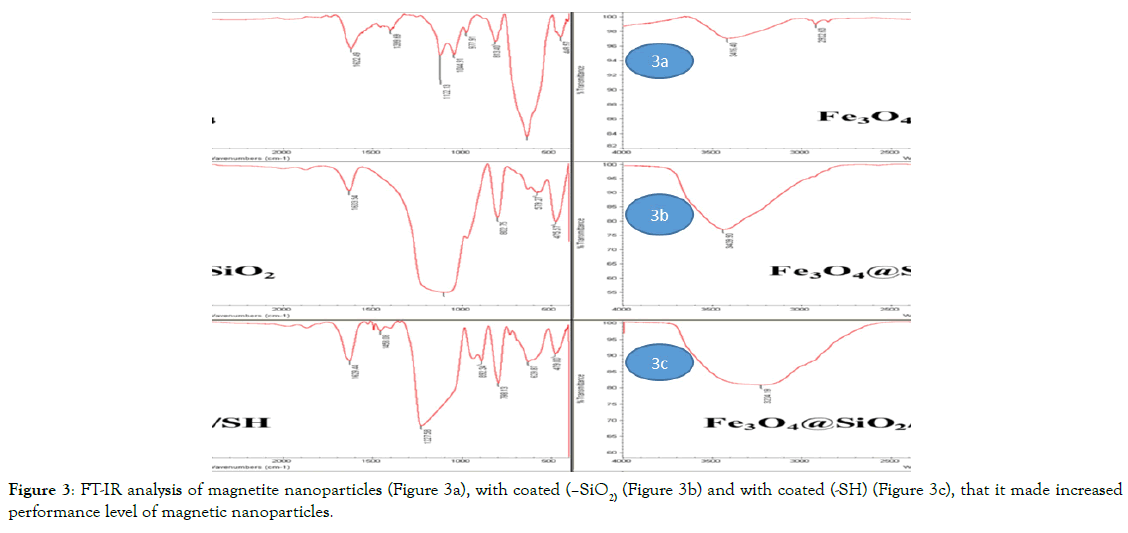
Figure 3. FT-IR analysis of magnetite nanoparticles (Figure 3a), with coated (–SiO2) (Figure 3b) and with coated (-SH) (Figure 3c), that it made increased performance level of magnetic nanoparticles
The XRD spectrum of Fe3O4@SiO2/SH
The XRD analysis, the X-ray diffraction, is an analysis that shows the number of electron transmissions using X-rays at energy levels in orbital layers and electron capacities. The synthesized Fe3O4 pixels are similar to 2 theta pixels, and in pixels between the processing of 30.1 and 35.4, 43.2, 53.7, 56.9, 62.9 and 220, 311, 422, 511, 440 cm-1 is visible in the Figure 4. Results indicate that Fe3O4 was synthesized and no changes were made in its crystalline structure. However, the pixels expressed for Fe3O4 are far from the standard pixels. Two new couriers were observed in 2 thetas of 40 and 46.7 cm-1, which are similar to the peaks of 111 and 200 cm-1, which confirm the completion of -SH group [2] to the surface of the magnetite magnetic nanoparticles with a silica substrate and factorized with the functional imidazoline hetropolymer.
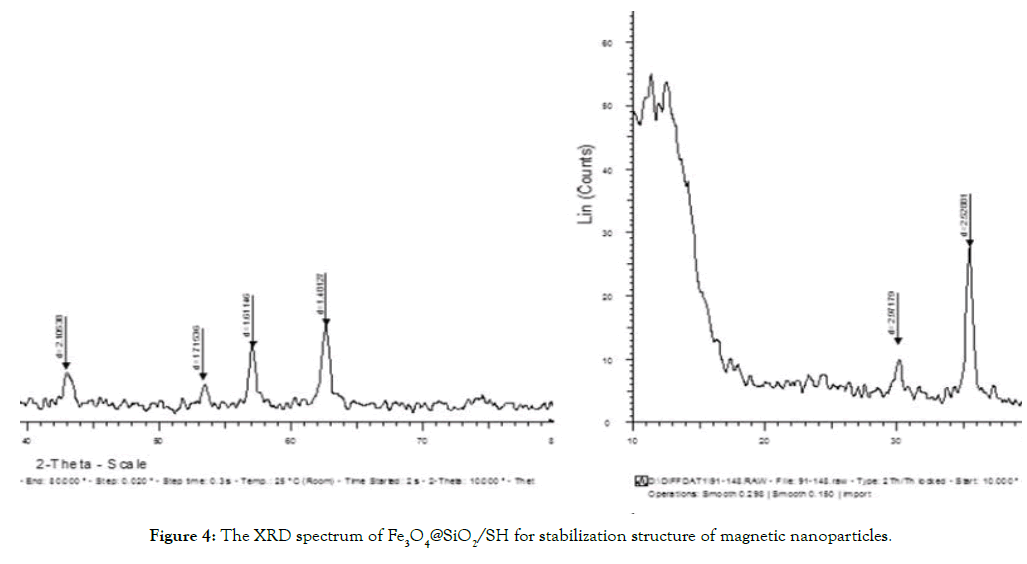
Figure 4. The XRD spectrum of Fe3O4@SiO2/SH for stabilization structure of magnetic nanoparticles
Adsorption studies
We want to check the absorption of ampicillin antibiotics in the electrostatic bond. So, we will use two analyzes EDX, FT-IR [11] to do this.
Discussion
Results of ampicillin loaded onto magnetic nanoparticles Fe3O4@SiO2 by spectrophotometry
In this section, we can calculate the absorption rate of ampicillin on the surface of magnetic nanoparticles using the Bradford equation and the Beyer-Lambert law [3]. In these two equations, the observed several spectrophotometry devices is multiplied by a constant number of 50 μg. ml-1 to get optical absorption. Now, before this test, 25 mg of nanoparticles should be analysed in the standard value obtained from the three equations for ampicillin (1 μg/ml) at 10, 50 and 100 μl. This test is performed for two nano sized particles with silica ligand and one with ligand N, the first bond being electrostatic. The standard time is 0.25 hours electrostatic. At 15-30 min, absorption was achieved on the MNPS-A and the MNPS-IHSPA nanoparticles 65% and 85%, respectively. At a time greater than 30 minutes, the result did not change, and that the presence of heterocyclic polymer in the ampicillin absorption results greatly affects both because the S group is by the same linker (-SH) is bonded with the -O linker from the silica substrate. The results of MNPS-IHSPA magnetic nanoparticles showed that absorption of ampicillin was higher than 85%, and ultimately the results of inhibition of bacterial growth were over 80%. As a result, this heterocyclic polymer with an SH linker stabilized on the silica substrate has created a specially made magnetic nanoparticles. Thus, by observing the percentages achieved at the end of the test, the ampicillin absorption rate in the electrostatic transplantation is up with 85 percent, (Figure 5). After analysing the data by the spectrophotometry analysis, the absorption rate for the electrostatic bond is revealed.
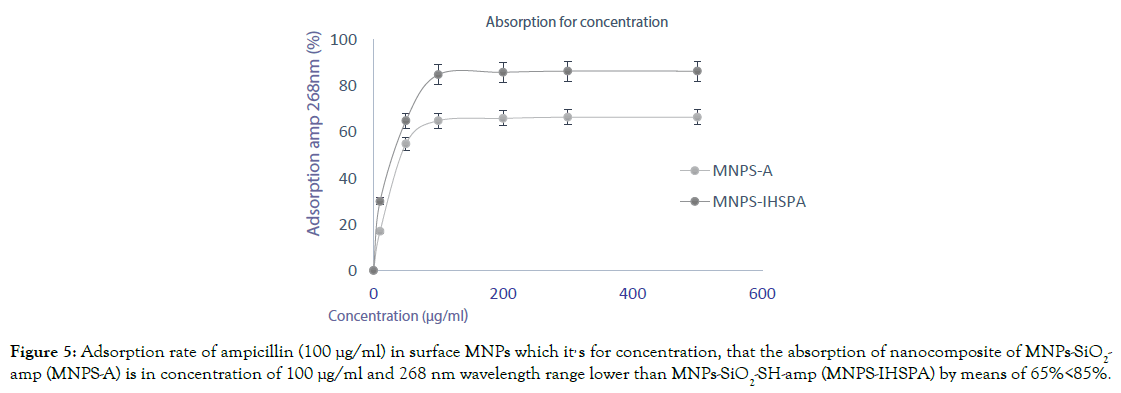
Figure 5. Adsorption rate of ampicillin (100 μg/ml) in surface MNPs which it, s for concentration, that the absorption of nanocomposite of MNPs-SiO2-amp (MNPS-A) is in concentration of 100 μg/ml and 268 nm wavelength range lower than MNPs-SiO2-SH-amp (MNPS-IHSPA) by means of 65%<85%.
Release of results and inhibition of bacterial growth by MNPSIHSPA magnetic nanoparticles: Finally, one of the most important analyses that clearly shows the difference between the two bonds is to check the bacterium in 3 concentrations of 70, 140 and 210 micrograms per microliter (Figure 6). For this, bacteria (10 μl) are cultured in culture in the presence of kanamycin for overnight. Same conditions as in the previous section are also present here, and after about half a day, with detailed examination and spectrophotometry analysis of UV-Visible (Figure 7), it was found that at a concentration of 210 μg/ml, 30 min, so bactericidal concentration was high and maximal, which was determined, it was done at a bactericidal rate of 85%. The bacterium used is a bacterial model. It is clear in Table 1, that when ampicillin (20 μl) was used alone (without the use of nanoparticles), its effectiveness in bacterial mortality was about 50%, when ampicillin was applied to coagulation magnetic nanoparticles (20 μl), This percentage has reached about 80 percent in 30 min. Results showed that MNPSIHSPA has antibacterial properties.
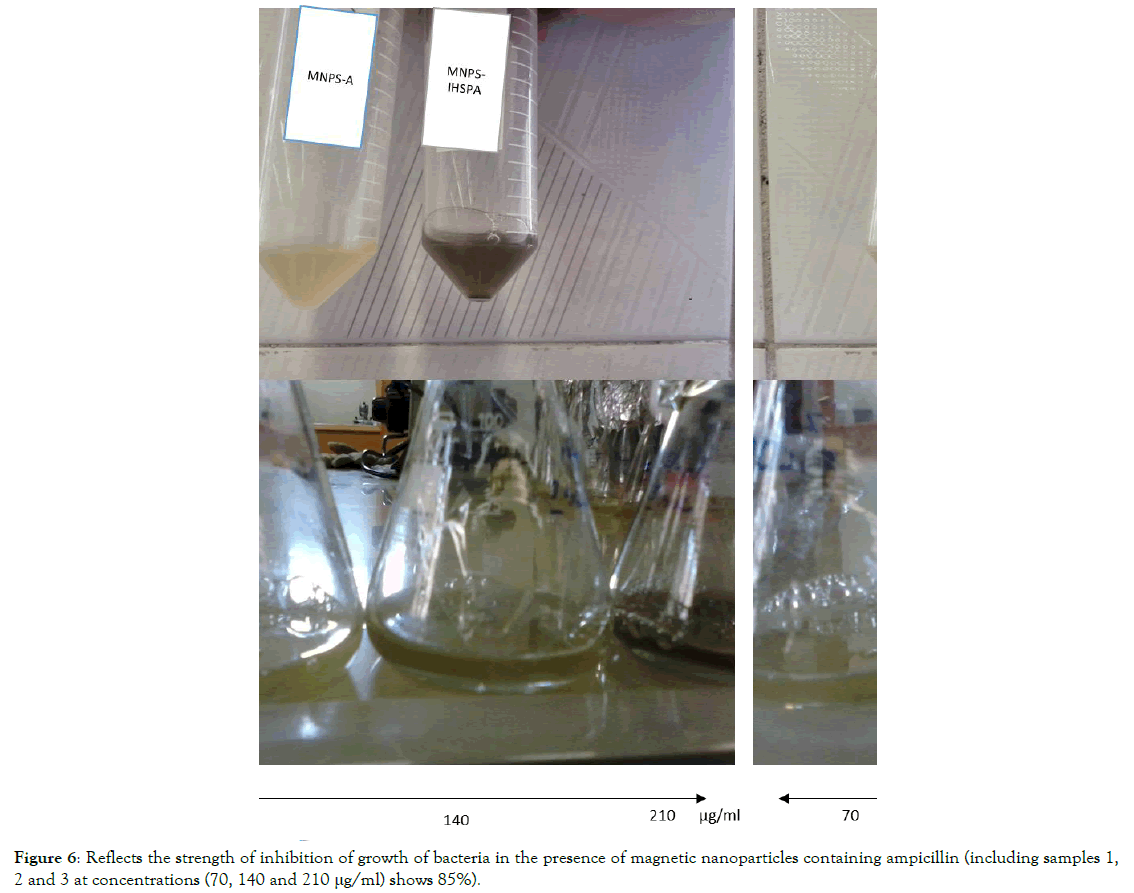
Figure 6. Reflects the strength of inhibition of growth of bacteria in the presence of magnetic nanoparticles containing ampicillin (including samples 1, 2 and 3 at concentrations (70, 140 and 210 μg/ml) shows 85%).
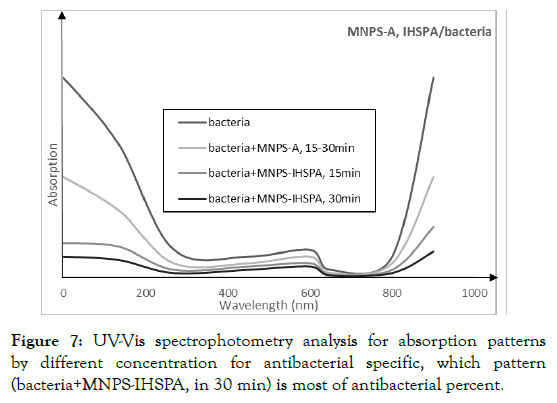
Figure 7. UV-Vis spectrophotometry analysis for absorption patterns by different concentration for antibacterial specific, which pattern (bacteria+MNPS-IHSPA, in 30 min) is most of antibacterial percent.
| Measures →µg/ml ↓µl/ml |
70 | 140 | 210 |
|---|---|---|---|
| Bacteria, 10 µl | 1.600 | 1.600 | 1.600 |
| 1) MNPS-A, 15-30 min, 20 µl | 0.845 | 0.645 | 0.355 |
| 2) MNPS-IHSPA, 15 min, 20 µl | 0.225 | 0.121 | 0.120 |
| 3) MNPS-IHSPA, 30 min, 20 µl | 0.127 | 0.052 | 0.010 |
Table 1: Evaluation of measured values (70, 140 and 210 μg/ml) at concentrations (210 μg/ml) is the inhibition of bacterial growth, for example 3 to 85% of the samples more ampicillin indicates the bactericidal properties of MNPs respectively.
Stabilization of amp on MNPs with electrostatic bonding: At this stage, we have two analyses to prove the claim that there are electrostatic. In the analysis of EDX, the aim is to decide the elemental frequency in the composition, which is the element that has a kW electron volt in a direction and has a plurality of graphs in a column indicating the bond of the element of con, which is here between (250-2500 keV), the volatile nitrogen functional group of ampicillin has established an electrostatic bond with the O, -SH functional groups of MNPS-IHSPA nanoparticles (Figure 8).
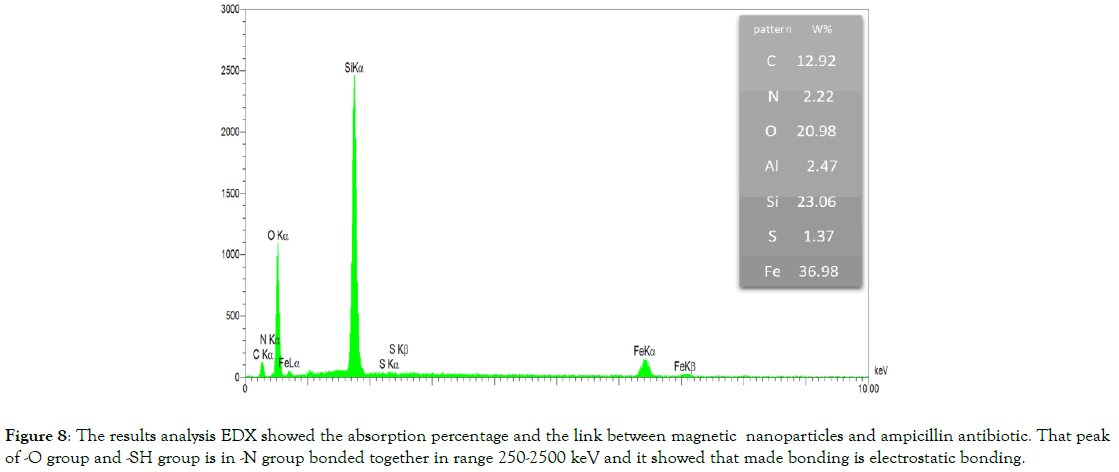
Figure 8. The results analysis EDX showed the absorption percentage and the link between magnetic nanoparticles and ampicillin antibiotic. That peak of -O group and -SH group is in -N group bonded together in range 250-2500 keV and it showed that made bonding is electrostatic bonding.
This project by helping the MNPS-IHSP nanocomposite could have received to basis of purpose that it is absorption and antibacterial agents that detected by UV spectrophotometry, EDX analysis.
Acknowledgments
All subjects received from various journals in the field of nanotechnology with biology and chemistry. In furtherance of the goal of attracting the magnetic nanoparticles were bio molecular antibiotics. Finally, thank you to the University of Maragheh to create the necessary facilities for starting and completing this project. Sincerely, I would like to express my gratitude to the hope that this project will bring the work of all those who have been on the road with the publication and use of this project.
Competing Interests
The authors declare that they have no competing interests.
Availability of Data and Materials
All data’s and values are fully measurable and availability.
Consent for Publication
That is applicable for this work.
Ethical Approval and Consent to Participate
Not applicable.
Funding
Authors did not receive any of funding for this work.
Conflict of Interest
The authors have no conflict of interest to declare.
REFERENCES
- Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62(3):284-306.
- Yang D, Hu J, Fu S. Controlled synthesis of magnetite-silica nanocomposites via a seeded sol-gel approach. J Phys Chem. 2009;113(18):7646-7651.
- Binandeh M. Frequency of High-Performance Magnetic Nanoparticles of Mid-Ampicillin, as Antibacterial Agents. J Antimicrob Agents. 2018;4(1):162-170.
- Pankhurst QA, Thanh NKT, Jones J, Dobson SK. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2003;42:22-27.
- Barza M. Challenges to Antibiotic Activity in Tissue. Clin Infect Dis. 1994;19(5):910-915.
- Suárez C, Gudiol F. Beta-lactam antibiotics. Infecc Microbiol Clin. 2009;27(2):116-129.
- Guan N, Wang Y, Sun D, Xu J. A simple one-pot synthesis of single crystalline magnetite hollow spheres from a single iron precursor. Nanotechnology. 2009;20(10):105603.
- Zeng SY, Tang KB, Li TW, Liang ZH, Wang D, Wang YK, et al. Hematite hollow spindles and microspheres; selective synthesis, growth mechanisms, and application in Li ion battery and water treatment. J Phys Chem C. 2007;111(28):102-117.
- Schwertmann U, Cornell RM. “The iron oxides”. New York: VCH. 1996.
- Ma M, Zhang Y, Yu W, Shen HY, Zhang HQ, GU N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Coll Surf A Physicochem Eng Aspects. 2003;212:219-221.
- Ulrich J, Krull, Thompson M. Encyclopedia of Physical science and Technology; Analytical Chemistry, 3rd Ed. 2001. Academic Press, ISBN 0-12-227410-5.
Citation: Binandeh M, Karimi F, Rostamnia S (2019) Use the Best of MNPS-IHSP Nanoparticles with Coating of Ampicillin Antibiotic, as Bactericidal Properties. J Microb Biochem Technol 11:416. doi: 10.4172/1948-5948.1000416
Copyright: © 2019 Binandeh M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

