Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 11, Issue 4
Time Course Evaluation of Nanozyme-Mediated Reversible/Irreversible Oxidation Reactions over Silver Nanoparticles as Peroxidase Alternatives
Saeed Reza Hormozi Jangi*Received: 07-Aug-2023, Manuscript No. MCA-23- 22487; Editor assigned: 09-Aug-2023, Pre QC No. MCA-23- 22487; Reviewed: 24-Aug-2023, QC No. MCA-23- 22487; Revised: 31-Aug-2023, Manuscript No. MCA-23- 22487; Published: 08-Sep-2023, DOI: 10.35248/2329-6798.23.11.426
Abstract
Silver nanoparticles were synthesized and then characterized by different characterization methods. The as-prepared nanozymes were found to be uniform in size and morphology with a mean size as small as 11.8 nm and high peroxidase-like activity. Considering the high intrinsic peroxidase-like activity of silver nanoparticles, the time course studies were performed toward both reversible and irreversible nanozyme-mediated oxidation reactions. 3,3’,5,5’-Tetramethylbenzidine (TMB) and 3,3’-Diaminobenzidine (DAB) were selected as model substrates to study the reversible and irreversible oxidations, in order. The results revealed that the maximal activity of the silver nanozymes was achieved within 3.0 min toward TMB oxidation while regarding DAB, the steady-state plateau was observed after a reaction time as long as 25.0 min, indicating that the active nodes of the silver nanoparticles were completely saturated by TMB molecules 6.5-fold faster than the DAB molecules. Regarding time-dependent activity measurements, the nanozyme activity reached about 32% of its maximal activity toward DAB oxidation after a long oxidation time of 300 sec while for TMB oxidation, 32% of maximal nanozyme activity was observed after 30 sec (i.e., 10.0-fold faster than that of DAB). More precisely, the magnified slope (i.e., rate of change) of the initial linear portion of the reaction time curve of TMB oxidation (slope=0.6286) shows about a 10-fold higher reaction rate than the DAB oxidation (slope=0.0636). As a consequence, the as-prepared silver nanoparticles are more efficient oxidizing catalysts for the oxidation of TMB than DAB.
Keywords
Time course study; Peroxidase alternatives; Silver nanoparticles; 3,3’,5,5’-Tetramethylbenzidine; 3,3’-Diaminobenzidine
Introduction
Nanozymes or nanoparticles with excellent enzyme-like activity are attracted much attention due to their stability and higher efficiency compared to natural enzymes [1-7]. Natural enzymes show several disadvantages such as low stability (thermal and narrow pH range) [8]. For overcoming these drawbacks, the enzyme immobilization process has been developed [9-13]. The recent progress in nanochemistry and material science opens a new door for developing high-performance nano-supports such as Metal- Organic Frameworks (MOFs), catalytic materials, and nanoparticles with enzyme-like activity [14-20]. Several of the above-mentioned nanoparticles reveal high peroxidase-like activity which can be used instead of enzymes in the reactions. Recently, nanozymes had been used for different applications for instance, analytical sensing of species, biocatalysis of reactions instead of natural enzymes, water treatment, dye degradation, sensing, and detection [21-24]. Since nanozymes can catalyze the oxidation of peroxidase substrates to their corresponding colored products, they have been used for analytical purposes [1-7]. Usually 3,3’,5,5’-Tetramethylbenzidine (TMB) and 3,3’-Diaminobenzidine (DAB) substrates have been used as the peroxidase substrates, and their corresponding oxidation products were utilized as the analytical probes for sensing aims [1-7]. In this regard, a wide variety of nanozymatic nanosensors were reported in the literature for determining different analytes, for instance, amino acids (such as cysteine and tryptophan), antioxidants (e.g., glutathione), heavy metals (e.g., mercury), explosives, sugars (e.g., glucose), enzyme substrates (e.g., xanthine), hydrogen peroxide, and so on [25-31]. However, regarding the majority of these nanozyme-based sensors, the analytical probe systems are based on TMB oxidation and the analytical probe is the blue cation radicals resulting from nanozyme-mediated oxidation of TMB molecules [2]. The sensing is based on probing the absorbance of blue-colored analytical probes as a function of analyte concentration for the Hydrogen Peroxide (HP) detection and for the HP-based nanozyme sensors. In fact, the HP-based nanozymatic sensors are a class of nanozyme-based sensors for indirect detection of some analytes (e.g., triacetone-triperoxide and glucose) via their conversion to hydrogen peroxide or via monitoring the hydrogen peroxide released from the reaction of the analyte with a certain reactant, as reported [2,4,23,30]. Besides the native absorbance of the analytical probe, the variation of the absorbance in the presence and the absence of a certain analyte had to be used as an analytical index for the quantification of different analytes using the nanozymatic sensors [3,7]. Besides the TMB, after the first report [7], of the application of DAB as a more stable probe than the TMB for developing nanozyme-based sensors with higher response stability and accuracy that the unstable responses of the resulted from cation radicals from TMB, several works were reported for DAB-based nanozymatic sensors for sensing of several analytes via the n-electron irreversible oxidation of 3,3′-Diaminobenzidine (DAB) to a stable brown colored indamine polymer rather than common cation radicals resulting from TMB [3,4,6,23]. Since the focus of the nanozyme field is on their application in different fields such as sensing and detection, the reports on the biochemical behavior of nanozyme-based systems are on limitation. As it is well-known that among different nanomaterials with intrinsic enzyme-like activity, noble metal nanoparticles such as gold and silver nanoparticles are considered excellent alternatives for the enzymes and had been widely used for different nanozymatic applications [32-37]. Hence, evaluation of their enzyme-like activity, biochemical properties, rate of nanozymatic reactions, and other enzymatic characteristics is necessary. Recently, our research group investigated the biochemical behavior and stability of gold nanoparticles toward nanozyme- catalyzed reactions [38]. Besides, in another report, the kinetic performances of BSA-gold nanozymes were evaluated for oxidation of different substrates, TMB and DAB [39]. Herein, considering the high intrinsic peroxidase-like activity of silver nanoparticles [40], the time course progress of oxidation of TMB and DAB over silver nanoparticles as peroxidase alternatives was studied. In this regard, silver nanoparticles were synthesized and then characterized by TEM, DLS, XRD, and UV-visible spectrophotometry. Considering the high intrinsic peroxidase-like activity of silver nanoparticles, the time course studies were performed toward both reversible and irreversible nanozyme-mediated oxidation reactions. TMB and DAB were selected as model substrates for studying the reversible and irreversible oxidations, in order. To explore a more precise and accurate comparison between TMB and DAB oxidation process over silver nanoparticles, the initial linear portion of the reaction time curve of both substrates was constructed and their linear equations were provided as an accurate index for evaluating the difference of their reaction rate [41]. The main objective of this study is to find compressive insight into the nanozymatic reaction rates over nanosilvers and their relation with substrate nature and oxidation mechanism (reversible or irreversible) using time course studies as a reliable index for this aim. Overall, the results of this work exhibited that the oxidation of TMB more efficiently proceeded over silver nanoparticles than DAB.
Materials and Methods
Synthesis of AgNPs
The synthesis was performed based on the process reported by Hormozi Jangi et al [34]. To do this, 5.0 mL of 10.0 mM AgNO3 was mixed with 5.0 mL sodium citrate (10.0 mM). Afterward, 89.0 mL DI water was added to the mixture, and the resulting solution was mixed for 20 minutes at room temperature. The synthesis process was followed by the quick addition of NaBH4 (8.8 mg) and stirring for 2.0 hours at the ambient temperature. After this time, the AgNP’s were collected and stored at 4.0 ± 1.0 °C
Time course study of oxidation reactions
To do the oxidation reactions, DAB and TMB were utilized as peroxidase substrates. Regarding the DAB oxidation process, 80.0 μL silver nanoparticles, 200.0 μL DAB solution, and 40.0 μL of 30% hydrogen peroxide were introduced into 680.0 μL phosphate buffer (pH=7.0 ± 0.1), followed by incubation for different times intervals over 5-30 minutes at room temperature. The colored products of the oxidation process with different incubation times were analyzed by UV-Vis spectrophotometer at 460.0 nm for monitoring resulted DAB- ox and calculation of the nanozymatic relative activity. For probing the TMB oxidation, 80.0 μL of nanozyme solution, 200.0 μL of TMB, and 40.0 μL of 30% hydrogen peroxide were added into 680.0 μL acetate buffer (pH=4.0 ± 0.1). The resulting mixtures were then incubated at ambient conditions for different time intervals over 0.5-10.0 minutes. After a certain reaction time, the resulting blue-colored TMB-ox was analyzed by recording its absorbance at 650.0 nm. It is notable that the relative nanozymatic activity (%) was calculated based on the following formula;
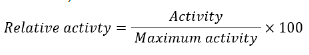
Instrumentation for material characterization
A CTChromTech UV 3300 UV-Visible spectrophotometer was utilized for time course studies. The TEM image and DLS pattern of the designed nanozymes were recorded by an EL10C Zeiss transmission electron microscope operating at an accelerating voltage of 80 kV and a Shimadzu SALD-301 V particle size analyzer model, in order. The XRD spectrum of the designed nanozymes was obtained using a Rigaku D/max-3C (Japan) with Cu-Kα (λ=1.54 nm) radiation power.
Results and Discussion
Characterization of silver nanozymes
TEM imaging for evaluation of size and morphology: Silver nanoparticles were synthesized and then characterized for their size and morphological properties. In this regard, the TEM image of the as-prepared nanozyme was recorded (Figure 1), as shown in this figure, the as- prepared silver nanoparticles showed uniform morphology with spherical particles. In addition, the as-prepared nanozymes showed a narrow size distribution over 8.3-12.6 nm with an average size of 11.4 nm.

Figure 1: TEM image of the as-prepared silver nanoparticles.
DLS analysis for accurate size calculation: In order to accurately report the size distribution of silver nanozymes due to the effect of nanozyme size on their peroxidase-like properties, the size distribution of the as-prepared silver nanozymes was estimated using DLS analysis (Figure 2), according to this figure, the as- synthesized nanozymes show a narrow size distribution range in the range of 8.2-13.2 with an average size of 11.8 nm, which is in good agreement with the results of TEM analysis.
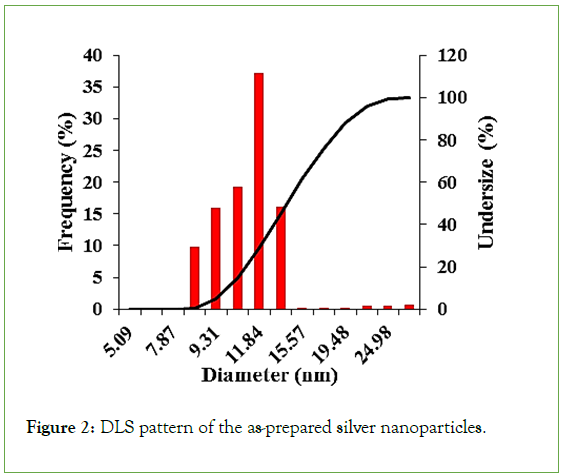
Figure 2: DLS pattern of the as-prepared silver nanoparticles.
XRD analysis for investigation of crystalline features: The crystalline properties of the synthesized silver nanozyme were investigated using XRD analysis (Figure 3). As can be seen in this figure, the results of XRD analysis indicate the presence of three peaks at the diffraction angles of 40.5°, 44.8°, and 59.9° for the as-synthesized silver nanozymes which are related to the cubic structure of silver.
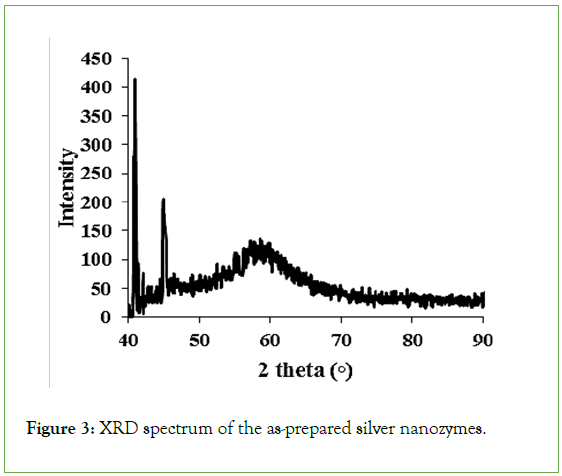
Figure 3: XRD spectrum of the as-prepared silver nanozymes.
UV-Vis spectrophotometry for evaluation of optical properties: The optical properties of silver nanozymes were investigated using visible-ultraviolet spectroscopy. For this purpose, the absorption spectrum of these nanozymes was recorded in the range of 330 nm-480 nm (Figure 4). As it is clear in this figure, the synthesized nanozyme shows a relatively symmetrical spectrum in the visible region with an absorption maximum at the wavelength of 390 nm.
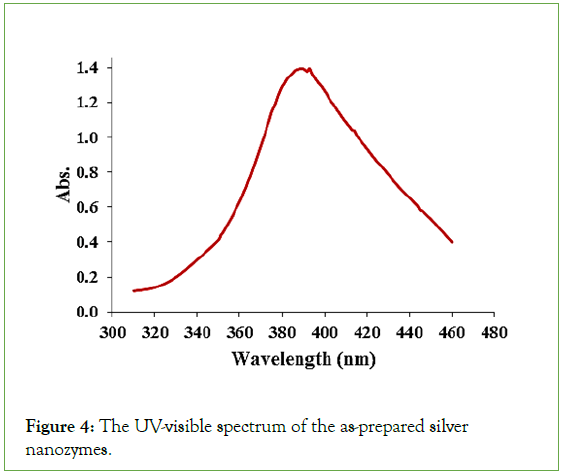
Figure 4: The UV-visible spectrum of the as-prepared silver nanozymes.
Time-course progress of TMB oxidation over silver nanoparticles
To evaluate the peroxidase-like activity of the as-prepared AgNPs, the oxidation of TMB was performed by hydrogen peroxide in the presence of AgNPs as peroxidase mimics. In this regard, the time course studies were performed by probing the blue-colored product via spectrophotometric detection at 650.0 nm. Afterward, the plot of oxidation of TMB in the presence of AgNPs as a function of time was constructed by plotting the absorbance at 650.0 nm as a function of reaction time (Figure 5A). As can be seen from this figure, the AgNPs can catalyze the oxidation of TMB to form a blue-colored product with a maximum absorbance at 650.0 nm. Based on the time-course studies, the oxidation of TMB was quickly proceeded by AgNPs, and the absorbance at 650 nm reached to 1.9 after a short reaction time of 3.0 minutes. To explore more precise on reporting of the nanozymatic activity of the as-prepared silver nanozymes toward nanozyme-catalyzed TMB oxidation as a function of incubation time, the relative activity of the as-prepared silver nanozymes was also quantified and utilized for investigating the effect of incubation (reaction) time on the TMB oxidation process and consequently on producing the corresponding blue-colored cation radicals of TMB (i.e., TMB- ox) (Figure 5B). According to these results, after a long oxidation time as long as five minutes, the nanozyme activity reached about 32% of its maximal activity toward DAB oxidation. The oxidation process slowly proceeded and the nanozyme activity reached about 54% after 12.0 minutes. The maximal activity of silver nanozymes was obtained after 20 minutes toward DAB oxidation. After this time, the incubation time could not affect the production of the poly (DAB), and therefore the relative activity of the nanozymes was leveled off. The results reveal that an incubation time over 25.0 min was enough for active nodes presented on the surface of the silver nanoparticles, to be blocked by the substrate (DAB) molecules. In fact, the active nodes on the surface of the silver nanoparticles were completely saturated in 25.0 minutes by the DAB molecules. Considering this fact that the active nodes (the binding sites) on the surface of the nanozymes are limited, the saturation of the DAB molecules leads to the leveling off of the relative activity of silver nanozymes (Figure 5).
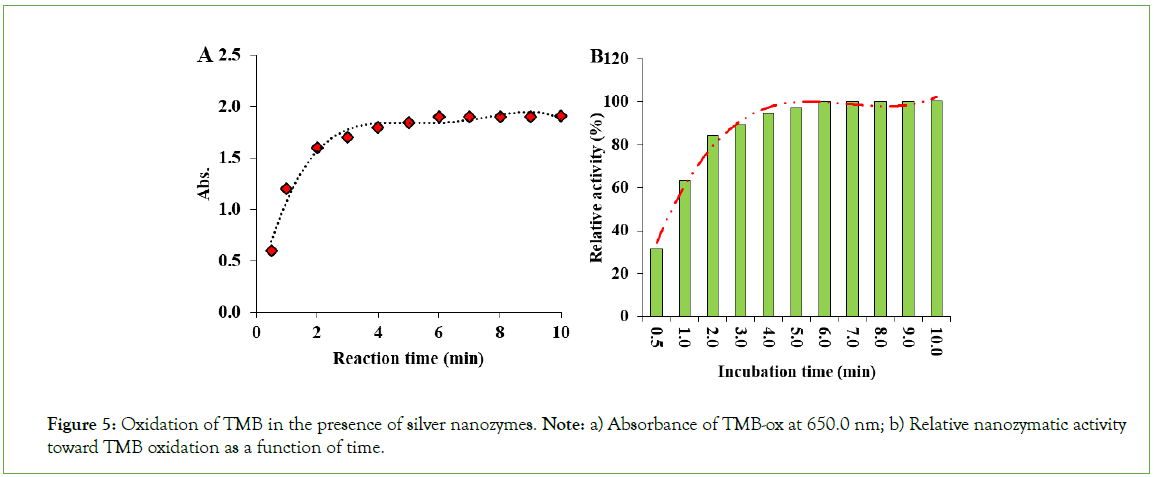
Figure 5: Oxidation of TMB in the presence of silver nanozymes. Note: a) Absorbance of TMB-ox at 650.0 nm; b) Relative nanozymatic activity toward TMB oxidation as a function of time.
Time-course progress of DAB oxidation over silver nanozymes
Besides, the peroxidase-like activity of silver nanoparticles toward DAB oxidation was also evaluated. To evaluate the peroxidase- like activity of the as-prepared AgNPs against DAB, the oxidation of DAB was performed by hydrogen peroxide in the presence of AgNPs as peroxidase mimics. In this regard, the time course studies were performed by probing the brown-colored product via spectrophotometric detection at 460.0 nm. Afterward, the plot of oxidation of DAB in the presence of AgNPs as a function of time was constructed by plotting the absorbance at 460.0 nm as a function of reaction time (Figure 6). As can be seen from this figure, the AgNPs can catalyze the oxidation of DAB to form a brown-colored product with a maximum absorbance at 460.0 nm. As can be seen from Figure 6A, the oxidation of DAB was found to be slower in rate than the TMB, reaching an absorbance of 1.5 after 20.0 minutes and then leveling off. To explore more precise on the nanozymatic activity of the as-prepared silver nanozymes toward oxidation of DAB at different incubation times, the relative activity of nanozymes was also calculated and used as an index for investigating the time effect on the oxidation process of DAB for producing the corresponding poly (DAB) (Figure 6B). The results showed that after a long oxidation time as long as five minutes, the nanozyme activity reached about 32% of its maximal activity toward DAB oxidation. The oxidation process slowly proceeded and the nanozyme activity reached about 54% after 12.0 minutes. The maximal activity of silver nanozymes was obtained after 20 minutes toward DAB oxidation. After this time, the incubation time could not affect the production of the poly (DAB), and therefore the relative activity of the nanozymes was leveled off. The results reveal that an incubation time over 25.0 minutes was enough for active nodes presented on the surface of the silver nanoparticles, to be blocked by the substrate (DAB) molecules. In fact, the active nodes on the surface of the silver nanoparticles were completely saturated in 25.0 minutes by the DAB molecules. Considering this fact that the active nodes (the binding sites) on the surface of the nanozymes are limited, the saturation of the DAB molecules leads to leveling off of the relative activity of silver nanozymes (Figure 6).
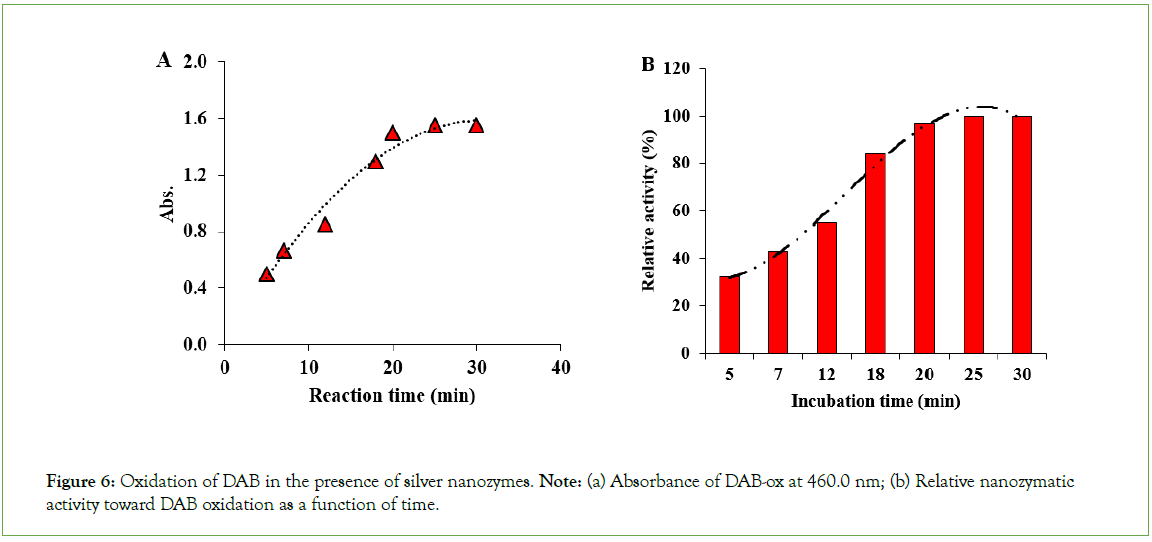
Figure 6: Oxidation of DAB in the presence of silver nanozymes. Note: (a) Absorbance of DAB-ox at 460.0 nm; (b) Relative nanozymatic activity toward DAB oxidation as a function of time.
Comparing the results of DAB and TMB oxidation over silver nanozymes
The time course curves revealed that the maximal activity of the silver nanozymes was achieved after a very short time of 3.0 minutes toward TMB oxidation while regarding DAB, the maximal activity was observed after 25.0 minutes which is 6.5-fold slower than that of the TMB oxidation. In fact, based on these results, it can be concluded that the active nodes of the silver nanoparticles were completely saturated 6.5-fold faster by TMB molecules than the DAB molecules. Moreover, the results showed that after a long oxidation time of as long as five minutes, the nanozyme activity reached about 32% of its maximal activity toward DAB oxidation while in the case of TMB oxidation, after a short reaction time as short as 3.0 minutes, 100% of the silver nanozymes activity was obtained and the time, 5.0 minutes, is in the range of steady-state region of the time course plot. Notably, for TMB oxidation on the surface of silver nanoparticles, 32% of maximal nanozymatic activity was observed within 30.0 seconds (10.0-fold faster than that of DAB), revealing a very higher oxidation rate of TMB over silver nanozymes than the DAB oxidation.
However, to explore more precision and accuracy in the comparison between TMB and DAB oxidation process over silver nanoparticles, the initial linear portion of the reaction time curve of both substrates was constructed and their linear equations were provided as an accurate index for evaluating the difference of their reaction rate (Figure 7). Based on this figure, the reaction time-dependent absorption spectra showed a faster increase in the oxidation of TMB than the oxidation of TMB over silver nanozymes and the magnified slope (i.e., rate of change) of the initial linear portion of the reaction time curve of TMB oxidation (slope=0.6286) shows about 10-fold higher reaction rate than the DAB oxidation (slope=0.0636), indicating that silver nanoparticles are more efficient oxidizing catalysts for oxidation of TMB than DAB. In fact, the oxidation of DAB has occurred via an n-electron irreversible pathway while the TMB oxidation has occurred through a simple 2-electron reversible pathway. Besides, DAB is a highly stable and less relative compound compared to TMB, resulting in different oxidation times, and different affinities for binding to nanozyme active nodes which lead to different nanozymatic activities. Abstractly, the difference between DAB and TMB can be assigned to their different reaction pathways and different reactivity, as well as the different affinity of these substrates for binding to the active nodes of nanozymes (Figure 7).
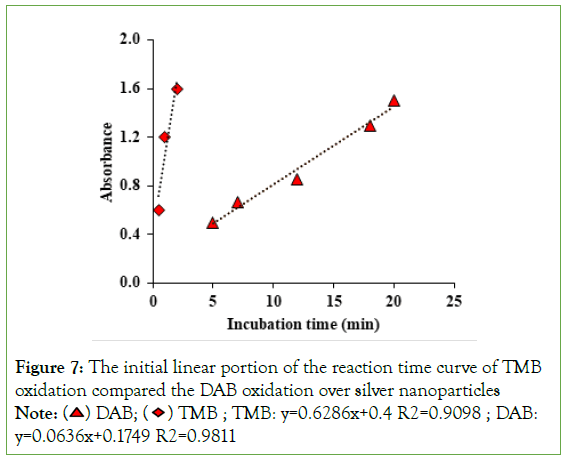
Figure 7: The initial linear portion of the reaction time curve of TMB
oxidation compared the DAB oxidation over silver nanoparticles Note:  TMB ; TMB: y=0.6286x+0.4 R2=0.9098 ; DAB: y=0.0636x+0.1749 R2=0.9811
TMB ; TMB: y=0.6286x+0.4 R2=0.9098 ; DAB: y=0.0636x+0.1749 R2=0.9811
Conclusion
Silver nanoparticles were synthesized and then characterized by different characterization methods. Based on TEM micrograph, DLS analysis, and UV-visible results, the as-prepared nanozymes were found to be uniform in size and morphology, have a mean size as small as 11.8 nm, and reveal a symmetrical spectrum with a λmax at 390.0 nm, in turn. Considering the high intrinsic peroxidase- like activity of silver nanoparticles, the time course studies were performed toward both reversible and irreversible nanozyme- mediated oxidation reactions. 3,3,5,5’-Tetramethylbenzidine (TMB) and 3,3’-Diaminobenzidine (DAB) were selected as model substrates to study the reversible and irreversible oxidations, in order. The results exhibited that the maximal activity of the silver nanozymes was achieved within 3.0 minutes toward TMB oxidation while regarding DAB, the steady-state plateau was observed after a reaction time as long as 25.0 minutes, indicating that the active nodes of the silver nanoparticles were completely saturated by TMB molecules 6.5-fold faster than the DAB molecules. Regarding time- dependent activity measurements, the nanozyme activity reached about 32% of its maximal activity toward DAB oxidation after a long oxidation time of 300 sec while for TMB oxidation, 32% of maximal nanozyme activity was observed after 30 sec (i.e., 10.0- fold faster than that of DAB). More precisely, the magnified slope (i.e., rate of change) of the initial linear portion of the reaction time curve of TMB oxidation (slope=0.6286) shows about a 10- fold higher reaction rate than the DAB oxidation (slope=0.0636). As a consequence, the as-prepared silver nanoparticles are more efficient oxidizing catalysts for the oxidation of TMB than DAB.
Acknowledgments
The authors gratefully thank the Hormozi Laboratory of Chemistry and Biochemistry (Zabol, Iran) for the support of this work.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li W, Chen B, Zhang H, Sun Y, Wang J, Zhang J, et al. BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury (II) ions. Biosens Bioelectron. 2015;66:251-258.
- Jangi AR, Jangi MR, Jangi SR. Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chin J Chem Eng. 2020;28(6):1492-1503.
- Jangi SR, Akhond M, Absalan G. A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Anal Chim Acta. 2020;1127:1-8.
- Hormozi Jangi SR, Akhond M, Absalan G. A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3,3′-diaminobenzidine. Mikrochim Acta. 2020;187:1-10.
- Huang Y, Ren J, Qu X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119(6):4357-4412.
- Jangi SR, Akhond M. Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchem. J. 2020;158:105328.
- Akhond M, Hormozi Jangi SR, Barzegar S, Absalan G. Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chem Pap. 2020;74:1321-1330.
- Schmid J, Heider D, Wendel NJ, Sperl N, Sieber V. Bacterial glycosyltransferases: challenges and opportunities of a highly diverse enzyme class toward tailoring natural products. Front Microbiol. 2016;7:182.
- Homaei AA, Sariri R, Vianello F, Stevanato R. Enzyme immobilization: An update. J Chem Biol. 2013;6:185-205.
- Jangi SR, Akhond M, Dehghani Z. High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochem. 2020;90:102-112.
- Jangi SR, Akhond M. Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochem. 2022;120:138-155.
- Garcia‐Galan C, Berenguer‐Murcia Á, Fernandez‐Lafuente R, Rodrigues RC. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal. 2011;353(16):2885-2904.
- Jangi SR, Akhond M. High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochem. 2021;105:79-90.
- Jangi SR. Introducing a high throughput nanozymatic method for eco-friendly nanozyme-mediated degradation of methylene blue in real water media. Sustain Chem Eng. 2023:90-99.
- Hormozi Jangi SR. Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: A new approach toward reuse instead of common mineralization. Chem Pap. 2023:1-5.
- Jangi SR, Akhond M. Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sens Actuators B Chem. 2021;339:129901.
- Hormozi Jangi SR, Akhond M. High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. J Chem Sci. 2020;132:1-8.
- Hormozi Jangi SR, Gholamhosseinzadeh E. Developing an ultra-reproducible and ultrasensitive label-free nanoassay for L-methionine quantification in biological samples toward application in homocystinuria diagnosis. Chem Pap. 2023:1-3.
- Dehghani Z, Akhond M, Jangi SR, Absalan G. Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes. Talanta. 2024;266:124977.
- Hormozi Jangi SR. Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials. 2023;1(4):1-9.
- Amany A, El-Rab SF, Gad F. Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Der Pharma Chem. 2012;4(1):53-65.
- Jangi SR. Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. 2023.
- Jangi SR, Davoudli HK, Delshad Y, Jangi MR, Jangi AR. A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surf Interfaces. 2020;21:100771.
- Ahmadi-Leilakouhi B, Hormozi Jangi SR, Khorshidi A. Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chem Pap. 2023;77(2):1033-1046.
- Zhang X, Wu D, Zhou X, Yu Y, Liu J, Hu N, et al. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. TrAC Trend Anal Chem. 2019;121:115668.
- Wang Q, Wei H, Zhang Z, Wang E, Dong S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trend Anal Chem. 2018;105:218-224.
- Feng WE, Xiao-Jun HA. Nanozymes and their application progress in biomedical detection. Chinese J Anal Chem. 2021;49(4):581-592.
- Unnikrishnan B, Lien CW, Chu HW, Huang CC. A review on metal nanozyme-based sensing of heavy metal ions: Challenges and future perspectives. J Hazard Mater. 2021;401:123397.
- Sun Q, Xu X, Yu J, Yin C, Wu M, Niu N, et al. Rice straw-derived carbon based nanozyme sensor: Application of identifying human urine xanthine content and study of active sites. Appl Surf Sci. 2022;602:154372.
- Chen M, Zhou H, Liu X, Yuan T, Wang W, Zhao C, et al. Single iron site nanozyme for ultrasensitive glucose detection. Small. 2020;16(31):2002343.
- Nsuamani ML, Zolotovskaya S, Abdolvand A, Daeid NN, Adegoke O. Thiolated gamma-cyclodextrin-polymer-functionalized CeFe3O4 magnetic nanocomposite as an intrinsic nanocatalyst for the selective and ultrasensitive colorimetric detection of triacetone triperoxide. Chemosphere. 2022;307:136108.
- Hormozi Jangi SR, Dehghani Z. Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials. 2023;2(1):15-23.
- Hormozi Jangi SR. Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios.2023;10.
- Hormozi Jangi SR, Dehghani Z. Kinetics and biochemical characterization of silver nanozymes and investigating impact of storage conditions on their activity and shelf-life. Chemical Research and Nanomaterials. 2023;1(4):25-33.
- Mishra S, Abdal‐hay A, Rather SU, Tripathi RM, Shekh FA. Recent advances in silver nanozymes: Concept, mechanism, and applications in detection. Adv Mater. 2022;9(30):2200928.
- Karim MN, Anderson SR, Singh S, Ramanathan R, Bansal V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens Bioelectron. 2018;110:8-15.
- Jiang Z, Li H, Deng Y, He Y. Blue light-gated reversible silver nanozyme reaction networks that achieve life-like adaptivity. ACS Sustain Chem Eng. 2020;8(13):5076-5081.
- Jangi SR. A Comparative Study on Kinetics Performances of BSA-gold Nanozymes for Nanozyme-Mediated Oxidation of 3,3’,5,5’-Tetramethylbenzidine and 3,3’-Diaminobenzidine.
- Hormozi Jangi SR. Evaluation of biochemical behavior and stability of gold nanoparticles with high intrinsic peroxidase-like activity. Petro Chem Indus Intern. 2023;6(4):234-239.
- Hormozi Jangi SR. Biochemical characterization of enzyme-like silver nanoparticles toward nanozyme-catalysed oxidation reactions. Micromaterials and Interfaces. 2023;1(1):2170.
- Gurmessa SK, Tufa LT, Kim J, Lee KI, Kim YM, Tran VT, et al. Colorimetric detection of Mycobacterium tuberculosis ESX-1 substrate protein in clinical samples using Au@ Pd nanoparticle-based magnetic enzyme-linked immunosorbent assay. ACS Appl Nano Mater. 2020;4(1):539-549.
Citation: Hormonzi Jangi SR (2023) Time Course Progess of Nanozyme-Mediated Reversible/Irreversible Oxidation Reactions over Silver Nanoparticles as Peroxidase Alternatives. Modern Chem App. 11:426.
Copyright: © 2023 Hormonzi Jangi SR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


