Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Publons
- International committee of medical journals editors (ICMJE)
- Geneva Foundation for Medical Education and Research
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 28, Issue 5
The Potential Importance of Electromagnetic Waves for The Treatment of Psychiatric Behavioral Disorders
Richard Ambron*Received: 22-Sep-2025, Manuscript No. JOP-25-29900; Editor assigned: 24-Sep-2025, Pre QC No. JOP-25-29900; Reviewed: 08-Oct-2025, QC No. JOP-25-29900; Revised: 15-Oct-2025, Manuscript No. JOP-25-29900; Published: 22-Oct-2025, DOI: 10.35248/2378-5756.24.28.764
Abstract
Efforts by psychiatrists to treat mental disorders are based on what is known about the relationship between the brain and the mind. The prevailing theory is that the functions of the mind responsible for normal and abnormal behavior emerge from the activity of the brain. Thus, disorders of the mind can be reduced to physical phenomena, i.e. the propagation of action potentials along neural networks and transmission across synapses and can therefore be explained in terms of these activities. However, this view cannot explain how higher mental attributes actually emerge from these activities, which is essential for understanding the origin of abnormal behavior. Studies of pain show that the mind and brain are separate entities, both functionally and spatially and this has major implications for treating mental disorders. Pain from an injury, encoded in APs, induces a Long-Term Potentiation (LTP) in a population of pyramidal neurons in the anterior cingulate cortex. The LTP activates NMDA receptors, resulting in the transformation of the information about pain into EM waves. The waves then communicate this information to consciousness and the mind where the pain is experienced. Pain-associated EM waves are also emitted from the amygdala and Nucleus accumbens so by extrapolation, each component of a behavior can be traced to an EM wave at a certain frequency and it is the summation of all sensory experiences transmitted by EM waves that provides the knowledge that guides behaviors. Since the creation and disemination of EM waves can be regulated by Transcranial Magnetic Stimulation (TMS) protocols, psychiatric disorders can be treated by targeting the aberrant waves that are responsible for the behavior. TMS protocols are already being used to treat schizophrenia and other mental disorders with some success and these new findings will provide important new information that should improve outcomes.
Keywords
Electromagnetic waves; Long term potentiation; NMDA receptors; Schizophrenia; Consciousness; Behavior; Amygdala; Nucleus accumbens; Action Potentials (APs); Transcranial Magnetic Stimulation (TMS)
Abbreviations
Electro Magnetic (EM) waves; Long Term Potentiation (LTP); N-Methyl-D-Aspartate (NMDA); cAMP Responsive Element-Binding protein (CREB); Local Field Potential (LFP); Nucleus Accumbens (NucA); Insula Cortex (IC); Anterior Cingulate Cortex (ACC)
Introduction
One of the great challenges in medicine is to understand the origins of neuropsychiatric disorders such as schizophrenia, addiction, and depression. These disorders of mind arise from misconceptions of the world, implying that the disorders are based at some point on faulty or incorrect information. We acquire information about objects and events in our world via our senses. The information from each sensation is encoded in Action Potentials (APs) that propagate along dedicated neural pathways to activate specific neuronal circuits distributed throughout the brain. The totality of this activity at each moment in time results in the knowledge that the mind uses to create ideas, plan for the future, formulate beliefs and make the decisions that guide our behavior and social interactions. When the knowledge of the world is based on false information, it leads to the abnormal behaviors that are subject of psychiatry. Thus, patients suffering from delusional disorders have a strong but false belief about their world despite evidence to the contrary. In Paranoid personality disorder patients believe that others are trying to harm or threaten them and in depressive disorders patients often believe that they have no control over their lives, or that they are worthless and a failure. These misconceptions often result in making wrong decisions, which is most obvious in patients with drug addiction who suffer from distortions in value assessment or reward that result in making decisions that are harmful.
Most efforts to treat these disorders are based on what we know about the relationship between the brain the mind. The prevailing theory is that the functions of the mind responsible for normal and abnormal behavior emerge from electrical activity of the brain. This is consistent with the traditional view of how the brain functions and with the various permutations of physicalism/materialism theory in which higher mental states are believed to be inseparable from the activities of the brain [1]. In other words, disorders of the mind can be reduced to physical phenomena-the propagation of APs along neural networks and transmission across synapses-and can therefore be explained in terms of these activities. This view favors a pharmacological approach to treating mental disorders, but not all disorders are susceptible to drug interventions and there is always the problem with unwanted and sometimes serious side-effects. However, there is a fundamental problem with the traditional, physicalist/materialist view because it cannot explain how knowledge and other attributes of the mind actually emerge from the functions of brain. There is now substantial evidence supporting an alternative view- that the mind and brain are actually separate entities, both functionally and spatially. This idea was originally proposed by the French philosopher and mathematician rene descartes in the early 1600s. Commonly known as Cartesian dualism, his theory was important, but it could not be proven experimentally and for centuries pondering the nature of the mind was relegated to philosophers. A major conceptual advance was to interpose consciousness between the brain and the mind [2]. Consciousness is the entity where sensory information becomes a sensory experience. In other words, it is where we become aware of the quality of a sensation, such as the color of a flower or the sound of a canary. Since the awareness of sensations is a precursor to knowledge in the mind, one approach to understanding the mind was to first determine how information acquired by our senses becomes sensory experiences in consciousness. Considerable progress toward this end has been made recently by studying pain and the findings have been surprising and have particular relevance to the treatment of psychiatric disorders.
Results and Discussion
Pain as a Sensory Experience
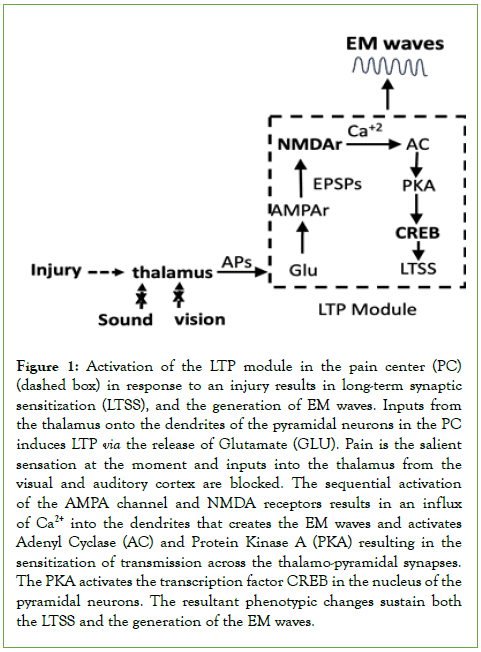
The initial approach to determining how we experience pain-the actual suffering- was to characterize the flow of information from the site of an injury to circuits in the brain. These findings have been published and are summarized here [3,4]. The perception of pain (aka, nociception), begins when agents released from a lesion evoke APs in the terminals of nociceptive neurons in the periphery. The APs encode information about the severity of the lesion: the more severe the lesion, the greater the number and frequency of the APs. The APs convey the information first to neuronal centers in the thalamus and then to neurons in the anterior cingulate cortex (ACC) that are necessary for experiencing pain from a physical injury [5-8], from viscera [9], as well as the suffering from psychological causes [10,11]. Located within the ACC of humans, other primates, and rodents is a pain center (PC) that contains a population of large lamina V pyramidal neurons that are associated specifically with experiencing pain [12,13]. The PC is located near areas in the ACC that mediate attention, pain avoidance, fear, and depression [14,15] and each of these contributes to a specific aspect of pain. After an injury, APs propagating along axons from the thalamus activate thousands of glutamatergic excitatory synapses on the pyramidal neurons in the PC. Notable is that the activation can be mimicked experimentally by stimulating the synapses using externally applied theta waves (4-8 Hz) [16]. The release of glutamate (Glu) at the thalamo-pyramidal synapses has two consequences. First is the induction of a Long-Term Potentiation (LTP) in the dendritic spines. Second is the appearance of both local field potential (LFP) and synchronized oscillating electromagnetic (EM) waves in the extra-neuronal space around the pyramidal neurons [17,18]. Both the LTP, LFP, and the EM waves are necessary for experiencing pain [3, 7,16, 19]. The LTP sensitizes the transmission across the thalamo-pyramidal synapses resulting in the allodynia that explains how pain can be present for hours or days after an injury. Since the duration of the LFP and EM waves depends on the sensitization, characterizing the events that unfold in the PC in response to the induction of the LTP is important for understanding persistent and perhaps chronic pain. Multiple electro-physiological and molecular events in both the presynaptic and postsynaptic terminals are involved [3,16], but it is the activation of N-Methyl-D-Aspartate (NMDA) receptors that is most significant because it results in an influx of Ca2+ into the postsynaptic terminal (Figure 1).
The Ca2+ initiates enzymatic reactions in the dendrites that culminate in the activation of the cAMP Responsive Element-Binding protein (CREB) in the nucleus of the pyramidal neurons [20,21]. CREB is a transcription factor that promotes the synthesis of proteins that alter the phenotype of the pyramidal neurons, thereby maintaining the LTP, the sensitization of the synapses, and the generation of the LFP and EM waves (Figure 1). The phenotypic changes strengthen transmission along the nociceptive pathway and can last for very long periods [22]. The reactions shown in figure 1 create a module that when activated will result in the generation of EM waves.
Figure 1: Activation of the LTP module in the pain center (PC) (dashed box) in response to an injury results in long-term synaptic sensitization (LTSS), and the generation of EM waves. Inputs from the thalamus onto the dendrites of the pyramidal neurons in the PC induces LTP via the release of Glutamate (GLU). Pain is the salient sensation at the moment and inputs into the thalamus from the visual and auditory cortex are blocked. The sequential activation of the AMPA channel and NMDA receptors results in an influx of Ca2+ into the dendrites that creates the EM waves and activates Adenyl Cyclase (AC) and Protein Kinase A (PKA) resulting in the sensitization of transmission across the thalamo-pyramidal synapses. The PKA activates the transcription factor CREB in the nucleus of the pyramidal neurons. The resultant phenotypic changes sustain both the LTSS and the generation of the EM waves.
It is evident that we can describe in great detail the electrophysiological and enzymatic events that result in painfulness, yet none of these can account for the suffering because they all occur in many areas of the cerebral cortex that are not involved in nociception. This reinforces the idea that neither the electrical activity coursing along neural circuits, nor the biochemical consequences of that activity can explain how sensory information becomes experiences in consciousness.
The other consequence of the injury-elicited activation of the pyramidal neurons is the creation of synchronized, oscillating theta EM waves. Since EM waves can travel rapidly throughout the brain and contain information about their source, these findings led to the theory that the information about pain that was encoded in APs in the brain was transformed into EM waves, which then conveyed the information to consciousness where the pain is experienced [4,21]. This idea was in accord with earlier proposals by Susan Pockett [23,24] and Johnjoe McFadden [25,26], and others [27,28], who argued that EM waves are intermediaries in consciousness [27,28]. As we shall see, this theory addresses a major issue in human cognition, namely, how sensory information from multiple sources is processed to provide an awareness of objects and events in our world, and it offers significant insights into understanding both normal and abnormal behavior.
EM Waves Integrate Sensory Experiences
In the situation discussed above, APs conveying information about the injury rapidly reached neurons in the PC and the intensity of the pain was commensurate with the severity of the injury. However, we live in an ever-changing world to which we must adapt to survive and there are several neuronal centers in the brain that can alter the degree of pain in order to accommodate the circumstances surrounding the injury. Thus, circuits in the Insula Cortex (IC) can divert attention away from pain toward some other more immediate relevant sensation [29]; circuits in the amygdala can impart emotional influences, such as fear [30], whereas those in the nucleus accumbens (NucA) contribute to a reward-value system that provides motivation to bear pain if the reward is deemed to be worthwhile [31,32]. In addition, there are several centers in the prefrontal cortex that together comprise a central executive network that imposes top-down control over the behavioral outcome [33,34].
The IC, amygdala, and the other centers that process nociceptive information are widely separated in the brain so how is the information from each center rapidly assembled into a single painful sensation? This is the so-called binding problem that was first recognized in the visual system where individual components of an image, such as color and various components of shape, are processed by separate areas in the cerebral cortex [35]. The solution came from studies showing that in addition to the theta EM waves from the PC, pain-associated theta waves are emitted from IC, NucA, and amygdala [16,36,37]. When waves of the same frequency and phase intersect, the resultant wave is the algebraic sum of the two, whereas if they are out of phase, they will cancel each other. In other words, the intensity of the pain that is ultimately experienced in consciousness is determined by the wave that emerges from the interactions among the theta waves from the IC, amygdala, and NucA [21].
The neuronal centers that participate in modulating pain also contribute to networks that are responsible for complex behaviors and it is important that the frequency of the EM waves emitted depends on the behavior. For example, fear of impending pain exacerbates pain intensity in a rodent pain model and generates theta waves from circuits in the amygdala. However, fear can also result from non-noxious stimuli and Zeng, et al. [38] detected high gamma (70-180 Hz) oscillations in the amygdala in response to seeing fearful faces. Likewise, theta waves were detected in the NucA in the context of pain, but circuits in the NucA also participate in networks that impart valuation and reward to making decisions [39] and Cohen, et al. [40] detected gamma (40-80 Hz) and alpha (8-12 Hz) oscillations from the human NucA in a reward paradigm. In addition to the gamma waves emitted from the amygdala and NucA in the context of fear and reward, respectively, gamma waves in the range 80-100 Hz are detected from many other areas of the brain, including the prefrontal cortex, the hippocampus, the primary visual cortex, and the striatum [41]. It is not surprising, therefore that they are believed to have a critical role in sensory processing and integration, working memory, the regulation of motor outputs, and emotions. It is also not surprising that disruption of the gamma oscillations induces aberrant neural activity and brain dysfunction.
Several important concepts emerge from the data presented above and from the references cited. First, our awareness of sensations, such as pain, depends on interactions among the EM waves that emerge from dedicated neural networks in the brain and it is the sum of all these experiences that results in our consciousness of our world. Second, is that the information that is processed within a given center is not confined to a particular network, but can be shared with other networks. Third, that the frequency of the EM waves that emerge from each neuronal center contains information that will contribute to the function of the network with which it interacts at each moment in time. Fourth, EM waves comprise a complex, dynamic, frequency-dependent mechanism that rapidly integrates and assembles sensory information acquired by defined neuronal centers within networks in the brain [21,42-45].
According to the scenario above EM waves contain information that is essential for behavior, which leads to the premise that both normal and abnormal behavior can be modified by altering the wave responsible for that behavior. Before discussing the implications of this premise, two points need to be made. First EM waves are not passive, but contain energy in photons. The amount of energy is determined by the frequency of the wave-the higher the frequency, the greater the energy and this energy can alter the activity of a receptive target. Second, the EM waves discussed above emerge from large clusters of neurons that fire in synchrony and are therefore readily detected. However, each category of EM waves includes waves with different frequencies. Thus, the 4-8 Hz theta and the 80-100 Hz gamma waves mentioned above will contain many EM waves with different frequencies. For example, Wang, et al. [46] recorded the frequency of the waves recorded in patients after various types of head trauma and detected specific frequencies ranging from 5.2 to 10.4 Hz. In addition, data from an important paper by Okonogi and Sasaki [47] show that there several frequencies within the theta range that have behavioral consequences. Finally, Brazdzionis, et al. [48] found that EM waves were even emitted from the motor cortex during a simple movement of the hand. What this means is that there is a yet to be determined number of EM waves with different frequencies that are conveying information from circuits in the brain to consciousness.
Modifying Behavior by Manipulating EM Waves
The findings above are important for the treatment of psychiatric disorders because they indicate that a specific behavior can be altered by manipulating the EM waves responsible for the behavior. Behavioral modification is possible by using Transcranial Magnetic Stimulation (TMS) and repetitive TMS (rTMS) methodologies that are non-invasive, well-tolerated, and do not have serious side-effects. Both employ a wire coil placed on the scalp to generate a magnetic field that alters the electrical activity of circuits in the cerebral cortex directly below the coil [49,50]. It has been documented for over a century that application of TMS to specific areas of the cortex elicits specific motor and sensory responses [51]. The effect depends on the frequency of the input and rTMS is more flexible because it provides pulses at a given frequency that induce rhythmic patterns of activity in the target area. The number of pulses, their intensity and duration can be adjusted. There are 3 ways in which TMS can be used to alter behavior.
As discussed above, the formation of EM waves depends on the induction of LTP (Figure 1) and it is significant that theta-burst rTMS protocols can influence the initiation and maintenance of LTP by altering the activity of AMPA and N-Methyl-D-aspartate (NMDA) receptors [52,53]. Other frequencies activate GABAergic inhibitory neurons that depress the LTP. Thus, it should be possible to use rTMS to initiate or block the production of EM waves and the associated behavior anywhere in the cortex that can be accessed by the coil. This is a very powerful approach because the events downstream of LTP will activate CREB and the effect will be long-lived (Figure 1). For example, conditioned fear in a mouse model depends on the induction of LTP and the creation of EM waves in the amygdala [54], which means that a rTMS protocol that prevents the induction of the LTP could be used to prevent the fear. An alternative approach is to generate an out-of-phase EM wave with a frequency that will cancel the wave responsible for the behavior [23]. This has great promise, but it depends on knowing which frequency to use and this should be the primary focus of future studies.
Lastly, there is evidence that aberrant gamma oscillations contribute to several mental disorders, including schizophrenia and depression [41,55]. TMS and rTMS protocols are being used to reinstate normal oscillating rhythms that are manifest as the synchronized oscillating EM waves. A literature review by Rizvi, et al. [55] discussed the use of high (10-20 Hz) frequency (TMS) applied to the dorsolateral prefrontal cortex (DLPFC). The DLPFC is an area that has is dysregulated in patients with major depression. Some success has been reported, but the neurobiological consequences of the treatment are not known.
A strong rationale for the use of TMS methodology to treat schizophrenia derives from landmark studies of showing that many of the symptoms of schizophrenia, such as delusions, hallucinations, and disorders of executive functions, appear in healthy subjects given low doses of the anesthetic ketamine. Ketamine is a well know NMDA receptor/channel antagonist and, as discussed above, it links glutamatergic synaptic activity to the production of EM waves and to the phenotypic changes responsible for experiencing sensations. Any disruption in the function of the receptor would therefore have profound effects on neuronal function. Indeed, studies have led to the conclusion that the symptoms of schizophrenia are due to hypoactivity of NMDA receptors located on inhibitory interneurons in the PFC [56,57]. The reduced activity of the inhibitory neurons results in the hyperactivation of cortical pyramidal neurons in the PFC and the generation of abnormal gamma oscillations that disrupt the functions of associated neural networks [58].
Consequently, rTMS procedures have been applied to the dorso-lateral PFC to restore normal gamma oscillations. The DLPFC is an area that is responsible for executive functions, but the results have been mixed. An extensive analysis of the literature by Wang, et al. [59] showed that rTMS was more effective in treating the symptoms of Schizophrenia than a sham procedure whereas Mo, et al. [60] evaluated the results from a modified procedure, deep TMS, and concluded that it was ineffective. Many of these discrepancies can likely be attributed to differences in protocols, especially the frequency that is applied, but there is a more fundamental problem, namely that it is not clear what is occurring at the circuit, cellular, and molecular levels in the targeted area. Since each frequency has a specific energy, slight changes in the applied frequency will activate different targets and can evoke different responses. That being said, TMS protocols have great promise for treating psychiatric disorders but the focus has to be on first identifying the frequency of the EM wave that is responsible for the target behavior. Once the frequency is known, there are several possibilities. For example, the behavior can be blocked by preventing the induction of the LTP, thereby preventing the formation of the wave, or the wave can be cancelled with an opposing wave.
Conclusions
There is strong evidence supporting a new theory that human behavior depends on communication of sensory information from the brain to consciousness that is mediated via EM waves. It is within consciousness that the quality of each sensation experienced and this information is then relayed to the mind where it guides the behaviors that enable us to navigate our world. This theory is consistent with studies showing that abnormal oscillating waves are associated with abnormal behavior. The theory upends the traditional view of brain function and has important implications for psychiatry because it indicates that a given behavior can be manipulated by altering the EM wave with which it is associated. Moreover, these manipulations can be implemented by TMS technologies that are non-invasive and well tolerated. TMS protocols have been used for years to treat various psychiatric conditions but with mixed results. Nevertheless, the theory validates the TMS approach and also provides details to improve the outcomes. The immediate need is to identify the frequency of the EM wave that is responsible for the target behavior. This will be challenging because there are potentially thousands of EM waves emitted due to the activity of the brain, but it is possible using the methodology introduced by Wang, et al. (46). Particularly important is to take advantage of the finding that LTP is necessary for the creation of the EM waves that contribute to behavioral outcomes in specific areas of the cerebral cortex, such as the amygdala. Since rTMS protocols at specific frequencies can control the induction of LTP means that they can be used to control the generation of the EM waves that emerge from areas of the cortex that are accessible to the coils. Cortical circuits in the prefrontal cortex are prime targets because they comprise the executive center that exerts control over behavior. The amygdala is another obvious target to influence the emotional aspects of behavior. This is a very powerful approach to modifying behavior and the result will be long lasting because it involves transcriptional programs mediated by CREB. Thus, TMS has a major advantage over drug treatments because it targets a small defined area in the cortex without side-effects. As such, modifying behavior by influencing EM waves at specific frequencies has great promise for treatment of psychiatric disorders.
Acknowledgements
Richard Ambron is solely responsible for all aspects of the paper. He did not receive any funding for the writing of this paper.
References
- Jiang Y. In What Sense is Physicalism a Materialism?. ProtoSociology. 2025;40:76.
- Ambron R. Dualism, Materialism, and the relationship between the brain and the mind in experiencing pain. Neuroscience. 2024;561:139-143.
[Crossref] [Google Scholar] [PubMed]
- Ambron R. Toward the unknown: Consciousness and pain. Neurosci Conscious. 2023;2023(1):niad002.
[Crossref] [Google Scholar] [PubMed]
- Ambron R. Synaptic sensitization in the anterior cingulate cortex sustains the consciousness of pain via synchronized oscillating electromagnetic waves. Front Hum Neurosci. 2024;18:1462211.
[Crossref] [Google Scholar] [PubMed]
- Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci Lett. 2012;520:174–181.
[Crossref] [Google Scholar] [PubMed]
- Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014;8:35.
[Crossref] [Google Scholar] [PubMed]
- Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130146.
[Crossref] [Google Scholar] [PubMed]
- Moon HC, Park YS. Optogenetic stimulation of the anterior cingulate cortex modulates the pain processing in neuropathic pain: A review. J Mol Neurosci. 2022;72:1–8.
[Crossref] [Google Scholar] [PubMed]
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607.
[Crossref] [Google Scholar] [PubMed]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434.
- Xiao X, Zhang YQ. A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev. 2018;90:200–211.
[Crossref] [Google Scholar] [PubMed]
- Shyu BC, Sikes RW, Vogt LJ, Vogt BA. Nociceptive processing by anterior cingulate pyramidal neurons. J Neurophysiol. 2010;103:3287–3301.
[Crossref] [Google Scholar] [PubMed]
- van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, et al. Where is cingulate cortex? A cross-species view. Trends Neurosci. 2020;43:285–299.
[Crossref] [Google Scholar] [PubMed]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190.
[Crossref] [Google Scholar] [PubMed]
- Journée SH, Mathis VP, Fillinger C, Veinante P, Yalcin I. Janus effect of the anterior cingulate cortex: Pain and emotion. Neurosci Biobehav Rev. 2023;153:105362.
[Crossref] [Google Scholar] [PubMed]
- Lee JHA, Chen Q, Zhuo M. Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines. 2022;10:2745.
[Crossref] [Google Scholar] [PubMed]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci. 2013;14:770–785.
[Crossref] [Google Scholar] [PubMed]
- Hales C, Pockett S. The relationship between local field potentials (LFPs) and the electromagnetic fields that give rise to them. Front Syst Neurosci. 2014;8:233.
[Crossref] [Google Scholar] [PubMed]
- Kang WB, et al. Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model. Mol Pain. 2016;12:1–9.
[Crossref] [Google Scholar] [PubMed]
- Toyoda H, et al. Calcium/calmodulin-dependent kinase IV contributes to translation-dependent early synaptic potentiation in the anterior cingulate cortex of adult mice. Mol Brain. 2010;3:27.
[Crossref] [Google Scholar] [PubMed]
- Ambron R. Experiencing pain: Electromagnetic waves, consciousness, and the mind. Front Hum Neurosci. 2025;19:1568019.
[Crossref] [Google Scholar] [PubMed]
- Ortega-Martínez SA. New perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015;8:46.
[Crossref] [Google Scholar] [PubMed]
- Pockett S. The electromagnetic field theory of consciousness: a testable hypothesis about the characteristics of conscious as opposed to non-conscious fields. J Conscious Stud. 2012;19:191–223.
- Pockett S. Field theories of consciousness. Scholarpedia. 2013;8:4951.
- McFadden J. Synchronous firing and its influence on the brain’s electromagnetic field: evidence for an electromagnetic theory of consciousness. J Conscious Stud. 2002;29:23–50.
- McFadden J. Integrating information in the brain’s EM field: the cemi field theory of consciousness. Neurosci Conscious. 2020;2020:niaa016.
[Crossref] [Google Scholar] [PubMed]
- Hunt T, Jones M. Fields or firings? Comparing the spike code and the electromagnetic field hypothesis. Front Psychol. 2023;14:1029715.
[Crossref] [Google Scholar] [PubMed]
- MacIver MB. Consciousness and inward electromagnetic field interactions. Front Hum Neurosci. 2022;16:1032339. doi:10.3389/fnhum.2022.1032339.
[Crossref] [Google Scholar] [PubMed]
- Molnar-Szakacs I, Uddin LQ. Anterior insula as a gatekeeper of executive control. Neurosci Biobehav Rev. 2022;139:104736.
[Crossref] [Google Scholar] [PubMed]
- Neugebauer V. Amygdala physiology in pain. Handb Behav Neurosci. 2012;26:101–113.
[Crossref] [Google Scholar] [PubMed]
- Sicre M, Meffre J, Louber D, Ambroggi F. The nucleus accumbens core is necessary for responding to incentive but not instructive stimuli. J Neurosci. 2020;40:1332–1343.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Lin Y, Yu M, Zhou K. The nucleus accumbens in reward and aversion processing: Insights and implications. Front Behav Neurosci. 2024;18:1420028.
[Crossref] [Google Scholar] [PubMed]
- Rushworth M, Noonan M, Boorman E, Walton M, et al. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069.
[Crossref] [Google Scholar] [PubMed]
- Otis JM, Namboodiri VM, Matan AM, Voets ES, et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543:103–107.
[Crossref] [Google Scholar] [PubMed]
- Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–126.
[Crossref] [Google Scholar] [PubMed]
- Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2005;23:721–731.
[Crossref] [Google Scholar] [PubMed]
- Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn Sci. 2017;21:100–110.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Anderson KL, Leal SL, Shestyuk A, et al. Amygdala-hippocampal dynamics during salient information processing. Nat Commun. 2017;8:14413.
[Crossref] [Google Scholar] [PubMed]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, et al. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–922.
[Crossref] [Google Scholar] [PubMed]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, et al. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci. 2007;21:875–889.
[Crossref] [Google Scholar] [PubMed]
- Deng Q, Wu C, Parker E, Zhu J, et al. Mystery of gamma wave stimulation in brain disorders. Mol Neurodegener. 2024;19:96.
[Crossref] [Google Scholar] [PubMed]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239.
[Crossref] [Google Scholar] [PubMed]
- Buzsáki G. Rhythms of the Brain. Oxford: Oxford University Press; 2006.
- Taub A, Paz R. Oscillations synchronize amygdala-to-prefrontal primate circuits during aversive learning. Neuron. 2018;97:291–298.e3.
[Crossref] [Google Scholar] [PubMed]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980.
[Crossref] [Google Scholar] [PubMed]
- Wang AS, Brazdzionis J, Rahman RK. Optimal frequency for cranial electromagnetic field stimulation. Cureus. 2025;17(3):e81436.
[Crossref] [Google Scholar] [PubMed]
- Okonogi T, Sasaki T. Theta-range oscillations in stress-induced mental disorders as an oscillotherapeutic target. Front Behav Neurosci. 2021;15:698753.
[Crossref] [Google Scholar] [PubMed]
- Brazdzionis J, Wiginton IV J, Patchana T, Savla P, et al. Evaluating the intrinsic electromagnetic field generated by neurons from repetitive motor activities in humans with a non-contact non-invasive electromagnetic helmet. Cureus. 2022;14:e23006. doi:10.7759/cureus.23006.
[Crossref] [Google Scholar] [PubMed]
- Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58:208–213.
[Crossref] [Google Scholar] [PubMed]
- Fröhlich F. Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci. 2014;16:93–102.
[Crossref] [Google Scholar] [PubMed]
- Cowey A. The Ferrier Lecture. What can transcranial magnetic stimulation tell us about how the brain works? Philos Trans R Soc Lond B Biol Sci. 2005;360:1185–1205.
[Crossref] [Google Scholar] [PubMed]
- Lenz M, Platschek S, Priesemann V, Becker D, Willems LM, et al. Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct Funct. 2015;220:3323–3337.
[Crossref] [Google Scholar] [PubMed]
- Komaki A, Khalili A, Salehi I, Shahidi S, Sarih AI. Effects of exposure to an extremely low frequency electromagnetic field on hippocampal long-term potentiation in rat. Brain Res. 2014;1564:1–8.
[Crossref] [Google Scholar] [PubMed]
- Kim WB, Cho JH. Encoding of discriminative fear memory by input-specific LTP in the amygdala. Neuron. 2017;95:1129–1146.e5.
[Crossref] [Google Scholar] [PubMed]
- Rizvi S, Khab AM. Use of transcranial magnetic stimulation for depression. Cureus. 2019;11(5):e4736.
[Crossref] [Google Scholar] [PubMed]
- Rebollo B, Perez-Zabalza M, Ruiz-Mejias M, Perez-Mendez L, et al. Beta and gamma oscillations in prefrontal cortex during NMDA hypofunction: an in vitro model of schizophrenia features. Neuroscience. 2018;383:138–149.
[Crossref] [Google Scholar] [PubMed]
- Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2019;205:107426.
[Crossref] [Google Scholar] [PubMed]
- Balu DT. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Adv Pharmacol. 2016;76:351–382.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Lu S, Hao L, Xia Y, et al. Placebo effects of repetitive transcranial magnetic stimulation on negative symptoms and cognition in patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Front Psychiatry. 2024;15:1377257.
[Crossref] [Google Scholar] [PubMed]
- Mo Y, Shi ZM, Yang XH, Lan XJ, et al. Deep transcranial magnetic stimulation for schizophrenia: a systematic review. Front Psychiatry. 2024;15:1390913.
[Crossref] [Google Scholar] [PubMed]
Citation: Ambron R (2025). The Potential Importance of Electromagnetic Waves for The Treatment of Psychiatric Behavioral Disorders. J Psychiatry. 28:764.
Copyright: © 2024 Ambron R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.