Citations : 2345
Dentistry received 2345 citations as per Google Scholar report
Indexed In
- Genamics JournalSeek
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 15, Issue 3
The Main Risk Factors for Implant Failure in Elderly Patients: A Clinical Trial Study
Rackel Goncalves1*, Valquiria Quinelato1, Marina Prado Fernandes Pinheiro1, Juliana Prazeres Castro1, Patricia Arriaga1, Esio de Oliveira Vieira1, Aldir Nascimento Machado1, Telma Aguiar1, Alexandre Campos Montenegro1,2 and Priscila Ladeira Casado12Department of Dentistry, São Leopoldo Mandic University, Brazil’s Navy, Rio de Janeiro, RJ, Brazil
Received: 26-Aug-2023, Manuscript No. DCR-23-22730; Editor assigned: 29-Aug-2023, Pre QC No. DCR-23-22730 (PQ); Reviewed: 12-Sep-2023, QC No. DCR-23-22730; Revised: 09-Jan-2025, Manuscript No. DCR-23-22730 (R); Published: 16-Jan-2025
Abstract
Background: Peri-implant disease is a multifactorial disease, with increasing prevalence in the population rehabilitated with endosseous implants and associated with numerous extrinsic and intrinsic risk factors such as systemic disease, smoking, genetic disorders, and previous periodontitis.
Objective: To identify risk factors associated with the presence of peri-implant disease and to analyze whether the biofilm of the prosthesis directly influences the development of peri-implantitis.
Methods: Fifty-one subjects rehabilitated with endosseous implants were included in this study. Risk factors were assessed through careful history (habit, systemic diseases, history of periodontitis). The clinical parameters evaluated were: clinical probing depth; presence of mobility; peri-implant bleeding; implant function time; presence of biofilm on the prosthesis and/or on the implant. Participants were divided into two groups: Health Group (HG) and Peri-Implant Disease (PID) group: Subdivided into mucositis and peri-implantitis.
Results: The average age was 65 ± 12, being 39 (76.4%) women and 12 (23.6%) men. The PID group showed a high incidence of smokers (p=0.02). Subjects with biofilm accumulation over the implant supportive prostheses showed 9 times more chance of developing mucositis (p=0.02) and while smokers had 3.5 times more chance of developing peri-implantitis (p=0.01) and dental implant loss (p=0.001).
Conclusion: The main risk factors associated with implant failure in elderly patients were: Smoking habit and biofilm accumulation in the prosthesis. Smoking is highly associated with implant loss.
Keywords
Peri-implantitis; Risk factors; Smokers; Periodontal diseases; Diabetes mellitus; Elderly patientsIntroduction
Peri-implant disease is a multifactorial disease characterized by the presence of soft tissue (mucositis) and hard (peri-implantitis) inflammation around the implants [1]. Its etiopathogenesis is related to the presence of numerous intrinsic and extrinsic factors that, together, can trigger an exacerbated inflammatory response around the implant, culminating in peri-implant bone resorption and even implant loss [2]. According to the 2019 Consensus of peri-implant disease, the prevalence of periimplant mucositis is 43% and peri-implantitis 22%, making treatment with endosseous implants, previously highly predictable in rehabilitation, unsuccessful.
The main theory related to the development of peri-implant disease is associated with the relationship with biofilm accumulation, which will culminate in an exacerbated inflammatory response to plaque-specific bacterial presence. Numerous pathogens are described as associated with the development of peri-implant disease, among them Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Porphyromonas gingivalis (P. gingivalis), and Tannerella forsythia (T. forsythia), which are key microorganisms in the etiology of periodontitis. The correlation of these pathogens with bone loss around implants has reaffirmed the relationship between peri-implant biofilm accumulation and periodontitis history as possible risk factors associated with implant failure [3].
However, in 2016, Albrektson et al. hypothesized that the involvement of peri-implant bone loss was related to a foreign body reaction, in which there is an imbalance between cells associated with the immune system and cells responsible for bone homeostasis. This recent theory has highlighted the possible presence of a biological imbalance that may be potentiated in the presence of other factors, such as: inadequate clinical management, systemic diseases, smoking, genetic disorders, previous periodontitis, increasing the possibility of marginal bone loss around the implant.
In order to understand the real clinical risk factors associated with possible pathological bone loss in implant dentistry, several Consensus have been performed, including numerous researchers responsible for the development of diagnostic techniques, therapeutics, prospective and biological studies, involving this topic [4].
Among the factors recognized as influencing the prevalence of peri-implant disease, deleterious habits and local and systemic conditions were considered as the main risk factors for the development of the disease, including accumulation of bacterial biofilm, exogenous irritants (residual cement), habit smoking, iatrogenic factors (misplaced or occlusal overloaded implants), medication use, and diabetes mellitus. In addition, prosthetic restorations are associated with peri-implant diseases. It is important to pay attention to the design of the implantsupported prosthesis because a convex restoration profile creates an additional risk for bone-level implants [5].
Other factors are still being explored, such as obesity, which has been proposed as a systemic risk factor associated with various complications, including implant failure. As well as genetic factors associated with implant loss and bone metabolism, keratinized mucosa band, prosthesis design, heart disease, former smokers, diabetes mellitus, alcohol consumption, osteoporosis, among other local factors, still remain with limited association with the development of peri-implant disease [6].
Thus, considering the multifactorial characteristic of periimplant disease, its increasing prevalence in the population rehabilitated with endosseous implants, and the importance of recognizing the main risk factors associated with its development, this study aimed to identify clinically risk factors associated with the presence of peri-implant disease.
Materials and Methods
This study is a descriptive cross-sectional clinical trial, approved by the Research Ethics Committee of the UFF-Antonio Pedro University Hospital (HUAP)-Faculty of Medicine, approved by the number 2,455,991, in accordance with the provisions of Resolution 466/2012 and its complements to the National Health Council and Resolution 441/2011.
Research participants
All patients from the implant dentistry post-graduation, who underwent dental implant placement treatment and finished with fixed full denture implants supported and attended periimplant supportive therapy, during 1 year, were invited to participate in the survey [7].
Inclusion criteria: Participants who underwent rehabilitation with endosseous implants at the implant dentistry specialization and who received implant prostheses, including total and partial, fixed and removable implants supported, mandibular or maxillary, single or multiple prostheses installed for at least 6 months were included in the study.
Exclusion criteria: Participants whose implants prostheses were broken or mismatched after radiographic analysis or who underwent peri-implant supportive therapy for less than 6 months were excluded from the study.
Clinical evaluation
Risk factors were assessed through careful anamnesis, which included a questionnaire with questions about the systemic and local health of the patients. The questions included aspects such as smoking habit, alcohol consumption, medication use, diseases such as diabetes, hypertension, and osteoporosis.
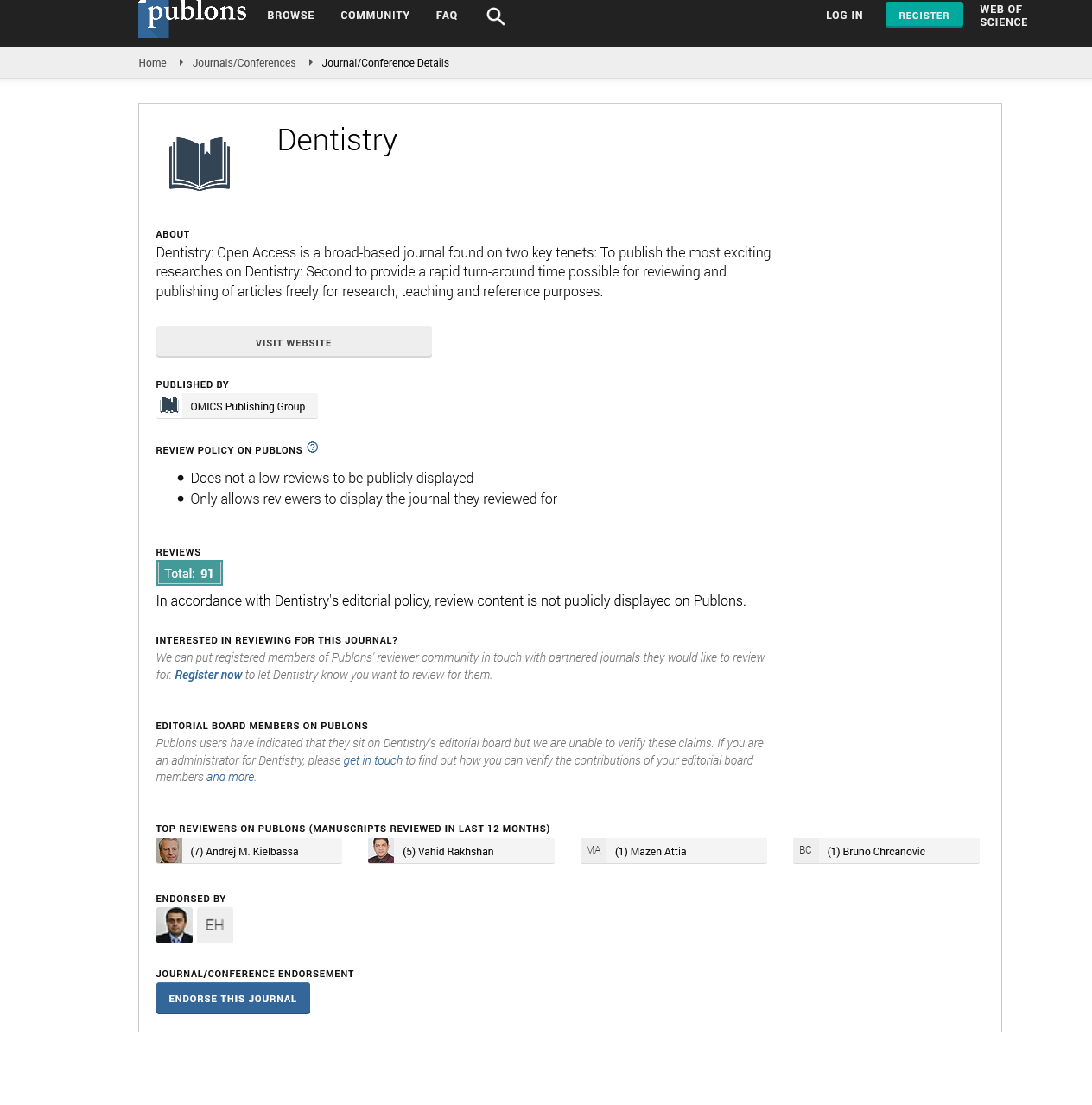
All screw-in fixed prostheses and implant-supported removable prostheses have been removed. Local aspects, such as the existence of plaque and calculus, color change, and integrity of the prosthesis were duly recorded (Figure 1). Unitary prostheses were not removed, being evaluated for the presence of a fracture. In these cases, the peri-implant tissue was submitted to the same clinical examination protocol.
The clinical parameters used to evaluate peri-implant tissues were: Clinical depth of peri-implantation probing; bleeding on probing and/or suppuration; spontaneous bleeding; the presence of mobility; presence of plaque; presence of keratinized tissue; peri-implant biotype; mucosal color change; presence of swollen area; exposure of implant threads; percussion sensitivity; implant function time. Peri-implant clinical examination was performed on the mesial, distal, buccal, and lingual surfaces of each implant using a North Carolina periodontal tube (PCPUNC15-6 Hufriedy from Brazil).
The implant function time was defined according to the years in function. Based on the study by Nobre et al. [8] the years in function were divided into two times (<4 years and ≥ 4 years), in order to investigate the correlation of peri-implant disease risk factors with early involvement.
Figure 1: Photographic images exemplify the clinical procedure. A) Front view of mandibular screw-in fixed prosthesis. B) Implants image after removal of the mandibular screw-in fixed prosthesis showing little calculus and biofilm.
Radiographic evaluation
All peri-implant regions were submitted to radiographic analysis to verify the presence of pathological bone loss when compared to the initial radiography. Radiographic examination consisted of digital periapical radiography using the Indicator Digital Shick Elite-Indusbello cone radiographic positioner (Londrina, PRBrazil). All radiographic shots were performed on the same x-ray machine DABI ATLANTE Spectro 70x (Ribeirão Preto, SP-Brazil) with the KODAK RVG5100 Digital Radiography System sensor (São José dos Campos, SP-Brazil) and using the KODAK imaging program software (São José dos Campos, SP-Brazil), through a single operator. At the time of the radiographic examination, the participants wore a lead apron and a thyroid protector, complying with Federal Ordinance 453/98 (01/06/1998).
However, extraoral panoramic radiography was requested in cases where it was not possible to perform intraoral radiography due to the difficulty in capturing the image and using the radiographic positioner.
Survey participants were divided into two groups based on clinical and radiographic examination. Health Group (HG): Presence of peri-implant health, with no clinical signs of inflammation and radiographs of peri-implant bone loss. Peri- Implant Disease (PID) group: Presence of pre-implant disease, subdivided into mucositis (clinical signs of peri-implant inflammation and absence of radiographic bone loss) and periimplantitis (peri-implant pathological bone loss). Physiological bone loss around the implant was characterized considering the normal bone loss of 1.0 mm during the first year after implant placement and 0.2 mm for subsequent years [9]. If total bone loss was greater than 1 mm and 0.2 mm per year compared to the initial radiograph after implant placement, the research participant was diagnosed with peri-implantitis (Table 1).
|
Groups |
Health group (GS) |
Defined with no probing and/or spontaneous bleeding, no edema, redness or purulent discharge, and no radiographically pathological bone loss. | |
|---|---|---|---|
|
Peri-implant disease (DPI) group |
Peri-implant mucositis |
Defined by the presence of inflammation characterized by redness and/or tissue edema, with or without spontaneous bleeding and always with the presence of bleeding on probing, absence of implant mobility and absence of pathological radiographic bone loss. | |
|
Peri-implantitis |
Defined by the presence of clinical inflammation as bleeding on probing, edema and redness, and pathological radiographic bone loss above 1 mm in the first year and above 0.2 mm per year after osseointegrated implants are installed. | ||
Table 1: Clinical-radiographic characteristics of the groups involved in the research.
Statistical analysis
Numerical variables were expressed as mean ± standard deviation and subjected to the normality test (Shapiro Test- Wilk): Normal (ANOVA e t-test) e-non-normal (Mann Whitney); Nominal variables were assessed by the chi-square test, including the odds ratio assessment with a 95% confidence interval. The p-value <0.05 was considered statistically significant. Microsoft Office 2013 Excel was used for data tabulation and Prisma GraphPad 6.0 software (GraphPad Software, La Jolla, CA-USA) for statistical calculations.
Results
From a total of 55 participants, 4 participants were excluded during anamnesis due to the presence of fractured prostheses. A total of 51 patients were included in the research. The average age of the participants was 65 ± 12, being 39 (76.4%) women and 12 (23.6%) men, with a mean implant prosthesis of 4.6 ± 3.6 years. The study participants had been without peri-implant supportive therapy for at least 6 months and all participants were rehabilitated with external hexagon implants of all patients the incidence of peri-implant disease was 54%. Eight (15.7%) participants had a history of implant loss [10]. Participants were divided into 2 groups according to the clinical and radiographic characteristics: Health-GS (n=23); peri-implant disease-DPI (n=28) (mucositis; n=15; peri-implantitis; n=13).
Considering the general characteristics of the research participants, such as age, gender, systemic disease, medication use (anxiolytics, antihypertensive drugs, diabetes control drugs), alcohol consumption and history of periodontitis, there was no statistically significant difference between groups. However, the DPI group showed a high incidence of smoking compared to the peri-implant health group (p=0.02), with higher incidence of smoking associated with peri-implantitis (p=0.01). Smokers had 3.5 times more chance of developing peri-implantitis (p=0.01) (Table 2).
| Parameter/ groups | Health group (n=23) | Peri-Implant Disease (PID) group | p-value (OR;CI) (HG X PID) |
||
|---|---|---|---|---|---|
| Mucositis (n=15) | Peri-implantitis (n=13) | Total PID (N=28) | |||
| Age | 62±16 | 69±7.7 | 64±9.1 | 66±8.6 | 0.73 |
| Gender | |||||
| Feminine | 17 (74%) | 10 (66.7%) | 12 (92.3%) | 22 (78.6%) | 0.8 (1.0;0.31-3.38) |
| Masculine | 6 (26%) | 5 (33.3%) | 1 (7.7%) | 6 (21.4%) | |
| Systemic disease | |||||
| Diabetes | 2 (8%) | 0 | 3 (23%) | 3 (10%) | 0.32 (0.31;0.04-2.21) |
| Hypertension | 9 (39%) | 7 (30%) | 4 (30%) | 11 (47%) | 0.72 (1.44;0.34-6.13) |
| Osteoporosis | 3 (13%) | 2 (13%) | 3 (23%) | 5 (17%) | 0.64 (0.5;0.08-2.93) |
| Medicines | 17 (73%) | 8 (53%) | 11 (84%) | 19 (67%) | 0.79 (0.72;0.23-2.26) |
| Smokers | 0 | 2 (13%) | 4 (30%) | 6 (21%) | *0.01 (RR** 3.55;2.04-6.18) |
| Alcohol consumption | 1 (0,4%) | 0 | 2 (15%) | 2 (0.7%) | 0.57 (0.59; 0.05-6.96) |
| Periodontitis History | 8 (34%) | 3 (2%) | 7 (53%) | 10 (35%) | 0.41 (1.81;0.6-5.44) |
| Note: *p value associated with peri-implantitis;**RR: Risk Ratio | |||||
Table 2: General clinical characteristics of the research participants.
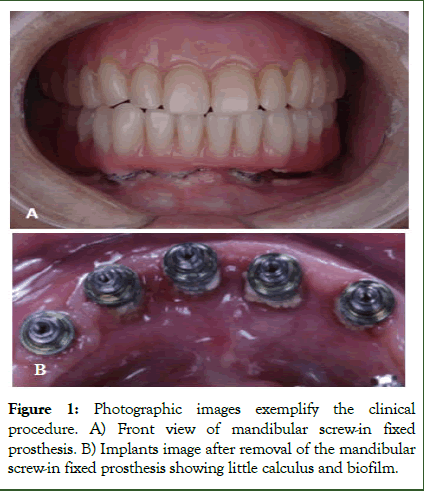
According to the characteristics analyzed in the peri-implant tissues and implant-supported prostheses, it was observed that the main prosthesis type were unitary (58.8%), protocol (31.4%) and overdenture (9.8%). Participants with biofilm accumulation over the implant supportive prostheses showed 9 times more chance of developing mucositis (p=0.02) (Table 3). Figure 2 shows the clinical relationship between prostheses biofilm accumulation and PID diagnosis [11]. Although the p-value was greater than 0.05 for the time in function, showing no significant difference, the odds ratio showed a 2.4 times tendency of individuals to develop peri-implant disease before 4 years of function.
| Parameters/groups | Health group (n=23) | Peri-implant Disease (PID) group | p-value (OR;CI) (HG X PID) | ||
|---|---|---|---|---|---|
| Mucositis (n=15) | Peri-implantitis (n=13) | Total PID (N=28) | |||
| Prior support therapy | 12 (52%) | 12 (80%) | 7 (53%) | 19 (67%) | 0.2 (0.51;0.16-1.61) |
| Protheses function time (years) | 5.25 ± 4 | 4.33 ± 3.8 | 4.2 ± 2.6 | 4.25 ± 3.25 | 0.46 |
| <4 anos | 9 (39.2%) | 9 (60%) | 10 (76.9%) | 17 (60.7%) | 0.10 (2.4; 0.77-7.44) |
| ≥ 4 anos | 14 (60.8%) | 6 (40%) | 3 (23.1%) | 11 (39.3%) | |
| Immediate charge | 5 (21%) | 3 (20%) | 0 | 3 (10%) | 0.70 (1.6;0.34-7.44) |
| Implant region | |||||
| Maxilla | 6 (26%) | 3 (2%) | 5 (38%) | 8 (28%) | 0.79 (0.98;0.31-3.06) |
| Mandible | 17 (73%) | 12 (52%) | 8 (61%) | 20 (71%) | |
| Prior implant loss | 0 | 2 (13%) | 5 (38%) | 7 (25%) | 0.45 (0.48; 0.10-2.27) |
| Antagonist | |||||
| Natural teeth | 9 (39%) | 4 (26%) | 8 (61%) | 12 (42%) | 0.6 |
| Implants | 3(13%) | 3 (20%) | 3 (23%) | 6 (21%) | |
| Dentures | 11(47%) | 8 (53%) | 2 (15%) | 10 (35%) | |
| Prosthesis characteristics | |||||
| Biofilm* | 14 (60%) | 14 (93%) | 10 (76%) | 24 (85%) | 0.02 (9.0;1.0-80.8) |
| Prosthesis-mucosa distance (mm)** | 1.1 ± 2.7 | 3.2 ± 4.3 | 0.31 ± 0.85 | 1.9 ± 3.5 | 0.43 |
| Cantilever (mm)** | 15±16 | 23±14 | 4.8±8.3 | 15±15 | 0.98 |
| Peri-implant characteristics | |||||
| Peri-implant biotype | |||||
| Thin | 6 (26%) | 4 (26%) | 1 (0.7%) | 5 (17%) | 0.51 (1.33;0.36-4.85) |
| Thick | 17(73%) | 11(73%) | 12 (92%) | 23 (82%) | |
| Biofilm | 13 (56%) | 12 (80%) | 10 (76%) | 22 (78%) | 0.08 (0.35;0.10-1.2) |
| Percussion sensitivity | 1(0,4%) | 1 (0.6%) | 0 | 1 (0.3%) | 1 |
| Spontaneous bleeding | 0 | 4 (26%) | 4 (30%) | 8 (34%) | 0,001 |
| Purulent secretion | 0 | 0 | 1(0,7%) | 1 (0.3% | 0.02 |
| PCS* | 2±1.5 | 2±1 | 3.5±2.2 | 2.7±1.8 | 0.12 |
| Thickness of ceratinized mucosae (mm) | 1.9±1.5 | 1.4±1.5 | 1.2±1.5 | 1.4±1.5 | 0.15 |
| Note: *presence of; **considering protocol type prosthese | |||||
Table 3: Characteristics of peri-implant regions.
Figure 2: Clinical and radiographic relationship between prostheses biofilm accumulation and PID diagnosis. Note that the higher biofilm prostheses accumulation was observed in mucositis sites but not in peri-implantitis sites.
In order to clarify the risks associated with implant loss, the clinical aspects of participants with peri-implantitis culminating in total implant failure (n=7) and participants with peri-implant health (n=23) were compared without any history of implant loss. It can be observed that smoking are high risk factors for implant loss (p<0.05) (Table 4) [12].
Individuals with diabetes are about 8 times more likely to lose the implant than non-diabetics (7.8; 0.97-63.31). There is still 9 times more chance of losing the implant up to 4 years after the function than above this period, representing the period of greatest risk for bone contact loss: Implant clinicallyradiographically (Table 4).
| Characteristics | Lost Implants (n=7) | Health (n=23) | p-value (OR; IC) |
|---|---|---|---|
| Diabetes | 3 (42%) | 2 (1%) | 0.06 (7.8; 0.97-63.31) |
| Smokers | 4 (57%) | 0 | 0.001 |
| Periodontitis History | 4 (57%) | 8 (34%) | 0.26 |
| Medicines | 5 (71%) | 17 (73%) | 0.66 |
| Prosthesis Type | |||
| Unitary | 2 (28%) | 8 (34%) | 0.39 |
| Protocol | 5 (71%) | 11 (47%) | |
| Overdenture | 0 | 4 (17%) | |
| Prosthesis characteristics | |||
| Biofilm* | 4 (57%) | 14 (60%) | 0.59 |
| Implant Region | |||
| Maxilla | 3 (42%) | 6 (26%) | 0.34 |
| Mandible | 4 (57%) | 17 (73%) | |
| Protheses function time (years) | 3 ± 2.66 | 5.25 ± 4 | 0.22 |
| <4 anos | 6 (85.7%) | 9 (39.2%) | 0.04 (9.33; 0.95-90.94) |
| ≥ 4 anos | 1 (14.3%) | 14 (60.8%) | |
| Previous support therapy | 6 (85%) | 12(52%) | 0.12 |
Table 4: Clinical differences associated with lost implants.
Discussion
A dental implant is a biomaterial used to replace missing teeth with a survival rate of around 98%. However, along with the emergence of implants, peri-implant diseases manifested, where mucositis affects around 43% and peri-implantitis 22% of implants in function [13]. This prevalence is closely related to numerous external and internal factors that characterize periimplant disease as a multifactorial disease. Among the factors already reported in the literature, smoking and the use of specific medications have been associated with the disease, while other factors, such as heart disease, alcohol use and prosthesisrelated aspects, are still under discussion or have not yet been addressed by the disease in the literature. Therefore, this research aimed to evaluate the factors that can clinically influence the development of peri-implant disease, considering the general aspects and habits of the elderly patient and the characteristics of the peri-implant tissue. Our main results showed that (1) Age, gender, drug use, alcohol consumption and history of periodontitis were not correlated with the presence of peri-implant disease; (2) Smoking is associated not only with the presence of peri-implantitis, but also with implant loss; (3) Biofilm accumulation in the implant-supported prosthesis increased the risk of having peri-implant mucositis; (4) The keratinized mucosa band and the peri-implant biotype showed no correlation with the disease.
Patients who receive implants and have a history of periodontitis show a destructive inflammatory response in the periodontal tissue, and in the presence of microbiota change, an exacerbated inflammatory response may be triggered leading to the development of DPI and, consequently, implant loss [14]. Several authors have concluded that patients with a history of periodontitis exhibited, over the long term, significantly greater probing depth, longer marginal bone loss, and a higher incidence of peri-implantitis compared with periodontally healthy patients [15,16]. In 2019, Meyle et al. reaffirmed that there is a statistically significantly greater risk of peri-implantitis and implant loss in patients with a history of treated periodontitis compared with patients without periodontitis. However, our results showed no correlation between history of periodontitis and the presence of DPI, emphasizing the hypothesis that the history of periodontitis is not a determinant for the development of peri-implant disease, but one of the factors triggered is that it will not always be leading cause of DPI.
In this study, both in the peri-implant disease group and in the peri-implant health group, most participants used some type of medication to control systemic diseases, such as osteoporosis, diabetes or depression. However, there was no statistically significant difference between the groups regarding this risk factor. However, Kumar et al. [17] stated that some medications for osteoporosis, depression or anti-inflammatory drugs alter bone metabolism, reducing bone formation or increasing bone resorption around implants.
Alcohol consumption above 5 units per day has recently been associated with a 2.3-fold increase in the incidence of DPI. However, according to the authors, studies are still needed.
There is widespread difficulty in knowing the exact influence of alcohol on the development of the disease, not only because the habit of drinking alcohol is still very much associated with smoking, but also in how to accurately quantify daily alcohol consumption by traditional means such as questionnaires. Our results did not find an association between alcohol consumption and the presence of DPI. We suggest further research as a way of detailing the isolated influence of alcohol consumption on periimplant tissue, in order to create more accurate guidance and prognosis protocols.
Among the risk factors evaluated in the present study, smoking prevailed among participants in the peri-implant disease group when compared to the health group, but was highly associated with peri-implantitis and loss of the endosseous implant, which characterizes the final stage of peri-implantitis and failure in rehabilitation. These findings corroborate studies described in the literature that state that smokers have a predisposition to inflammation of peri-implant tissues more clearly than nonsmokers, independent of the patient's age.
Although some researchers have found a correlation between smoking and a higher prevalence of peri-implant disease, controversial data on the negative influence of smoking on the prevalence of peri-implantitis have also been reported recently [18].
In a study, Casado et al. evidenced the influence of smoking on the healing process around the implants and observed that smoking can interrupt the healing process of peri-implant tissues. The literature reviewed showed smoking as a real risk factor for susceptibility to peri-implantitis. Therefore, patients in this category should be warned of the increased risk of implant failure, particularly when this habit is associated with a history of prior periodontal disease. However, it is still necessary to explore numerous issues associated with the smoking habit. How many daily cigarettes influence osseointegration? Does being a former smoker increase the risk of DPI? Is the smoking habit potentiated by other local and systemic factors?
In the present study, biofilm was a potential risk factor for periimplant disease. Of 28 sick participants, 24 had biofilm accumulation in implant prostheses, either single, protocol or overdenture prostheses. The presence of periodontopathic bacteria has been proposed as a risk indicator for peri-implant mucositis. The microenvironment around implants may favor colonization of anaerobic gram-negative bacteria. An interaction within the biofilm can also contribute to the aggregation of new microorganisms causing the destruction of peri-implant tissues. The microbiota associated with peri-implant disease can be described as a polymicrobial anaerobic association. This shows that the etiology of peri-implant mucositis is directly related to biofilm accumulation. However, peri-implantitis had no association with biofilm prostheses accumulation, which can demonstrate the presence of different pathogens tigger the disease that need to be investigate.
From a mechanical point of view, PID, as a chronic condition, is influenced not only by local and systemic factors, but also by occlusal overload that directly interferes with long-term osseointegrated bone remodeling [19]. Corroborating the findings of Nobre et al., our study showed that there was a 9 times greater chance of losing the implant within 4 years after the function than above this period. This finding may be based on the fact that many implants with a longer installation time than this period may have undergone prior peri-implant evaluation or peri-implant tissue adaptation after this period do not find the same regenerative conditions in response to mechanical factors. However, future studies are needed to clarify such correlation. However, there is sufficient evidence to explore in the dental clinic and change clinical protocols for further supportive therapy within the first 4 years of function to minimize the possible effects associated with implant loss.
Numerous studies have associated the presence of diabetes mellitus with the development of DPI. This is because diabetes is a disease capable of interfering with the healing response, hindering the tissue remodeling necessary for the maintenance of peri-implant health [20]. Therefore, there is a consensus in the literature to report survival rates of smaller implants for patients with diabetes. On the other hand, it has also been shown that well-controlled diabetes is not a contraindication to implant treatment. Thus, for diabetics, it seems indisputable that patient knowledge and glycemic level control should be considered when implant treatment is required. In our study, there wasn't an association of this disease with implant loss. Elderly participants with diabetes were found in both the health and DPI groups, also showing that there is an association with implant loss, but not necessarily with the lack of peri-implant health. Many diabetic patients underwent endosseous implant rehabilitation treatment in this study remained in peri-implant health condition, corroborating the recent study by Kumar [18], where it was stated that well-controlled diabetic patients demonstrated a similar outcome when compared to non-diabetic patients in unitary implants.
Although current evidence does not allow a definitive conclusion that diabetic patients have a higher prevalence of peri-implantitis, it is known that the presence of diabetes may be considered a potential risk factor for the development of the disease. In order to clarify our results, it would be important to accurately measure blood glucose at the time of clinical onset of DPI, which often would have a diagnostic and scientific clinical unfeasibility. In addition, futures studies should consider the characterization of biofilm accumulation around prostheses in order to clarify the association of specific microorganism with peri-implant disease development.
Conclusion
Given the above, it was concluded that in the elderly population studied, smoking is a potential risk factors for the development and severity of the peri-implant disease. Smoking patients were more likely to lose dental implants than healthy patients. Biofilm accumulation was directly related to peri-implant mucositis but not peri-implantitis.Acknowledgment
The authors are gratefully acknowledging the assistance of the Course of Pos-graduation in Implant Dentistry at the school of dentistry, Universidade Federal Fluminense, Niteroi.
Author Contributions
All authors contributed to the study's conception and design. The physical examination of the implant's oral cavity and data collection were performed by (RG, MPFP, JPC, TA and PA). Data analysis and interpretation of data were performed by (ANM, PLC and VQ). The first draft of the manuscript was written by (RG, TA and VQ). They were revising it critically for important intellectual content (EOV, ANM and PLC). All authors read and approved the final manuscript.
Funding
None.Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.Ethical Approval and Informed Consent Statements
This study was conducted in accordance with the Declaration of Helsinki (WMA) and its ethical principle and was approved by the Research Ethics Committee of the Hospital Universitário AntônioPedro/Universidade Federal Fluminense by number 2.455.991. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all the patients.
Consent for Publication
Not applicable.Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- Lindhe J, Meyle J. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35:282-285.
[Crossref] [Google Scholar] [PubMed]
- Giovannoli JL, Roccuzzo M, Albouy JP, Duffau F, Lin GH, Serino G. Local risk indicators-Consensus report of working group 2. Int Dent J. 2019;69:7-11.
[Crossref] [Google Scholar] [PubMed]
- Meyle J, Casado P, Fourmousis I, Kumar P, Quirynen M, Salvi GE. General genetic and acquired risk factors, and prevalence of peri-implant diseases–Consensus report of working group 1. Int Dent J. 2019;69:3-6.
[Crossref] [Google Scholar] [PubMed]
- Schwarz F, Alcoforado G, Guerrero A, Jonsson D, Klinge B, Lang N, et al. Peri-implantitis: Summary and consensus statements of group 3. The 6th EAO Consensus Conference 2021. Clin Oral Implant Res. 2021;32:245-253.
[Google Scholar] [PubMed]
- Albrektsson T, Canullo L, Cochran D, de Bruyn H. Peri-implantitis: A complication of a foreign body or a man-made disease. Facts and fiction. Clin Implant Dent Relat Res. 2016;18(4):840-849.
[Crossref] [Google Scholar] [PubMed]
- Casado PL, Aguiar T, Pinheiro MP, Machado A, da Rosa Pinheiro A. Smoking as a risk factor for the development of periimplant diseases. Implant Dent. 2019;28(2):120-124.
[Crossref] [Google Scholar] [PubMed]
- Renvert S, Hirooka H, Polyzois I, Kelekis-Cholakis A, Wang HL. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants–Consensus report of working group 3. Int Dent J. 2019;69:12-17.
[Crossref] [Google Scholar] [PubMed]
- Khoury F, Keeve PL, Ramanauskaite A, Schwarz F, Koo KT, Sculean A, Romanos G. Surgical treatment of peri-implantitis-Consensus report of working group 4. Int Dent J. 2019;69:18-22.
[Crossref] [Google Scholar] [PubMed]
- Polymeri A, Loos BG, Aronovich S, Steigmann L, Inglehart MR. Risk factors, diagnosis, and treatment ofperi-implantitis: A cross-cultural comparison of US and European periodontists’ considerations. J Periodontol. 2022;93:481-492.
[Crossref]
- Rokaya D, Srimaneepong V, Wisitrasameewon W, Humagain M, Thunyakitpisal P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur J Dent. 2020;14(4):672-682.
[Crossref] [Google Scholar] [PubMed]
- Vohra F, Alkhudhairy F, Al-Kheraif AA, Akram Z, Javed F. Peri-implant parameters and C-reactive protein levels among patients with different obesity levels. Clin Implant Dent Relat Res. 2018;20(2):130-136.
[Crossref] [Google Scholar] [PubMed]
- Ladeira Casado P, Villas-Boas R, de Mello W, Leite Duarte ME, Mauro Granjeiro J. Peri-implant disease and chronic periodontitis: is interleukin-6 gene promoter polymorphism the common risk factor in a Brazilian population?. Int J Oral Maxillofac Implants. 2013;28(1):35-43.
[Crossref] [Google Scholar] [PubMed]
- Lucarini G, Zizzi A, Rubini C, Ciolino F, Aspriello SD. VEGF, microvessel density, and CD44 as inflammation markers in peri-implant healthy mucosa, peri-implant mucositis, and peri-implantitis: impact of age, smoking, PPD and obesity. Inflammation. 2019;42:682-689.
[Crossref] [Google Scholar] [PubMed]
- Nobre MA, Salvado F, Nogueira P, Rocha E, Ilg P, Malo P. A prognostic model for the outcome of nobel biocare dental implants with peri-implant disease after one year. J Clin Med. 2019;8:1352.
[Crossref] [Google Scholar] [PubMed]
- Roos J, Sennerby L, Lekholm UL, Jemt T, Gröndahl K, Albrektsson T. A qualitative and quantitative method for evaluating implant success: a 5-year retrospective analysis of the Brånemark implant. Int J Oral Maxillofac Implants. 1997;12(4):504-514.
[Google Scholar] [PubMed]
- Altay MA, Tozoglu S, Yildirimyan N, Ozarslan MM. Is History of Periodontitis a Risk Factor for Peri-implant Disease? A Pilot Study. Int J Oral Maxillofac Implants. 2018;33(1):152–160.
[Crossref] [Google Scholar] [PubMed]
- Roccuzzo M, Bonino L, Dalmasso P, Aglietta M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res. 2014;25(10):1105-1112.
[Crossref] [Google Scholar] [PubMed]
- Kumar PS. Systemic risk factors for the development of periimplant diseases. Implant Dent. 2019;28(2):115-119.
[Crossref] [Google Scholar] [PubMed]
- Mombelli A, Decaillet F. The characteristics of biofilms in peri-implant disease. J ClinPeriodontol. 2011;38:203-213.
[Crossref] [Google Scholar] [PubMed]
- Rinke S, Ohl S, Ziebolz D, Lange K, Eickholz P. Prevalence of periimplant disease in partially edentulous patients: a practice-based cross-sectional study. Clin Oral Implants Res. 2011;22(8):826-833.
[Crossref] [Google Scholar] [PubMed]
Citation: Goncalves R, Quinelato V, Pinheiro MPF, Castro JP, Arriaga P, Vieira EO, et al. (2025) The Main Risk Factors for Implant Failure in Elderly Patients: A Clinical Trial Study. J Dentistry. 15:727.
Copyright: © 2025 Goncalves R, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.