Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 0, Issue 0
The Latest Evidence of Risks and Management of Venous Thromboembolism in Pregnancy: A Comprehensive Review
Salvatore Felis*, Beatrice Marchese and Irene GaviniReceived: 19-Jul-2023, Manuscript No. JBDT-23-22288; Editor assigned: 21-Jul-2023, Pre QC No. JBDT-23-22288 (PQ); Reviewed: 11-Aug-2023, QC No. JBDT-23-22288; Revised: 18-Aug-2023, Manuscript No. JBDT-23-22288 (R); Published: 25-Aug-2023, DOI: 10.4172/2155-9864.23.S2.006
Abstract
Venous Thromboembolism (VTE) is a major cause of maternal morbidity and mortality during pregnancy or early after delivery and it remains a diagnostic and therapeutic challenge. The latest Confidential Enquiry into Maternal Deaths showed that VTE is now the third leading cause of direct maternal mortality, beside sepsis and hypertension. In particular the prevalence of VTE has been estimated to be 1 per 1000-2000 pregnancies. The risk of VTE is five times higher in a pregnant woman than in non-pregnant woman of similar age and postpartum VTE is more common than antepartum VTE. This review aimed to provide an update of whole current literature on VTE in pregnancy highlighting the most recent findings in diagnostic and therapeutic strategies, considering in detail risks and benefits of various techniques and drug classes, for both mother and fetus. Large trials of anticoagulants administration in pregnancy are lacking and recommendations are mainly based on case series and on expert opinions. Nonetheless, anticoagulants are believed to improve the outcome of pregnancy for women with current or previous VTE.
Keywords
Venous thromboembolism; Pregnancy; Blood; Pulmonary embolism
Introduction
Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) are the two components of a single disease called Venous Thromboembolism (VTE). Approximately 30% of apparently isolated cases of PE are associated with DVT, and in patients with symptoms of DVT, the frequency of silent pulmonary embolism ranges from 40 to 50% [1,2]. VTE during pregnancy remains the leading medical cause of maternal mortality in the developed world, with an incidence of 0.85 per 100,000 pregnancies, as reported in the latest UK report MBRRACE-UK-Saving Lives, Improving Mothers' Care 2017 [3].
Literature Review
Pathogenesis
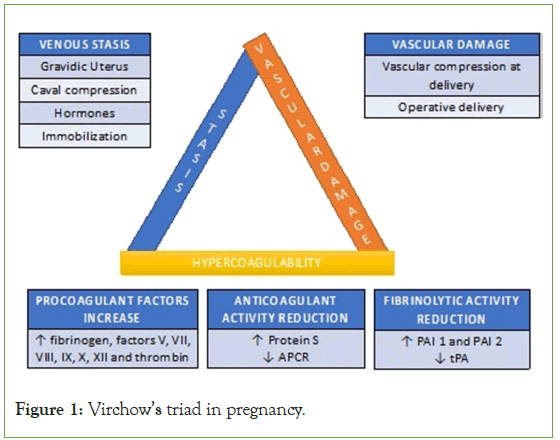
VTE complicates 1 in 1000 pregnancies and has a frequency 10 times higher than in the non-pregnant population [4]. This is due to the hypercoagulable state characteristic of pregnancy, which begins at conception and persists for over 8 weeks after delivery [5,6]. The Virchow's triad, characterized by hypercoagulability, vascular damage, and venous stasis, is amplified during pregnancy, resulting in a relative risk of 4.3 (95% confidence interval [CI], 3.5 to 5.2) for VTE in pregnant or postpartum women compared to non-pregnant women (Figure 1) [3].
Figure 1: Virchow’s triad in pregnancy.
Venous stasis
As pregnancy progresses, there is an expansion of blood volume and an increase in maternal cardiac output. At term, uterine blood flow increases from 700 mL/min to 900 mL/min, which represents 10%-12% of the maternal cardiac output. The gravid uterus compresses the inferior vena cava and other structures (e.g., ureter). This caval compression leads to venous stasis distal to the compression in the pelvis and lower limbs.
Hypercoagulability
Pregnancy is associated with rapid turnover of platelets, coagulation, and fibrinolysis. Platelet count remains unchanged or seemingly reduced due to hemodilution. There is an increase in the concentrations of fibrinogen, factors V, VII, VIII, IX, X, XII and thrombin. In addition to these, additional factors may coexist, exposing the pregnant woman to a higher risk of thromboembolism, such as Factor V Leiden mutation (which causes increased resistance to Protein C), the G20210A mutation of the prothrombin gene, protein S and C deficiencies, and antithrombin III deficiency. Pregnancy can be defined, from a coagulative perspective, as a state of accelerated but compensated intravascular coagulation.
Vascular damage
During placental separation, microvascular injuries occur, which can trigger a series of events responsible for the acceleration of coagulation activity. This prothrombotic activity accounts for the increased incidence of thromboembolic events in the postpartum period. Cesarean delivery carries an eight-fold increased risk of thromboembolic events compared to vaginal delivery.
Furthermore, the presence of inherited thrombophilia and antiphospholipid syndrome, as well as a history of previous thrombosis, increase the risk of VTE during pregnancy and the postpartum period [7].
Risk factors
The risk of VTE increases by 2-6 times during pregnancy and approximately 20 times in the postpartum period, persisting for at least 12 weeks afterward, although most thromboembolic events occur within 3 weeks after delivery. This risk is increased in women who are:
• Over 35 years old,
• Obese,
• Have thrombophilic abnormalities,
• Have a history of previous VTE,
• Undergo cesarean section (especially emergency cesarean section during labor).
Most thromboembolic events occur during pregnancy, with an equal distribution across the three trimesters.
DVTs account for 75%-80% of prenatal thromboembolism [7-9]. Unlike non-pregnant women, DVTs during pregnancy involve the iliofemoral veins, with the main site of thrombus localization being the popliteal-femoral region.
Approximately 90% of DVTs during pregnancy affect the left lower limb, compared to 55% in non-pregnant women. This difference reflects the course of the iliac artery, which crosses the internal iliac vein on the left side and explains why, during pregnancy, most DVTs are iliofemoral rather than femoropopliteal (72% vs. 9%) [10].
For the assessment of thromboembolic risk during pregnancy, a thorough personal and family medical history is of fundamental importance, aimed at identifying any previous thromboembolic events in the patient or her first-degree relatives [11]. This helps determine the need for pharmacological antithrombotic prevention measures and the opportunity for further diagnostic investigation.
For the evaluation of thromboembolic risk during pregnancy. See (Appendix Table 1).
| Contraindications/Precuations for the use of LMWH |
|---|
| Known bleeding disorder (e.g., hemophilia, Von Willebrand disease or acquired coagulopathy) |
| Active prenatal or postpartum bleeding |
| Women with a high risk of bleeding (e.g., placenta previa) |
| Severe thrombocytopenia (platelet count<75 x 109 / L) |
| Acute stroke within the past 4 weeks (hemorrhagic or ischemic) |
| Severe renal insufficiency (glomerula filtration rate<30 mL / minute ) |
| Severe liver insufficiency (prothrombin time above the normal range or known varices) |
| Uncontrolled hypertension (systolic blood pressure>200 mmHg or diastolic blood pressure>120 mmHg diastolica) |
Table 1: Contraindications for the use of EBPM.
Discussion
Diagnosis of acute VTE during pregnancy
The clinical diagnosis of VTE, which is already challenging in the general population, is even more complicated in pregnant women due to the physiological changes that occur during pregnancy, which can mimic signs and symptoms of VTE itself (Figure 2).
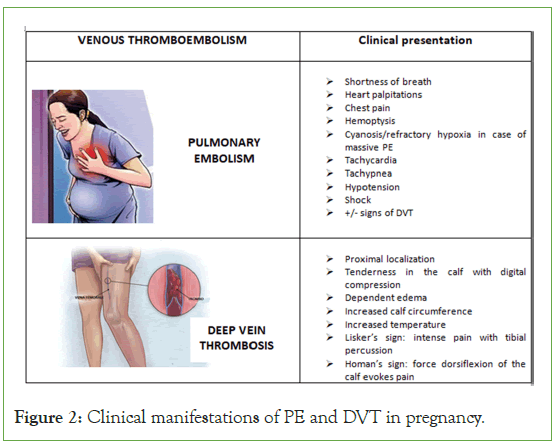
Figure 2: Clinical manifestations of PE and DVT in pregnancy.
Diagnostic clinical tests used in the general population, such as the Wells score, cannot be applied during pregnancy due to their low positive predictive value [12]. In recent years, a specific scoring system for pregnancy, the LEFTt test, has been proposed as a predictive test for DVT. This test combines three variables:
• Symptoms reported in the left lower limb (L)
• Difference ≥ 2 cm in calf circumference (E)
• Onset of symptoms in the first trimester (Ft)
Further future studies are needed to prospectively validate this test and define its role in a diagnostic algorithm for DVT during pregnancy [13].
Diagnostic investigations, particularly for pulmonary embolism, involve radiation exposure to both the mother and fetus, raising concerns, mostly unfounded, about the effects of radiation on the fetus, resulting in delayed confirmation of a diagnosis.
Diagnosis of deep vein thrombosis
In case of signs and symptoms suggestive of DVT:
• Initiate therapy with (LMWH) Low-Molecular-Weight Heparin,
• Make lower limb Doppler ultrasound [14].
If the Doppler ultrasound is negative and signs and symptoms are not highly suggestive of DVT:
• Therapy can be discontinued.
If the Doppler ultrasound is negative but signs and symptoms are highly suggestive of DVT:
• Repeat Doppler ultrasound after one week,
• Continue therapy with LMWH until a negative Doppler ultrasound is obtained.
In cases of suspected iliac vein thrombosis, with pain in the entire limb and abdominal region, magnetic resonance venography should be performed.
Diagnosis of pulmonary embolism
In cases of suspected PE:
• Complete blood count, electrolytes, liver and kidney function tests.
• D-Dimer: Outside of pregnancy, D-Dimer testing is used in the diagnosis of VTE, with high sensitivity, moderate specificity, and a high negative predictive value. During pregnancy, D-Dimer levels progressively increase, with elevated values at term and in the postpartum period in healthy women [15]. D-Dimer levels are significantly increased in multiple pregnancies, following cesarean section, in major bleeding, and in conjunction with pre-eclampsia [16,17]. In pregnancy, a positive D-Dimer does not necessarily confirm the diagnosis of VTE; its role remains controversial [18]. The guidelines of the European Society of Cardiology recommend D-Dimer testing, stating that normal levels exclude PE in pregnancy as in other patients, although it is unlikely to find levels within the normal range during pregnancy. In contrast, the guidelines of the American Society of Thoracic Radiology recommend not using D-Dimer testing for the diagnosis of VTE in pregnancy [19].
• Electrocardiogram (ECG): The most common abnormalities observed are inverted T waves, the classic S1Q3T3 pattern, and right bundle branch block (18% during pregnancy and 4.2% in the postpartum period) [20].
• Arterial Blood Gas Analysis (ABG): Only 10% of cases show PO2 values below 60 mmHg, and only 2.9% have saturation below 90% [21]. These results indicate a diagnostic role for ECG in women with suspected acute PE, while blood gas analysis has limited diagnostic value.
• Chest X-ray (differential diagnosis with pneumonia, pneumothorax, Acute respiratory distress syndrome (ARDS)): while the chest X-ray is normal in over half of pregnant women with confirmed PE, abnormal features caused by PE include atelectasis, effusion, focal opacities, and pulmonary edema [22]. The radiation dose from a chest X-ray to which the fetus is exposed at any stage of pregnancy is negligible (less than 0.01 mSv) [23].
• Lower limb Doppler ultrasound: necessary in cases of suspected PE and if the pregnant woman has signs and symptoms suggestive of DVT [24,25]. Given the higher incidence of isolated iliac vein thrombosis in pregnancy, an increased probability of false negatives has been observed using Doppler methodology [26]. Therefore, while a positive result is valuable, in the case of a negative result, Doppler ultrasound does not help exclude PE. If the Doppler ultrasound is:
• Positive: No further investigations are necessary as the anticoagulant therapy is the same.
• Negative but with strong clinical suspicion: Perform ventilation-perfusion (V/Q) scan or Computed Tomography Pulmonary Angiography (CTPA).
• V/Q scan versus CTPA: The choice between these two diagnostic tests depends on local availability and the diagnostic protocol approved by individual centers. CTPA has potential advantages over V/Q scan, such as:
1. More readily available
2. Higher sensitivity and specificity
3. Lower fetal radiation exposure
With both techniques, the doses used are well below accepted thresholds for teratogenicity, fetal death, and fetal growth retardation [27,28]. The main concern for the fetus is a minimal increased risk of childhood cancer.
While CTPA is associated with a low risk of radiation to the fetus, the maternal breast tissue receives a relatively high radiation dose (up to 20 mGy), resulting in an increased risk of breast cancer. The estimated radiation dose for CTPA is 20 to 100 times higher than for V/Q scan [29].
A radiation dose of 10 mGy to the breast of a pregnant woman increases the risk of developing breast cancer by 13.6%.
Treatment of acute VTE
In the presence of signs and symptoms suggestive of DVT or PE, it is mandatory to initiate therapy with LMWH until the diagnosis is confirmed or unless treatment is strongly contraindicated (Table 1) [30].
If left DVT is untreated, 15%-25% of patients may develop PE. PE during pregnancy can be fatal in 15% of patients, and in 66% of these cases, death occurs within 30 minutes of the thromboembolic event [31,32].
LMWH therapy
Advantages of LMWH compared to (UFH) Unfractionated Heparin:
• Greater efficacy.
• Lower risk of bleeding [33].
• Lower mortality.
• Lower incidence of heparin-induced thrombocytopenia and osteoporosis [34,35].
• Half-life of 4-6 hours.
The therapeutic dose of LMWH should be titrated according to the woman's pre-pregnancy weight (Table 2). see (Appendix Table 2) (Figures 3-5).
| EBPM | Daily dosage | |
|---|---|---|
| Prophylactic dosage | Therapeutic dosage | |
| Enoxaparin | 4000 U/die | 100 U/Kg every 12 h |
| Nadroparin | 2859 U/die up to 70 Kg 3800 U/Kg>70 Kg | 180 U/Kg every 24 h |
| Dalteparin | 5000 U/24 h | 200 U/Kg every 24 h |
Table 2: Therapeutic dose of Enoxaparin, Nadroparin, and Dalteparin Italian Official Gazette (G.U.) August 6, 2016: AIFA Determination regarding the prescription of LMWH in pregnancy and bridging therapy.
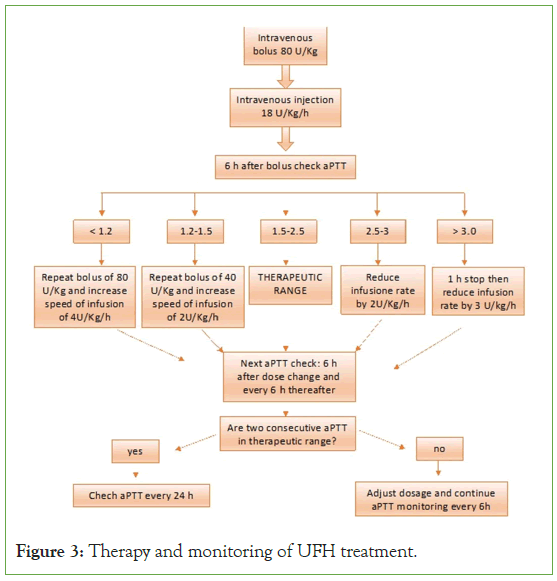
Figure 3: Therapy and monitoring of UFH treatment.
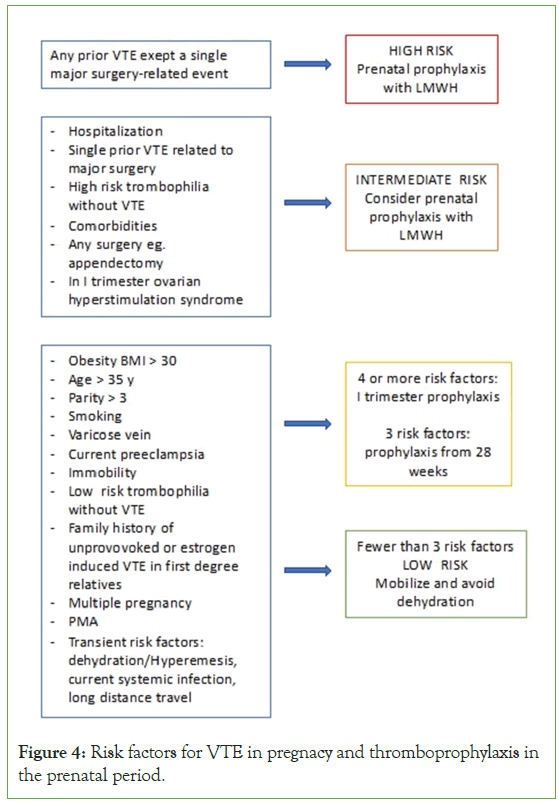
Figure 4: Risk factors for VTE in pregnacy and thromboprophylaxis in the prenatal period.
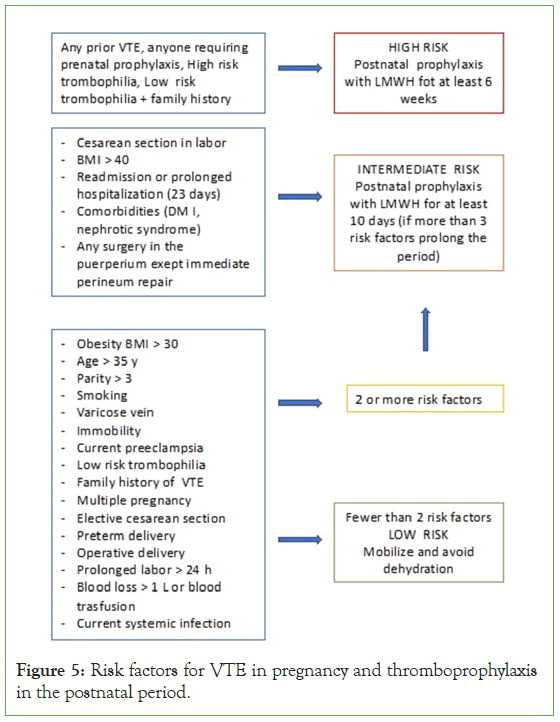
Figure 5: Risk factors for VTE in pregnancy and thromboprophylaxis in the postnatal period.
During pregnancy, changes in volume distribution and glomerular filtration can affect the pharmacokinetics of LMWHs. If creatinine clearance is below 30 mL/minute, lower doses of LMWH should be administered.
There are no recommendations for monitoring anti-Xa activity during LMWH treatment, except in women with body weight <50 kg or >90 kg or with other concurrent factors (e.g., renal insufficiency or recurrent VTE);39 and platelet count monitoring is not necessary [36-39].
If a pregnant woman is considered at high risk of bleeding, anticoagulant therapy should be managed with intravenous UFH until the bleeding risk is resolved [40,41].
UFH has a shorter half-life compared to LMWHs, and its activity can be antagonized by protamine sulfate or chloride. If a woman develops a bleeding issue during LMWH therapy, treatment should be discontinued, and hematological consultation should be sought.
UFH therapy
Massive PE is a medical emergency that can present with shock, refractory hypoxemia, and/or right ventricular dysfunction. If cardiac arrest occurs, maternal resuscitation should begin following the principles of (CAB) (Compressions Airway Breathing) and manual left uterine displacement should be performed. Perimortem cesarean section should be performed within 5 minutes if resuscitation is unsuccessful, and the pregnancy is beyond 20 weeks.
Intravenous UFH is the initial treatment in massive PE due to its rapid onset of action and because it can be more easily titrated if thrombolytic therapy is administered [42].
How to administer UFH:
• Loading dose of 80 IU/kg followed by;
• Continuous intravenous infusion of 18 IU/kg/h
• If the patient has undergone thrombolysis, the loading dose can be omitted, and the continuous intravenous infusion of 18 IU/ kg/h is continued [43,44].
Monitoring of a Partial Thromboplastin Time (PTT) during UFH treatment is technically problematic, especially in late pregnancy when there is apparent heparin resistance due to increased levels of fibrinogen and factor VIII, which influence aPTT. This can result in excessively high levels of UFH and subsequent bleeding issues. Therefore, the involvement of a hematologist in managing pregnant women receiving UFH treatment is desirable. It may be helpful to determine the anti-Xa level as a measure of heparin dose [45,46].
In the case of potentially life-threatening massive PE with hemodynamic compromise (or ischemic complications of an extremity due to extensive iliofemoral venous thrombosis), thrombolytic therapy should be considered as anticoagulation alone will not reduce obstruction in the circulation. A meta- analysis has shown that the use of thrombolytic agents for PE is more effective than heparin therapy in reducing clot burden and improving hemodynamics [47]. These studies, however, did not demonstrate any impact on long-term survival compared to conventional therapy with UFH or LMWH, and the thrombolytic agent did not prove to be superior to any other [48]. However, when Wan et al., limited their analysis to trials involving massive PE, they identified a significant reduction in recurrent PE or death from 19.0% with heparin alone to 9.4% with thrombolysis (OR 0.45, 95% CI 0.22-0.90) [48].
Protamine sulfate or chloride reverses the anti-IIa fraction of LMWH, but it does not completely reverse the anti-Xa effect; therefore, protamine cannot be used as an antagonist for LMWH.
Protamine dosage:
1 mg of protamine chloride or sulfate per 100 IU of LMWH, administered as a slow intravenous infusion, up to a maximum of 50 mg in 10 minutes [49].
Therapeutic scheme:
• Intravenous UFH administered for a few minutes: 1-1.5 mg of protamine chloride per 100 IU of heparin.
• Intravenous UFH administered for 30-60 minutes: 0.50 mg-0.75 mg of protamine chloride per 100 IU of heparin.
• Intravenous UFH administered for 2 hours: 0.25 mg-0.37 mg of protamine chloride per 100 IU of heparin.
• Continuous intravenous UFH infusion: 25 mg-50 mg of protamine chloride immediately after discontinuation of the infusion, with the remaining dose calculated for continuous infusion over 8-16 hours.
Role of elastic compression
In the initial treatment of DVT, the affected leg should be elevated, and graduated compression elastic stockings should be applied to reduce edema [50]. Randomized studies have shown that early ambulation with leg compression does not increase the risk of PE, does not cause embolus migration, and improves pain and edema more quickly compared to patients with limited mobility.
Thigh-high elastic compression stockings appear to prevent post- phlebitic syndrome to a greater extent than compression exerted below the knee [51,52].
Indications for the use of graduated compression elastic stockings are:
a) Hospitalized pregnant women in whom LMWH therapy is contraindicated.
b) Post-cesarean section hospitalized women (also receiving LMWH prophylaxis) at particularly high risk of VTE (previous VTE, presence of more than three risk factors for VTE).
c) Pregnant women with a history of VTE (currently undergoing LMWH treatment).
d) Pregnant women undertaking journeys lasting more than 4 hours.
Role of caval filters
Consideration should be given to the use of a temporary inferior vena cava filter in the peripartum period for patients with iliac vein thrombosis to reduce the risk of PE or in patients with diagnosed DVT who have recurrent PE despite adequate anticoagulant therapy.
The long-term safety of inferior vena cava filters is uncertain; the main complications associated with inferior vena cava filters are migration, increased risk of caval and lower extremity DVT, and rarely, infection.
Maintenance of anticoagulant therapy during pregnancy
LMWHs are the preferred drugs for the treatment of VTE in pregnancy. Anticoagulant therapy should be continued for at least six weeks after delivery, for a minimum total treatment duration of three months [53].
The rationale for recommending therapeutic doses of LMWH rather than prophylactic doses for the whole duration of pregnancy is based on the continuous risk of recurrent VTE during the gestational period, which is influenced by:
• Changes in the hemocoagulative profile,
• Reduced venous flow velocity,
• A higher incidence of isolated iliac vein DVT,
• At least 50% of patients will have a thrombophilia.
Role of vitamin K antagonists
The use of coumarins in pregnancy is limited to a few situations where heparin is considered unsuitable, such as in women with older generation mechanical heart valves who have an increased risk of thrombosis even during anticoagulant therapy with UFH or LMWH [14].
Coumarins cross the placenta, leading to:
• Fetal anticoagulation: fetal bleeding
• Spontaneous abortions: fetal death
They also have teratogenic effects:
• Risk of 5%
• Dose-related risk: risk reduced if<5 mg/day
• Between the 6th and 12th week, they can cause the appearance of the so-called "warfarin embryopathy," characterized by nasal hypoplasia, optic atrophy, varying degrees of mental retardation, microcephaly, microphthalmia.
The risk of embryopathy is minimized if coumarins are replaced with LMWH between the 6th and 12th week of gestation.
Coumarin therapy appears to be reasonably safe in the second and third trimesters of pregnancy. It is advisable to switch to the more manageable heparin therapy near delivery (4 weeks before the estimated due date) to reduce the risk of maternal or neonatal bleeding complications.
The administration of oral anticoagulants is absolutely compatible with breastfeeding, considering the minimal passage of coumarins into breast milk.
Role of new oral anticoagulants
Fondaparinux (a selective factor Xa inhibitor) is recommended in pregnancy as an alternative to LMWH when heparin-induced thrombocytopenia occurs or in case of allergy to heparin [54]. Fondaparinux has a long half-life (18 hours), so there should be a gap of 36-42 hours from the previous dose administered before a neuraxial block can be performed. Fondaparinux treatment is not recommended during breastfeeding [55,57].
There are no indications for the use of oral vitamin K antagonists Non-Vitamin K antagonist Oral Anticoagulants (NOACs): rivaroxaban, apixaban, betrixaban and dabigatran) in pregnancy or postpartum as they are likely to cross the placenta and have potential direct fetal effects, so they should be avoided during the prenatal period.
Anticoagulant therapy during labor and delivery
When VTE occurs at term pregnancy, the use of intravenous UFH should be considered because it is more manageable [58].
Pregnant women receiving LMWH should discontinue its administration in case of labor or vaginal bleeding.
If elective cesarean section or labor induction is planned, LMWH therapy should be stopped 24 hours before.
In case of labor induction or potential neuraxial block, UFH therapy:
• Should be discontinued 12 hours before if administered subcutaneously,
• Should be discontinued 6 hours before if administered intravenously.
If women receiving therapeutic doses of UFH experience spontaneous labor, aPTT monitoring is required. If a significantly prolonged aPTT is observed near delivery, the administration of protamine sulfate may be necessary to reduce the risk of bleeding [59].
When VTE occurs at term, the risk of recurrent thrombosis may increase if anticoagulant therapy is interrupted to allow for planned labor induction or cesarean section. The risk of recurrent VTE is higher if the discontinuation occurred within 2 weeks of the initial thrombotic event. The use of intravenous UFH, which is more easily manageable and minimizes the duration without anticoagulant therapy due to its short half-life, may be considered [60].
However, as noted earlier, there are challenges in monitoring UFH in pregnancy using aPTT.
Safety of neuraxial blocks in obstetrics
The incidence of spinal hematoma in the obstetric population, with or without anticoagulant therapy, is unknown. Obstetric patients have a lower incidence of spinal hematoma compared to elderly patients.
The introduction of thromboprophylaxis and antiplatelet therapy in parturients raises concerns about the safety of neuraxial anesthesia [61,62]. On the other hand, excluding the use of neuraxial techniques in parturients receiving prophylactic or therapeutic antithrombotic treatment not only lacks rationale but is also undesirable considering the well-known benefits of central blocks in obstetrics.
When a pregnant woman is on therapeutic LMWH treatment, neuraxial techniques should not be performed for at least 24 hours from the last dose of LMWH [63].
Strict adherence to time intervals for LMWH administration should be observed:
▪ Time 0: Performance of neuraxial technique with placement of an epidural catheter.
▪ At 12 hours: Prophylactic dose of LMWH may be administered, followed by the next dose at 24 hours from the previous one.
▪ Removal of the epidural catheter should occur at least 10- 12 hours after the administration of a prophylactic dose of LMWH.
▪ After 6 hours from the removal of the epidural catheter, a dose of LMWH may be administered.
▪ Administration of LMWH twice daily or at therapeutic doses is incompatible with the placement and/or maintenance of an epidural catheter.
These recommendations are based on the observation that LMWH, in addition to its anticoagulant and antiplatelet activity, also has significant fibrinolytic activity, which contributes to its antithrombotic effect but can increase the risk of spinal bleeding (as it can lyse an already formed clot in an epidural arterial vessel that may have been damaged by puncture or an epidural catheter).
This fibrinolytic effect is related to the release of tissue Plasminogen Activator (t-PA) and becomes significant starting from the third day of LMWH administration [63]. In fact, it is known that 50% of hematomas occur 3 days after the initiation of LMWH administration, typically about 12 hours after the removal of the epidural catheter. This is considered a particularly critical time (likely more dangerous than the catheter insertion itself) because the patient has already received 2-3 doses of LMWH, and thus, the drug concentration approaches steady-state conditions.
Anticoagulant therapy in the postpartum period
Anticoagulant therapy should be continued for the duration of pregnancy and at least 6 weeks after delivery, with a total treatment duration of 3 months [64]. Both LMWH and coumarins can be used in the postpartum period.
Warfarin after delivery should be avoided for at least the first five days, and longer in women with an increased risk of bleeding.
Prevention of post-thrombotic syndrome
Post-thrombotic syndrome is characterized by leg edema, persistent chronic pain, cyanosis, telangiectasia, eczema, varicose veins, and, in severe cases, venous ulceration.
For the prevention of Post-Thrombotic Syndrome (PTS), the use of graduated compression elastic stockings with a pressure of 30 mmHg-40 mmHg is recommended for over 2 years after the acute event.
Conclusion
In conclusion, Venous Thromboembolism (VTE) is a serious condition that occurs when a blood clot forms in a vein, usually in the legs or lungs. It can cause pain, swelling, and even death if the clot breaks off and travels to the heart or brain. VTE is especially dangerous for pregnant women and their babies, as it is the third leading cause of direct maternal mortality. This means that VTE causes more deaths among pregnant women than any other cause except bleeding and infection. This review has provided valuable insights into the latest diagnostic and therapeutic strategies for VTE in pregnancy, highlighting the importance of considering both maternal and fetal outcomes.
References
- Turkstra F, Kuijer PM, van Beek EJ. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775-781.
[Crossref] [Google Scholar] [PubMed]
- Meignan M, Rosso J, Gauthier H. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch Intern Med. 2000;160:159-164.
[Crossref] [Google Scholar] [PubMed]
- MBRRACE-UK Saving Lives, Improving Mothers’ Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15 December 2017. 2018.
- Andersen BS, Steffensen FH, Sorensen HT. The cumulative incidence of venous thromboembolism during pregnancy and puerperium–an 11 year Danish population-based study of 63,300 pregnancies. Acta Obstet Gynecol Scand. 1998; 77: 170–173.
[Crossref] [Google Scholar] [PubMed]
- Anderson FA Jr, Wheeler HB, Goldberg RJ. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991; 151: 933–938.
[Crossref] [Google Scholar] [PubMed]
- White RH. The epidemiology of venous thromboembolism. Circulation. 2003; 107(23): I4–I8.
[Crossref] [Google Scholar] [PubMed]
- James AH, Jamison MG, Brancazio LR, Myers MR. Venous thromboembolism during pregnancy and the postpartum period: Incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311-1315.
[Crossref] [Google Scholar] [PubMed]
- Idem. Venous thromboembolic disease in obstetrics and gynaecology: The Scottish experience. Scott Med J. 1996;41:83-86.
[Crossref] [Google Scholar] [PubMed]
- Larsen TB, Sorensen HT, Gislum M, Johnsen SP. Maternal smoking, obesity, and risk of venous Thromboembolism during pregnancy and the puerperium: A population-based nested case-control study. Thromb Res. 2007;120:505-509.
[Crossref] [Google Scholar] [PubMed]
- Ginsberg JS, Brill-Edwards P, Burrows RF. Venous thrombosis during pregnancy: leg andtrimester of presentation. Thromb Haemost. 1992;67:519-520.
- McColl M, Ramsay JE, Tait RC. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost. 1997; 78: 1183–1188.
[Crossref] [Google Scholar] [PubMed]
- Bourjeily G, Paidas M, Khalil H. Pulmonary embolism in pregnancy. Lancet. 2010; 375: 500–512.
[Crossref] [Google Scholar] [PubMed]
- Rodger MA, Le Gal G, Wells P. Clinical decision rules and D-Dimer in venous thromboembolism: current controversies and future research priorities. Thromb Res. 2014; 134: 763–768.
[Crossref] [Google Scholar] [PubMed]
- RCOG Thrombosis and Embolism during Pregnancy and the Puerperium, the Acute Management of (Green-top Guideline No. 37b). 2015.
- Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Eng J Med. 2003;349:1227–1235.
[Crossref] [Google Scholar] [PubMed]
- Khalafallah AA, Morse M, Al-Barzan AM, Adams M, Dennis A, Bates G, et al. D-Dimer levels at different stages of pregnancy in Australian women: A single centre study using two different immunoturbidimetric assays. Thromb Res. 2012;130:171–177.
[Crossref] [Google Scholar] [PubMed]
- Yamada T, Kawaguchi S, Araki N, Takeda M, Nishida R, Yamada T, et al. Difference in the D-dimer rise between women with singleton and multifetal pregnancies. Thromb Res. 2013;131:493–496.
[Crossref] [Google Scholar] [PubMed]
- Francalanci I, Comeglio P, Liotta AA, Cellai AP, Fedi S, Parretti E, et al. D-Dimer in intra-uterine growth retardation and gestational hypertension. Thromb Res. 1995;80:89–92.
[Crossref] [Google Scholar] [PubMed]
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: The task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276–2315.
[Crossref] [Google Scholar] [PubMed]
- Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM, et al. ATS/STR Committee on pulmonary embolism in Pregnancy. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: Evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med. 2011;184:1200–1208.
[Crossref] [Google Scholar] [PubMed]
- Rodger MA, Carrier M, Jones GN, Rasuli P, Raymond F, Djunaedi H, et al. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am J Respir Crit Care Med 2000;162:2105–2108.
[Crossref] [Google Scholar] [PubMed]
- Blanco-Molina A, Rota LL, Di Micco P, Brenner B, Trujillo- Santos J, Ruiz-Gamietea A, et al. RIETE Investigators. Venous thromboembolism during pregnancy, postpartum or during contraceptive use. Thromb Haemost. 2010;103:306–311.
[Crossref] [Google Scholar] [PubMed]
- Fidler JL, Patz EF Jr, Ravin CE. Cardiopulmonary complications of pregnancy: Radiographic findings. AJR Am J Roentgenol. 1993;161:937–942.
[Crossref] [Google Scholar] [PubMed]
- Damilakis J, Perisinakis K, Prassopoulos P, Dimovasili E, Varveris H, Gourtsoyiannis N. Conceptus radiation dose and risk from chest screen-film radiography. Eur Radiol. 2003;13:406–412.
- Nguyen CP, Goodman LH. Fetal risk in diagnostic radiology. Eur Radiol. 2012;33:4–10.
[Crossref] [Google Scholar] [PubMed]
- James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol. 2005;193:216–219.
[Crossref] [Google Scholar] [PubMed]
- Bhargavan M, Sunshine JH, Hervey SL, Jha S, Vializ J, Owen JB. The actual role of CT and ventilation–perfusion scanning in workup for suspected pulmonary embolism: Evidence from hospitals. AJR Am J Roentgenol. 2009;193:1324–1332.
[Crossref] [Google Scholar] [PubMed]
- Vinayakamoorthy V, Geary S, Ganatra R. A United Kingdom based survey of clinical practice in the diagnosis of suspected pulmonary embolism. Nucl Med Commun. 2010;31:112–120.
[Crossref] [Google Scholar] [PubMed]
- Hurwitz LM, Reiman RE, Yoshizumi TT, Goodman PC, Toncheva G, Nguyen G, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology 2007;245:742–750.
[Crossref] [Google Scholar] [PubMed]
- Remy-Jardin M, Remy J. Spiral CT angiography of the pulmonary circulation. Radiology. 1999;212:615–636.
[Crossref] [Google Scholar] [PubMed]
- Rutherford SE, Phelan JP. Deep venous thrombosis and pulmonary embolism in pregnancy. Obstet Gynecol Clin North Am. 1991;18:345–370.
[Crossref] [Google Scholar] [PubMed]
- Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730–734.
[Crossref] [Google Scholar] [PubMed]
- Lennox C, Marr L. Reproductive Health Programme, Healthcare Improvement Scotland. Scottish Confidential Audit of Severe Maternal Morbidity. 9th Annual Report. Healthcare Improvement Scotland. 2013.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: A systematic review of safety and efficacy. Blood. 2005;106:401–407.
[Crossref] [Google Scholar] [PubMed]
- Dahlman TC. Osteoporotic fractures and the recurrence of thromboembolism during pregnancy and the puerperium in 184 women undergoing thromboprophylaxis with heparin. Am J Obstet Gynecol. 1993;168:1265–1270.
[Crossref] [Google Scholar] [PubMed]
- Blomback M, Bremme K, Hellgren M, Lindberg H. A pharmacokinetic study of dalteparin (Fragmin) during late pregnancy. Blood Coagul Fibrinolysis. 1998;9:343–350.
[Crossref] [Google Scholar] [PubMed]
- Kitchen S, Iampietro R, Woolley AM, Preston FE. Anti Xa monitoring during treatment with low molecular weight heparin or danaparoid: Inter-assay variability. Thromb Haemost. 1999;82:1289–1293.
[Crossref] [Google Scholar] [PubMed]
- Greer I, Hunt BJ. Low molecular weight heparin in pregnancy: Current issues. Br J Haematol. 2005;128:593–601.
[Crossref] [Google Scholar] [PubMed]
- Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: Available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43:1064–1083.
[Crossref] [Google Scholar] [PubMed]
- Watson H, Davidson S, Keeling D. Haemostasis and thrombosis task force of the British committee for standards in haematology. guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528–540.
[Crossref] [Google Scholar] [PubMed]
- Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):495S-530S.
- Chu J, Johnston TA, Geoghegan J. Royal college of obstetricians and gynaecologists. Maternal collapse in pregnancy and the puerperium: green‐top guideline No. 56. BJOG. Int J Obs Gyn. 2020;127(5):14-52.
- Guideline Development Group. Prevention and management of venous thromboembolism. SIGN publication no. 122. Edinburgh: SIGN; 2010.
- Raschke RA, Gollihare B, Peirce JC. The effectiveness of implementing the weight-based heparin nomogram as a practice guideline. Arch Int Med. 1996;156(15):1645-1649.
- Hyers TM, Hull RD, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest. 1995;108(4):335S-351S.
- Raschke RA, Guidry JR, Foley MR. Apparent heparin resistance from elevated factor VIII during pregnancy. Obs Gyn. 2000;96(5):804-806.
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: A meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Van Veen JJ, Maclean RM, Hampton KK, Laidlaw S, Kitchen S, Toth P, et al. Protamine reversal of low molecular weight heparin: Clinically effective?. Blood Coagul Fibrinolysis. 2011;22(7):565-570.
- Aschwanden M, Engel H, Schwob A, Jeanneret C, Mueller-Brand J, Jaeger KA. Acute deep vein thrombosis: Early mobilization does not increase the frequency of pulmonary embolism. Thromb Haem. 2001;85(1):42-46.
- Trujillo-Santos J, Perea-Milla E, Jimenez-Puente A, Sanchez-Cantalejo E, Del Toro J, Grau E, et al. Bed rest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE registry. Chest. 2005;127(5):1631-1636.
- National Institute for Health and Clinical Excellence. Venous thromboembolic diseases: The management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. Manchester: NICE; 2012.
- McLintock C. Thromboembolism in pregnancy: challenges and controversies in the prevention of pregnancy-associated venous thromboembolism and management of anticoagulation in women with mechanical prosthetic heart valves. Best Pract Res Clin Obstet Gynaecol. 2014;28(4):519-536.
- Hajj-Chahine J, Jayle C, Tomasi J, Corbi P. Successful surgical management of massive pulmonary embolism during the second trimester in a parturient with heparin-induced thrombocytopenia. Interact Cardiovasc Thorac Surg. 2010;11(5):679-681.
- Ciurzynski M, Jankowski K, Pietrzak B, Mazanowska N, Rzewuska E, Kowalik R, et al. Use of fondaparinux in a pregnant woman with pulmonary embolism and heparin-induced thrombocytopenia. Med Sci Monitor: Int Med J Exp Clin Res. 2011;17(5):56- 59.
- Nagler M, Haslauer M, Wuillemin WA. Fondaparinux–data on efficacy and safety in special situations. Thromb Res. 2012;129(4):407-417.
- Tang AW, Greer I. A systematic review on the use of new anticoagulants in pregnancy. Obs Med. 2013;6(2):64-71.
- Gogarten W, Vandermeulen E, Van Aken H, Kozek S, Llau JV, Samama CM. Regional anaesthesia and antithrombotic agents: Recommendations of the European Society of Anaesthesiology. Eur J Anaesthesiology. 2010;27(12):999-1015.
- Buller HR, Gent M, Gallus AS, Ginsberg J, Prins MH, Baildon R. Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med. 1997;337(10):657-662.
- Crawford JS. Some maternal complications of epidural analgesia for labour. Anaesthesia. 1985;40:1219–1225.
[Crossref] [Google Scholar] [Pubmed]
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–1999. Anesthesiology. 2004;101:950–959.
[Crossref] [Google Scholar] [Pubmed]
- Gris JC, Neveu S, Tailland ML, Courtieu Ch, Mares P, Schved JF. Use of a low-molecular weight heparin (enoxaparin) or of a phenormin-like substance (moroxydine chloride) in primary early recurrent aborts with an impaired fibrinolytic capacity. Thromb Haemost. 1995;73:362-367.
[Crossref] [Google Scholar] [Pubmed]
- Kirchmaier CM, Lindhoff-Last E, Ruebesam D, Scharrer I, Vitgh Z, Mosch G, et al. Regression of deep vein thrombosis by i.v. administration of a low molecular weight heparin. Results of a pilot study. Thromb Res. 1994;73:337-348.
[Crossref] [Google Scholar] [Pubmed]
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6):454-545.
[Crossref] [Google Scholar] [Pubmed]
Citation: Felis S, Marchese B, Gavini I (2023) The Latest Evidence of Risks and Management of Venous Thromboembolism in Pregnancy: A Comprehensive Review. J Blood Disord Transfus. S2.006.
Copyright: © 2023 Felis S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.