Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 1
The Effectiveness and Compliance of Routine Antenatal RhIG Prophylaxis using Different Strategies: A Systematic Review and Meta-analysis
Kar Men Tee1 and Denise E Jackson1,2*2Department of Laboratory Medicine, RMIT University, Bundoora, Victoria, Australia
Received: 15-Jan-2024, Manuscript No. JBDT-24-24624; Editor assigned: 17-Jan-2024, Pre QC No. JBDT-24-24624 (PQ); Reviewed: 07-Feb-2024, QC No. JBDT-24-24624; Revised: 15-Feb-2024, Manuscript No. JBDT-24-24624 (R); Published: 21-Feb-2024, DOI: 10.4172/2155-9864.24.15.573
Abstract
Routine antenatal anti-D prophylaxis has successfully reduced the incidence of RhD alloimmunisation and Haemolytic Disease of the Foetus and Newborn (HDFN). However, there are different strategies and guidelines available across different countries. This systematic review and meta-analysis aimed to investigate the effectiveness and compliance of routine antenatal prophylaxis of different strategies by measuring detectable anti-D levels at delivery, RhD alloimmunisation rate and compliance. PubMed, SCOPUS, EMBASE and Cochrane Library were searched up until 30 July 2023. Binary random effects meta-analysis using maximum likelihood method was performed in Open Meta Analyst software. 17 studies were eligible for meta-analysis. More women had detectable anti-D at delivery after two-dose regimen (24.5%; estimate 0.532, 95% CI [0.181, 0.866], P<0.001) than one-dose regimen (18.35%; estimate 0.252, 95% CI [0.107, 0.433], P<0.001). The rate of RhD alloimmunisation was higher for two-dose regimen (0.33%; estimate 0.003, 95% CI [0.002, 0.005], P<0.001) when compared to one-dose regimen (0.13%; estimate 0.002, 95% CI [0.000, 0.005], P<0.001). Targeted antenatal Rh Immune Globulin (RhIG) had the highest compliance (90.01%; estimate 0.903, 95% CI [0.880, 0.924], P<0.001). One-dose had higher compliance when compared to two-dose regimens (88.73% vs. 61.49%; P=0.004). Both universal one-dose and two-dose regimens were equally effective for the prevention of RhD alloimmunisation. However, one-dose regimen would perform better than two-dose regimen due to higher compliance. The targeted regimen which uses a single dose of RhIG is considered the best strategy for routine antenatal prophylaxis with the highest compliance.
Keywords
Routine antenatal prophylaxis; Rhig; One-dose; Two-dose; Targeted; Detectable anti-D; Rhd alloimmunisation; Compliance
Introduction
Since the introduction of Rhesus D (RhD) Immunoglobulin (Ig) in the late 1960s, the prevalence of anti-D mediated Haemolytic Disease of the Foetus and Newborn (HDFN) and RhD alloimmunisation has significantly declined [1]. HDFN is a condition known as the destruction of foetal/neonatal erythroid cells caused by RhD alloimmunisation in RhD-negative women, which may result in anaemia, jaundice, kernicterus, hydrops foetalis, stillbirth and neonatal death in severe cases [2]. Rh Immunoprophylaxis (RhIG) is produced by purifying human polyclonal anti-D Immunoglobulin G (IgG) from pooled plasma of hyperimmunised male donors. RhIG was first introduced for postpartum administration, where a standard dose is given within 72 hours to all non-sensitised RhD-negative women after delivery of RhD-positive infant.
Postnatal administration of RhIG successfully reduced the risk of Rh alloimmunisation from 13% to 1-2% [3]. However, studies reported failure of postnatal RhIG due to sensitising events during pregnancy and highlighted the potential of antenatal RhIG [4,5]. Some studies demonstrated the likelihood of sensitising events during the third trimester of pregnancy without visible clinical symptoms [6-9]. Antenatal prophylaxis was proposed with the combined use of postnatal prophylaxis and further reduced the overall sensitisation rate to 0.07%-0.3% [10-15]. Many countries, mainly developed countries, have implemented different guidelines and strategies for routine antenatal prophylaxis [9,16].
Routine antenatal anti-D prophylaxis
The purpose of antenatal prophylaxis is to administer RhIG to all non-immunised RhD-negative pregnant women during pregnancy who have the risk of RhD alloimmunisation regardless of the foetal RhD status. The burden of HDFN is most prevalent in Caucasians with an estimated 15% of the population being RhD-negative [17]. Of all pregnancies, there are approximately 10% RhD-negative women delivered an RhD-positive foetus with the risk of sensitisation by Fetomaternal Haemorrhage (FMH) [7]. Prevention of RhD alloimmunisation by RhIG usually focuses on the first pregnancy to avoid complications in subsequent pregnancies with an increased risk of HDFN. In the first pregnancy, approximately 60% RhD-negative women will deliver an RhD-positive infant [7]. Development of anti-D IgG antibodies requires a transplacental haemorrhage sufficient to trigger an immune response, where the majority occurs after 28th week of gestations [9]. Thus, antenatal RhIG is administered routinely in the third trimester of pregnancy to suppress potential RhD alloimmunisation by passively removing any foetal D antigen in the mother [6]. The possible routes of administration are either intramuscularly or intravenously, where intramuscular injections are more commonly used.
Universal one-dose vs. two-dose regimens
There is no one standardised routine antenatal prophylaxis programme globally. The British Committee for Standards in Haematology (BCSH) guidelines and the Society of Obstetricians and Gynaecologists of Canada (SOGC) recommend either one 1500 International Units (IU) at 28 weeks of gestation or 500 IU doses at 28 and 34 weeks [18,19]. The American Congress of Obstetricians and Gynecologists (ACOG) recommends administrating a single 1500 IU dose at 28 weeks of gestation [20]. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) recommend 625 IU doses at 28 and 34 weeks [21]. The National Prevention Program of the Netherlands recommends 1000 IU at 30 weeks’ gestation, Germany routinely administers a single 300 µg dose at 28-30 weeks’ gestation, and different strategies for other European countries [8,14,22]. Therefore, the two main approaches for antenatal administration of RhIG are either the one-dose regimen of 1000 IU- 1500 IU dose between 28-30 weeks’ gestation or the two-dose regimen of 500-625 IU doses at 28 and 34 weeks’ gestation.
Targeted regimens
Recently, many have suggested prenatal screening of foetal RHD genotype using a Non-Invasive Prenatal Test (NIPT) to guide targeted administration of antenatal RhIG. In the late 1990s, Lo and his colleagues first discovered low concentrations of cell-free foetal Deoxyribonucleic acid (cfDNA) present in maternal plasma without the use of invasive procedures [23,24]. Detection of the foetal RHD gene in maternal plasma using a whole Ethylenediaminetetraacetic Acid (EDTA) blood sample provides high sensitivity and accuracy. This new regimen can avoid unnecessary exposure to RhIG derived from human plasma, reduce wasteful use of RhIG and increase the utilisation of RhIG to only RhD-negative women pregnant with an RhD-positive foetus [25]. After the success of further clinical trials, Denmark was the first country to implement a national prenatal screening for foetal RHD before the administration of antenatal RhIG in January 2010 [26]. Followed by the Netherlands in July 2011, Finland in October 2013, Norway in September 2016 as well as regional Sweden [27-31]. The general practice is a NIPT of all non-immunised RhD-negative pregnant women at 24 weeks’ gestation followed by a single 1250-1500 IU dose at 28-30 weeks to RhD-negative pregnant women carrying a RhD-positive foetus [25,29]. Australia is still evaluating the feasibility of introducing routine NIPT as part of antenatal care [32].
Scope of study
Several strategies are available: universal one-dose, universal two-dose, and the latest targeted regimens, where different countries and locations adopted different regimens. Some suggested the one-dose regimen is superior to the two-dose regimen while others suggested otherwise. Despite the efforts of routine antenatal and postnatal prophylaxis, there are residual cases of RhD alloimmunisation. Compliance with routine antenatal prophylaxis regimen may contribute to failure of the regimens. Missing or incorrect doses of routine antenatal prophylaxis or given at the wrong timing may put non-immunised RhD-negative pregnant at risk of RhD alloimmunisation. Therefore, this study aims to investigate the following primary question: Is the one-dose antenatal anti-D prophylaxis regimen more effective than the two-dose regimen in protecting non-immunised RhD-negative pregnant women against RhD immunisation? With a secondary question: Does the one-dose regimen have higher compliance than two-dose routine antenatal anti-D prophylaxis? An addition question: How does targeted regimen perform when compared to one-dose and two-dose regimen? This study evaluates the effectiveness and compliance of routine antenatal prophylaxis of different strategies for the prevention of RhD alloimmunisation and HDFN with regard to the level of detectable anti-D at delivery, the rate of RhD alloimmunisation/sensitisation after delivery, and the rate of compliance.
Materials and Methods
This meta-analysis was prepared according to the protocols outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [33].
Search strategy and study selection
To identify eligible literature for this meta-analysis, the following databases were systematically searched for: PubMed, SCOPUS, EMBASE and Cochrane Library. The latest search was conducted on 30th July 2023. The following keywords and phrases in various combinations were used in accordance with the search strategy: anti-D prophylaxis, RhIG prophylaxis, antenatal RhIG, antenatal prophylaxis, anti-D immunoglobulin, RhD immune globulin, Rhesus prophylaxis, single-dose, one-dose, and two-dose. Additional literature was included by hand searching reference lists of potential articles. Articles were retrieved based on the search strategy and duplicates were removed. The titles and abstracts were screened for relevancy based on the predefined exclusion and inclusion criteria to identify potentially eligible studies for meta-analysis.
Exclusion and inclusion criteria
Eligible studies must include non-immunised RhD-negative pregnant women given one dose, two doses, or targeted routine antenatal RhIG intramuscularly during pregnancy. Studies should investigate a single dose of 1500 IU administered at 28-30 weeks’ gestation for one-dose regimen, two doses of 500-625 IU administered at 28 and 34 weeks’ gestations for two-dose regimen, and a single 1250 IU- 1500 IU administered at 28-30 weeks’ gestation guided by a NIPT for targeted regimen. Review articles, guidelines, meta-analyses, editorials, letters, and case reports were excluded. Non-English articles without translation available were excluded. Any studies that only evaluated the effect of postnatal prophylaxis, RhIG given antenatally due to complicated pregnancies, or intravenous administration were excluded. Abstract-only publications and full text not available were excluded.
Quality assessment of eligible studies
All eligible studies were assessed for reporting quality using the recommendations outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [34].
Data extraction
Information on study design, location, study period, sample size, type of regimen and measurements were extracted to summarise the characteristics of each eligible study. Relevant data were extracted from each eligible study and grouped based on the measurements for further investigation and comparison.
Statistical analysis
Extracted data was analysed in Open Meta Analyst software (version 12.11.14) from the Brown University website, and results were presented in forest plots. One-arm using arcsine transformed proportion metric were employed to explore the effects of detectable anti-D, RhD alloimmunisation and compliance of one-dose, two-dose and targeted regimens. Two-arm using difference of arcsine transformed proportions metric was employed the explore the relationship of a single measurement between regimens, if applicable. For both metrics, a binary random effects meta-analysis was conducted using maximum likelihood method. Multiple forest plots were generated, displaying the estimated 95% confidence intervals, P-values, and heterogeneity for each study and overall pooled effect of meta-analysis. The magnitude of heterogeneity was measured as I2 statistic, where below 40% may suggest not important heterogeneity, 30%-60% suggest moderate heterogeneity and above 50% suggest substantial heterogeneity [35]. Statistical significance was expressed as P-value, where P ≤ 0.05 defined as ‘statistically significant’.
Results
Study selection
Overall, 5124 citations were retrieved from PubMed, SCOPUS, EMBASE and Cochrane Library. After removing 1863 duplicates, 2291 articles were excluded by title screening and 779 based on abstract, yielding a total of 191 articles to assess for eligibility. Of the 191 articles, 172 were excluded based on the predefined exclusion criteria and 18 studies were included after final review. One additional eligible article was manually searched from reference lists. A total of 19 studies were included in this review but only 17 were included for meta-analysis (Figure 1).
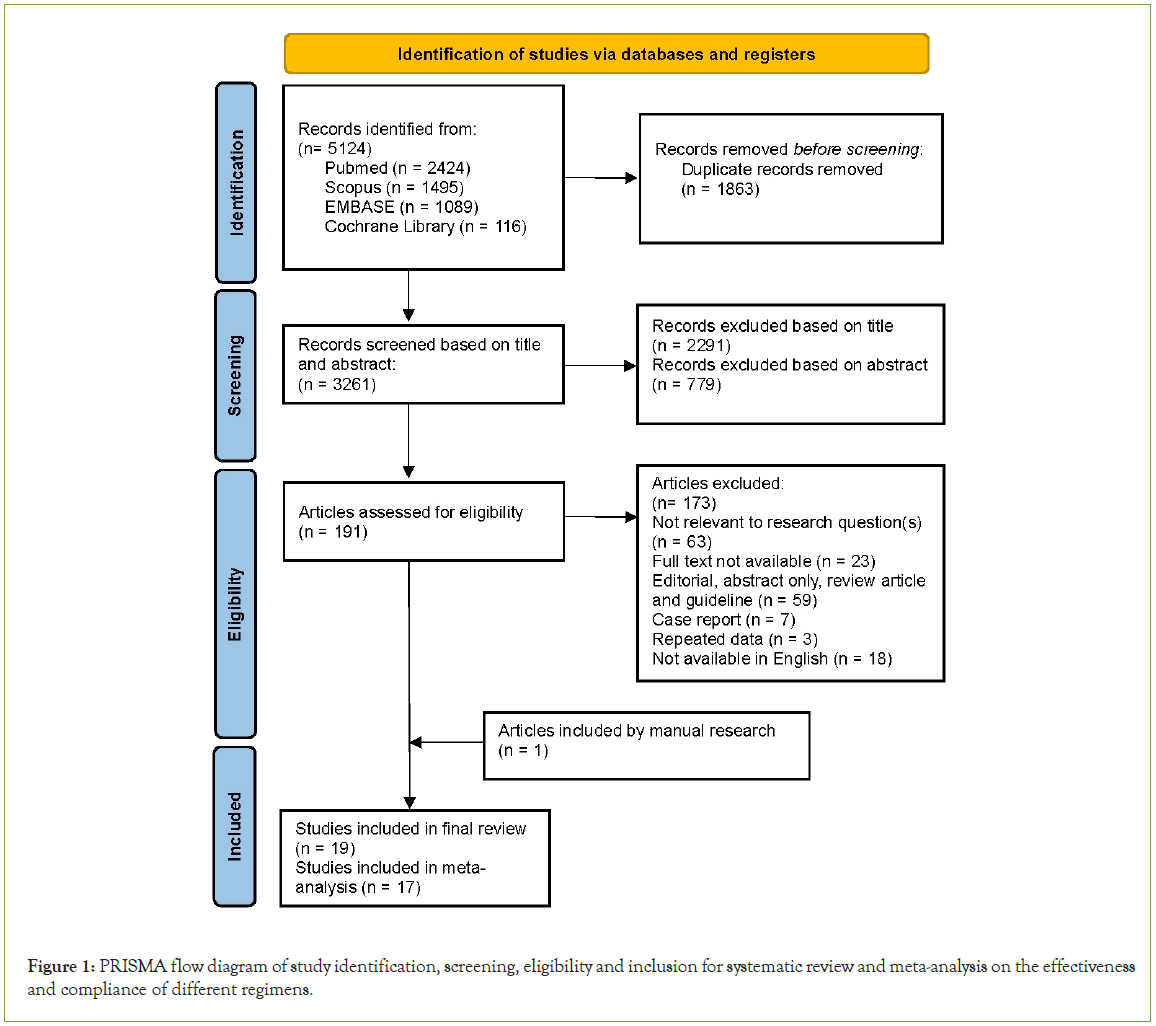
Figure 1: PRISMA flow diagram of study identification, screening, eligibility and inclusion for systematic review and meta-analysis on the effectiveness and compliance of different regimens.
Study characteristics
As shown in Table 1, the eligible studies included nine retrospective studies [8,13,30] four prospective studies [25] five clinical trials [11,22] and one did not specify its study design (Table 1) [36-48]. These studies were conducted across eight countries in 1975-2019. One study was missing information on study period [22].
| Study | Country | Study Design | Study Period | Study Size | Regimen | Measure(s) |
|---|---|---|---|---|---|---|
| Bowman and Pollock; 1978 [36] | Canada | Clinical trial | 1975 | 1086 | One-dose | RhD alloimmunisation |
| Chaffe et al.; 2007 [37] | UK | Retrospective audit | 2004 | 207 | Two-dose | Compliance |
| Clausen et al.; 2014 [38] | Denmark | Retrospective | 2010-2012 | 690 | Targeted | Compliance |
| Damkjaer et al.; 2012 [39] | Denmark | Retrospective | 2010 | 239 | Targeted | Compliance |
| Davies et al.; 2011 [8] | UK | Retrospective | 2009 | 157 | One-dose | Detectable anti-D |
| 407 | Two-dose | Detectable anti-D | ||||
| Glazebrook et al.; 2020 [40] | Australia | Retrospective | 2017-2018 | 939 | Two-dose | Compliance |
| MacKenzie et al.; 1999 [41] | UK | Prospective | 1990-1996 | 3332 | One-dose | RhD alloimmunisation |
| MacKenzie et al.; 2004 [22] | UK, USA | Clinical trial | - | 216 | Two-dose | RhD alloimmunisation |
| MacKenzie et al.; 2006 [42] | UK | Prospective | 1992-2003 | 546 | Two-dose | Compliance |
| MacKenzie et al.; 2011 [43] | UK | Prospective | 2009 | 144 | One-dose | Compliance |
| 500 | Two-dose | Compliance | ||||
| Mayne et al.; 1997 [13] | UK | Retrospective | 1993-1995 | 1425 | Two-dose | RhD alloimmunisation |
| Rowley et al.; 2014 [44] | UK | Retrospective audit | 2012 | 4887 | One-dose | Compliance |
| 389 | Two-dose | Compliance | ||||
| Rudensky et al.; 2003 [45] | Israel | - | 2000 | 150 | One-dose | Detectable anti-D |
| Sorensen et al.; 2022 [30] | Norway | Retrospective | 2017-2019 | 984 | One-dose | Detectable anti-D |
| Tiblad et al.; 2013 [25] | Sweden | Prospective | 2009-2012 | 9380 | Targeted | RhD alloimmunisation, Compliance |
| Tovey et al.; 1983 [11] | UK | Clinical trial | 1980-1981 | 2069 | Two-dose | Detectable anti-D, RhD alloimmunisation |
| Trolle 1989 [46] | Denmark | Clinical trial | 1980-1985 | 609 | One-dose | Detectable anti-D, RhD alloimmunisation |
| White et al.; 2019 [47] | Australia | Randomised clinical trial | 2013-2015 | 138 | One-dose | Detectable anti-D, Compliance |
| 139 | Two-dose | Detectable anti-D, Compliance | ||||
| Wikman et al.; 2021 [48] | Sweden | Retrospective | 2010-2012 | 4280 | Targeted | Detectable anti-D |
Table 1: Characteristics of eligible studies for the analysis of effectiveness and compliance of different regimens.
For the detection of anti-D at delivery, two studies used the DiaMed gel method, one study used papain-treated antibody screen with passive anti-D quantified as<0.1 IU/mL, one study used Ortho BioVue column agglutination technique, and one study did not specify the technique for antibody screen [8,11,45-47]. To assess the rate of RhD alloimmunisation, women who delivered a RhD-positive infants were followed up in 6-12 months in three studies [22,36,46]. Besides, two studies measured sensitised second pregnancies, and one study followed up to 12 months after delivery and subsequent sensitised second pregnancies [11,13,41].
To access compliance, the definition of ‘appropriate timing’ for injection slightly varied between studies. For one-dose regimen, two studies assessed injection given at 28 weeks’ gestation with 2 weeks intervals, while one study assessed injection given within a week of 28 weeks’ gestation [43,44,47]. For two-dose regimen, two studies assessed injections given within a week of 28 and 34 weeks’ gestation, two studies assessed first injection at 28 ± 1 weeks’ gestation and second injection given after 6 ± 1 weeks, and other two studies given with a 2-week interval for both injections (28-30 and 34-36 weeks’ gestation) [37,40,42-44,47]. However, targeted regimen had a broader definition for compliance, which was assessed by the total number of RhDnegative pregnant women carrying a RhD-positive foetus received recommended antenatal prophylaxis [25,38,39]. Each included study recorded at least one measurement for effectiveness and/or compliance (Table 2).
| Study | Regimen | Detectable anti-D at delivery, n/N | RhD alloimmunisation, n/N | Compliance, n/N |
|---|---|---|---|---|
| Bowman and Pollock; 1978 [36] | One-dose | - | 2/1086 | - |
| Chaffe et al.; 2007 [37] | Two-dose | - | - | 179/207 |
| Clausen et al.; 2014 [38] | Targeted | - | - | 330/354 |
| Damkjaer et al.; 2012 [39] | Targeted | - | - | 126/147 |
| Davies et al.; 2011 [8] | One-dose | 34/157 | - | - |
| Two-dose | 247/407 | - | - | |
| Glazebrook et al.; 2020 [40] | Two-dose | - | - | 681/920 |
| MacKenzie et al.; 1999 [41] | Two-dose | - | Dec-20 | - |
| MacKenzie et al.; 2004 [22] | One-dose | 0/194 | ||
| MacKenzie et al.; 2006 [42] | Two-dose | - | - | 232/401 |
| MacKenzie et al.; 2011 [43] | One-dose | - | - | 108/139 |
| Two-dose | - | - | 304/451 | |
| Mayne et al.; 1997 [13] | Two-dose | - | 4/1425 | - |
| Rowley et al.; 2014 [44] | One-dose | - | - | 4388/4887 |
| Two-dose | - | - | 228/389 | |
| Rudensky et al.; 2003 [45] | One-dose | 32/150 | - | - |
| Tiblad et al.; 2013 [25] | Targeted | - | 14/9380 | 4590/5104 |
| Tovey et al.; 1983 [11] | Two-dose | 282/2069 | 4/1238 | - |
| Trolle 1989 [46] | One-dose | 55/609 | 0/291 | - |
| White et al.; 2019 [47] | One-dose | 70/125 | - | 86/138 |
| Two-dose | 111/129 | - | 70/139 |
Note: N = total number of cases tested for the respective measurements; n = number of cases identified for the respective measurements.
Table 2: Data of included studies in meta-analysis portraying in proportions.
Study quality assessment
The included studies were assessed for their methodological quality according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines, with the most relevant criteria selected and summarised in Table 3. Of 19 included studies [22,25,30], only nine studies had fulfilled all criteria [44,47,48] and were considered as high quality [39,40,43]. The remaining ten studies were considered of lower quality as most studies failed to describe statistical methods and address potential limitations. However, two studies were further identified as poor quality due to only stating rationale without explaining the background of the study and the lack of an abstract in one of them [13,45]. Studies that did not describe statistical methods [8,11,13,22] and discuss potential limitations [36- 38,41] were mostly conducted in the late 1900s and early 2000s, except the study by MacKenzie and colleagues which was conducted in 2004 [42,45,46]. A possible reason could be that guidelines and practices for standardised reporting may not be well established and with poor adherence. However, the studies conducted in the early period provided the fundamental evidence used to form the early guidelines for the national routine antenatal prophylaxis programme. Thus, considering the impact of these early studies, some studies of lower quality were still included in this meta-analysis.
| Title and abstract | Introduction | Methods | Results | Discussion | |||
|---|---|---|---|---|---|---|---|
| Clear title and abstract with information on study design | Explain scientific background and rationale | Provide detailed study method | Provide eligibility criteria and selection of participants | Describe statistical methods | Report numbers of individuals at each stage of study | Summarise key results and discuss potential limitations | |
| Bowman and Pollock; 1978 [36] | Y | Y | Y | Y | N | Y | Na |
| Chaffe et al.; 2007 [37] | Y | Y | Y | Y | Nb | Y | Na |
| Clausen et al.; 2014 [38] | Y | Y | Y | Y | Y | Y | Na |
| Damkjaer et al.; 2012 [39] | Y | Y | Y | Y | Y | Y | Y |
| Davies et al.; 2011 [8] | Y | Y | Y | Y | Y | Y | Na |
| Glazebrook et al.; 2020 [40] | Y | Y | Y | Y | Y | Y | Y |
| MacKenzie et al.; 1999 [41] | Y | Y | Y | Y | Y | Y | Na |
| MacKenzie et al.; 2004 [22] | Y | Y | Y | Y | Y | Y | Y |
| MacKenzie et al.; 2006 [42] | Y | Y | Y | Y | N | Y | Na |
| MacKenzie et al.; 2011 [43] | Y | Y | Y | Y | Y | Y | Y |
| Mayne et al.; 1997 [13] | Nc | Nd | Y | Y | N | Y | Na |
| Rowley et al.; 2014 [44] | Y | Y | Y | Y | Y | Y | Y |
| Rudensky et al.; 2003 [45] | Y | Nd | Y | Y | N | Y | Na |
| Sorensen et al.; 2022 [30] | Y | Y | Y | Y | Y | Y | Y |
| Tiblad et al.; 2013 [25] | Y | Y | Y | Y | Y | Y | Y |
| Tovey et al.; 1983 [11] | Y | Y | Y | Y | N | Y | Na |
| Trolle 1989 [46] | Y | Y | Y | Y | N | Y | Na |
| White et al.; 2019 [47] | Y | Y | Y | Y | Y | Y | Y |
| Wikman et al.; 2021 [48] | Y | Y | Y | Y | Y | Y | Y |
Note: Y = criteria fulfilled; N = criteria unfulfilled. a No limitation discussed; b Audit standards were defined; c No abstract included; d Only stated the rationale.
Table 3: Evaluation of methodological quality of eligible studies according to the STROBE checklist.
Meta-analysis of detectable anti-D at delivery
Meta-analysis on detectable anti-D at delivery was performed for one-dose and two-dose regimens, with results presented in forest plots (Figure 2). The one-arm proportion analyses were statistically significant for one-dose (estimate 0.252, 95% CI [0.107, 0.433], P<0.001), and two-dose regimens (estimate 0.532, 95% CI [0.181, 0.866], P<0.001). The overall results showed that 18.35% of women had detectable anti-D after one-dose regimen, while 24.57% of women had detectable anti-D after two-dose regimen. The results were highly heterogeneous for the one-dose regimen (I2=96.87%, P<0.001) and for the two-dose regimen (I2=99.50%, P<0.001), indicating variability in data for studies included in the meta-analyses. Overall, the results suggested that two-dose regimen had higher detectable anti-D at delivery compared to one-dose regimen.
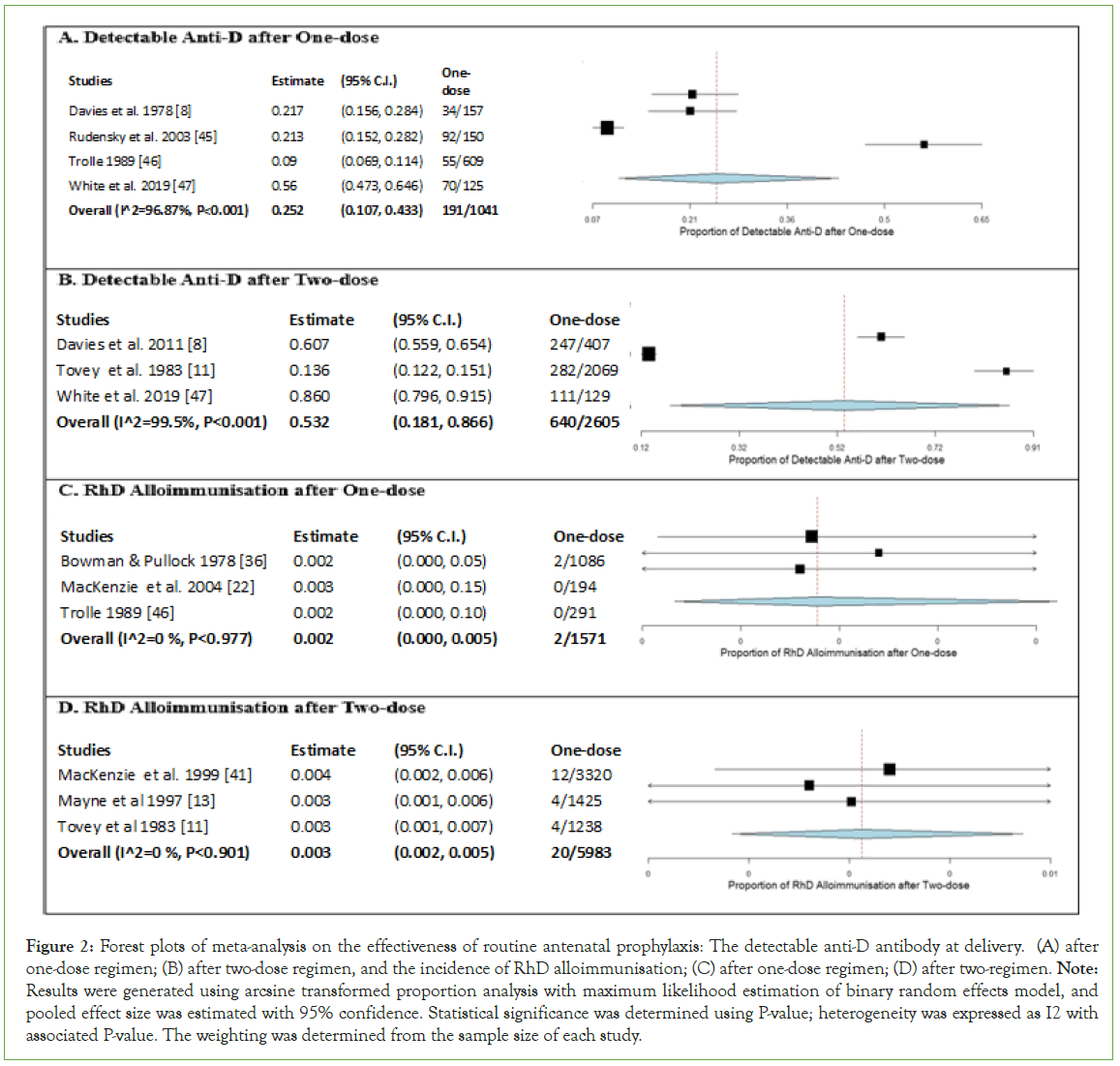
Figure 2: Forest plots of meta-analysis on the effectiveness of routine antenatal prophylaxis: The detectable anti-D antibody at delivery. (A) after one-dose regimen; (B) after two-dose regimen, and the incidence of RhD alloimmunisation; (C) after one-dose regimen; (D) after two-regimen. Note: Results were generated using arcsine transformed proportion analysis with maximum likelihood estimation of binary random effects model, and pooled effect size was estimated with 95% confidence. Statistical significance was determined using P-value; heterogeneity was expressed as I2 with associated P-value. The weighting was determined from the sample size of each study.
Meta-analysis of RhD alloimmunisation after delivery
Meta-analysis on RhD alloimmunisation after delivery was performed for one-dose and two-dose regimens, with results presented in forest plots. The one-arm proportion analyses were statistically significant for one-dose (estimate 0.002, 95% CI [0.000, 0.005], P<0.001), and two-dose regimens (estimate 0.003, 95% CI [0.002, 0.005], P<0.001). The overall results showed that 0.13% of women were immunised after one-dose regimen, while 0.33% of women were immunised after two-dose regimen. The results found no heterogeneity for one-dose (I2=0%, P=0.977) and two-dose regimen (I2=0%, P=0.901), indicating no variability in data for studies included in the meta-analyses. Overall, the results suggested that there was a slightly higher rate of RhD alloimmunisation after two-dose regimen than one-dose regimen.
Meta-analysis of compliance
A meta-analysis was performed on compliance with one-dose, two-dose, and targeted regimens, with results presented in forest plot (Figure 3). The one-arm proportion analyses were statistically significant for one-dose (estimate 0.781, 95% CI [0.639, 0.895], P<0.001), twodose (estimate 0.665, 95% CI [0.564, 0.759], P<0.001), and targeted regimens (estimate 0.903, 95% CI [0.880, 0.924], P<0.001), with total compliance of 88.73%, 67.57% and 90.01%, respectively (Figures 3A- 3C). The results were highly heterogeneous for one-dose (I2=95.07%, P<0.001) and two-dose regimens (I2=96.17%, P<0.001) while moderately heterogeneous for targeted regimen (I2=51.77%, P=0.025), indicating variability in data for studies included in the meta-analyses. Overall, the results suggested that the targeted regimen has the highest compliance, followed by one-dose and two-dose regimens. In Figure 3D, a two-arm proportion analysis was performed to compare the compliance of one-dose and two-dose regimens. The results of three included studies demonstrated a significant difference between the compliance of one-dose and two-dose regimens (estimate 0.212, 95% CI [0.069, 0.354], P=0.004), where one-dose regimen has higher compliance than two-dose regimen (88.73% compared to 61.49%). The heterogeneity was substantial (I2=87.85%, P<0.001).
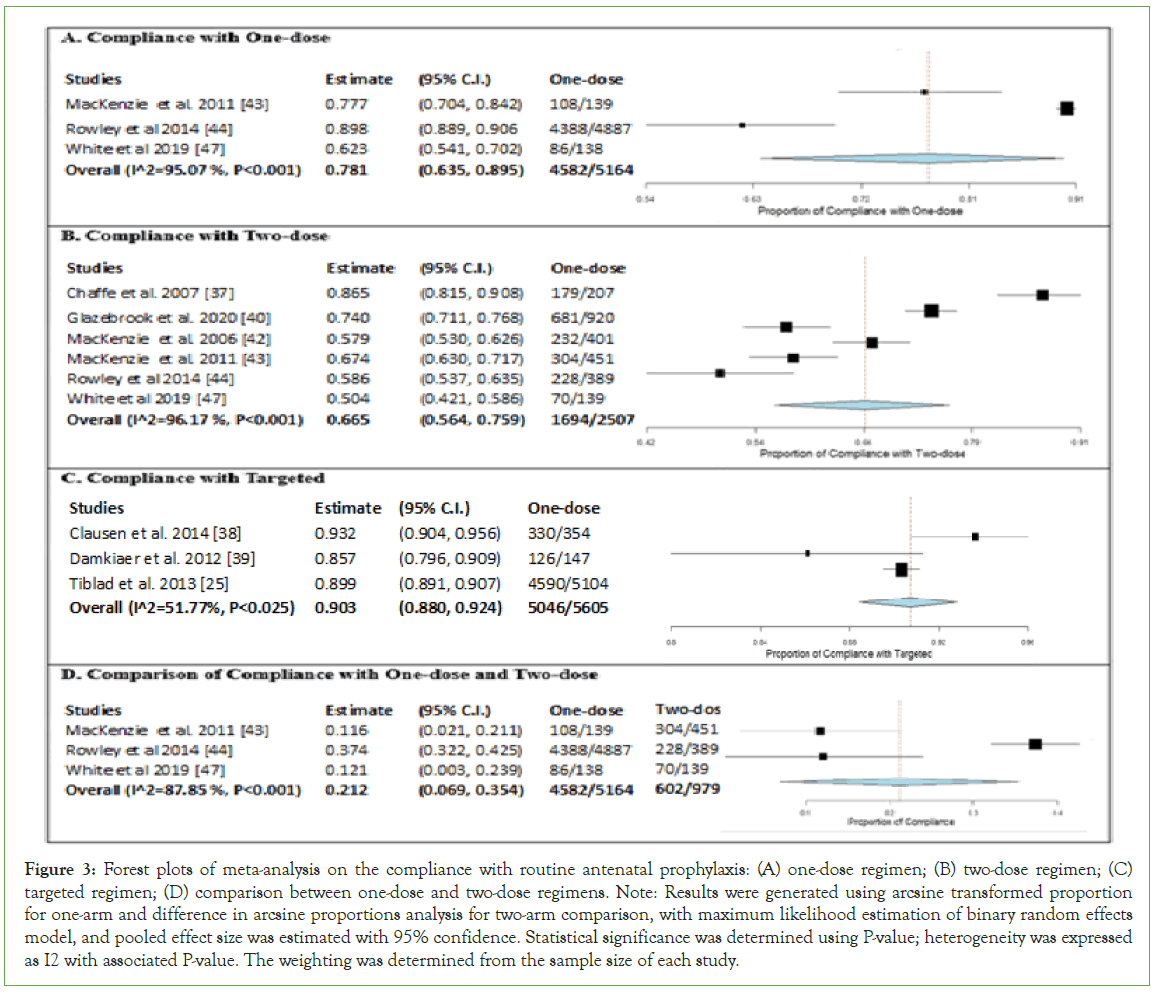
Figure 3: Forest plots of meta-analysis on the compliance with routine antenatal prophylaxis: (A) one-dose regimen; (B) two-dose regimen; (C) targeted regimen; (D) comparison between one-dose and two-dose regimens. Note: Results were generated using arcsine transformed proportion for one-arm and difference in arcsine proportions analysis for two-arm comparison, with maximum likelihood estimation of binary random effects model, and pooled effect size was estimated with 95% confidence. Statistical significance was determined using P-value; heterogeneity was expressed as I2 with associated P-value. The weighting was determined from the sample size of each study.
Discussion
Effectiveness of routine antenatal prophylaxis
The results showed that 18.35% of women after one-dose regimen and 24.57% of women after two-dose had detectable anti-D at delivery, suggesting two-dose regimen was relatively more effective than onedose regimen. Theoretically, the half-life of circulating anti-D was 17-21 days [49,50]. Thus, the residual dose by calculation would be higher after 12 weeks of administrating the primary dose for twodose regimen than one-dose regimen. With a half-life of 21 days, the calculated residual dose after 12 weeks was 18.75 μg after a single dose of 300 μg (1500 IU), and 31.75 μg after two doses of 100 μg (500 IU) at 28 and 34 weeks’ gestation [51]. Therefore, two-dose regimen proposed a theoretical advantage over one-dose regimen as more women were at risk of potential RhD sensitisation after one-dose regimen due to reduced protection from RhIG.
The World Health Organisation (WHO) suggested a minimal dose of 25 μg (100 IU) for the prevention of RhD sensitisation with 1 mL of RhD-positive foetal red cells or approximately 2 mL of foetal whole blood [52]. However, it was believed that an unprovoked transplacental haemorrhage during pregnancy unlikely to be as large as the estimated FMH of 1 mL foetal red cells at delivery [51]. Hence, both regimens were considered as effective. In addition, both regimens showed approximately 80% of women with no detectable anti-D. Rudensky et al. hypothesised that the non-detectable of passive anti-D was a result of RhIG binding to foetal red cells leading to either the clearance from maternal circulation before stimulating maternal immune system or presenting foetal red cells in a non-stimulatory form [45]. Therefore, the lack of detectable anti-D may reflect the effectiveness of antenatal RhIG.
Interestingly, the results showed more women were RhD alloimmunised after two-dose regimen compared to one-dose regimen. If more women had detectable anti-D at delivery after two-dose regimen, the RhD alloimmunisation rate would be expected to be lower with two-dose regimen given better protection from RhIG. Thus, suggesting other probable contributing factors leading to compromising efficacy of RhIG. A lower compliance rate with two-dose regimen may have resulted in an increased incidence of RhD alloimmunisation. Missing second dose and incorrect timing for administrating second dose may lead to a lower concentration of RhIG in maternal circulation resulting in inadequate protection against RhD sensitisation until delivery.
Despite receiving antenatal according to recommended guidelines, RhD alloimmunisation was still prevalent indicating failure of routine antenatal RhIG. Davies et al. found that women who delivered after 40 weeks’ gestation had 1.5-3.5 times more risk of RhD sensitisation post administration of antenatal RhIG at 28 weeks’ gestation [8]. In addition, some studies observed a shorter half-life of RhIG in majority of pregnant women where a decline in detectable anti-D happened before 40 weeks’ gestation [41,42]. Other proposed explanations for the failure of antenatal RhIG included inadequate uptake of RhIG after intramuscular administration and failure to recognise silent massive FMH [50-53].
Compliance of routine antenatal prophylaxis
The results revealed suboptimal compliance with all three regimens. There were several reasons for declining routine antenatal prophylaxis program including fear of infection, religious reason, concern about additives, partner was known to be RhD negative, last planned pregnancy, and no documented reason [37,40,43]. As for noncompliant with guidelines, the most common reason may be due to poor documentation of RhIG administration [39,40,42,44]. Appropriate RhIG administration documentation is critical for traceability and accessibility of detailed patient information by laboratories and clinical staff to ensure RhD-negative women have been properly managed [40]. Another reason for non-compliant for all three regimens was incorrect timing [37,40,42-44,47]. Administration of RhIG before recommended at 28 weeks can lead to lower level of passive anti-D in circulation close to delivery, while administration of RhIG too late can lead to potential sensitisation by silent FMH [44].
Glazebrook et al. demonstrated that lack of appropriate education on routine antenatal prophylaxis by responsible midwives and clinicians to RhD-negative pregnant women for making an informed decision [40]. It is significant that RhD-negative women understand the risk associated with being RhD negative, the significance of RhD alloimmunisation, and possibly affecting subsequent pregnancies. White et al. concluded that poor compliance for both one-dose and two-dose regimens were a result of systematic errors [47].
The compliance rate for targeted regimen was probably compromised as studies were conducted in the first few months after implementation and sources of information regarding antenatal NIPT and targeted antenatal RhIG may not be well known [38,39]. Improvement in compliance was observed over time [25]. However, targeted regimen had the highest compliance rate among the three regimens. The possible explanation could be that knowing the RHD status of foetus served as a motivating factor for receiving the antenatal RhIG at 28 weeks’ gestation.
The results from meta-analysis revealed that one-dose regimen had a higher compliance than two-dose regimen (95% CI 0.069, 0.354, P<0.01). Such results were expected as the second dose of a twodose regimen increased the chances of non-compliant due to several reasons. The most rational reason that women were not as compliant with two-dose regimen is women experienced early labour or potential sensitising events before second dose [44,47]. However, there are other concerning reasons including forgetting to attend appointment for second dose, lack of continuity of care, and underestimating the consequence of omission of second dose. White et al. found that missing a dose and receiving a dose late were common in two-dose regimen than one-dose regimen [47]. Missing of second dose may result in insufficient protection against sensitisation and increases the risk of RhD alloimmunisation in women which can be avoided [32,54]. The importance of routine antenatal RhIG at 28 and 34 weeks’ gestation should be emphasised thus encouraging RhD-negative women to attend the designed appointments on time to minimise potential risk for RhD alloimmunisation [55,56].
Limitations
The major limitation of this study is most included studies were retrospective in nature, which may introduce bias and/or confounding leading to controversies about the validity of the results. The information on potential confounding factors may be absent as the data were collected in the past. Differential losses to follow up leading to bias which may compromise the validity of the study [54,57]. Therefore, the quality of historical records is dependent on the accuracy and completeness of the available data recorded in the healthcare databases. Moreover, the difference in methodologies and dosing and substantial heterogeneity of the included studies may limit the certainty of the overall effect size of the meta-analysis. Therefore, one should be cautious when interpreting the results. In addition, this study only evaluated compliance with targeted regimen with a broad definition. Therefore, further study is required to investigate full compliance with targeted regimen by considering the uptake of NIPT by women and targeted antenatal RhIG given at the correct gestational age. This is critical as eligible pregnant women who declined or missed NIPT will not be identified for antenatal RhIG.
Conclusion
Overall, both one-dose and two-dose regimens were equally effective although slight differences in detectable anti-D and RhD alloimmunisation were observed. However, the one-dose regimen had higher compliance than the two-dose regimen, thus one-dose regimen may have performed better than two-dose regimen. Given that the current targeted regimen uses a single dose of antenatal prophylaxis which is similar to the one-dose regimen, one could conclude that the targeted regimen is the best strategy for routine antenatal prophylaxis with the highest compliance. The targeted regimen can avoid approximately 40% unnecessary administration of antenatal RhIG and conserve RhIG for pregnant women who are at risk of RhD sensitisation. Several studies performed cost analysis on targeted regimen and suggested that targeted regimen might not be sufficiently cost effective to be implemented routinely. While others suggested that targeted regimen enables cost saving from omitting cord blood serology, and reduced use of anti-D products and its associated risks. The risks and benefits should be carefully assessed before the implementation of targeted regimen. However, the scarcity of RhIG may encourage the implementation of targeted regimen by many countries in the near future.
Conflict of Interest
Authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- de Haas M, Finning K, Massey E, Roberts DJ. Anti-D prophylaxis: Past, present and future. Transfus Med. 2014;24(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: Managing the mother, fetus, and newborn. Hematology Am Soc Hematol Educ Program. 2015;2015(1):146-151.
[Crossref] [Google Scholar] [PubMed]
- Crowther C, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev. 2000;1997(2):CD000021.
[Crossref] [Google Scholar] [PubMed]
- Eklund J, Nevanlinna HR. Rh prevention: A report and analysis of a national programme. J Med Genet. 1973;10(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Portmann C, Ludlow J, Joyce A, Chan FY. Antecedents to and outcomes of Rh(D) isoimmunization: Mater Mothers Hospital, Brisbane, 1988-1995. Aust N Z J Obstet Gynaecol. 1997;37(1):12-16.
[Crossref] [Google Scholar] [PubMed]
- Contreras M. The prevention of Rh haemolytic disease of the fetus and newborn-general background. Br J Obstet Gynaecol. 1998;105(18):7-10.
[Crossref] [Google Scholar] [PubMed]
- Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al. A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus-negative. Health Technol Assess. 2003;7(4):62.
[Crossref] [Google Scholar] [PubMed]
- Davies J, Chant R, Simpson S, Powell R. Routine antenatal anti-D prophylaxis-is the protection adequate? Transfus Med. 2011;21(6):421-426.
[Crossref] [Google Scholar] [PubMed]
- McBain RD, Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev. 2015;2015(9):CD000020.
[Crossref] [Google Scholar] [PubMed]
- Hermann M, Kjellman H. Rh-Prophylaxis with immunoglobulin anti-D administered during pregnancy and after delivery. Acta Obstet Gynecol Scand Suppl. 1976;49:1-11.
[Crossref] [Google Scholar] [PubMed]
- Tovey LA, Townley A, Stevenson BJ, Taverner J. The Yorkshire antenatal anti-D immunoglobulin trial in primigravidae. Lancet. 1983;2(8344):244-246.
[Crossref] [Google Scholar] [PubMed]
- Thornton JG, Page C, Foote G, Arthur GR, Tovey LAD, Scott JS. Efficacy and long term effects of antenatal prophylaxis with anti-D immunoglobulin. BMJ. 1989;298(6689):1671-1673.
[Crossref] [Google Scholar] [PubMed]
- Mayne S, Parker JH, Harden TA, Dodds SD, Beale JA. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315(7122):1588.
[Crossref] [Google Scholar] [PubMed]
- Koelewijn JM, de Haas M, Vrijkotte TG, Bonsel GJ, van der Schoot CE. One single dose of 200 microg of antenatal RhIG halves the risk of anti-D immunization and hemolytic disease of the fetus and newborn in the next pregnancy. Transfusion. 2008;48(8):1721-1729.
[Crossref] [Google Scholar] [PubMed]
- Hamel C, Esmaeilisaraji L, Thuku M, Michaud A, Sikora L, Fung-Kee-Fung K. Antenatal and postpartum prevention of Rh alloimmunization: A systematic review and GRADE analysis. PLoS One. 2020;15(9):e0238844.
[Crossref] [Google Scholar] [PubMed]
- Engelfriet CP, Reesink HW, Judd WJ, Ulander VM, Kuosmanen M, Koskinen S, et al. Current status of immunoprophylaxis with anti-D immunoglobin. Vox Sang. 2003;85(4):328-337.
[Crossref] [Google Scholar] [PubMed]
- Huszti AM, Gica N, Botezatu R, Peltecu G, Panaitescu AM. Hemolytic disease of the newborn, beyond the Rhesus disease. Rom Med J. 2022;69(2):45-49.
- Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med. 2014;24(1):8-20.
[Crossref] [Google Scholar] [PubMed]
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40(1):e1-e10.
[Crossref] [Google Scholar] [PubMed]
- Practice Bulletin No. 181: Prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130(2):e57-e70.
[Crossref] [Google Scholar] [PubMed]
- RANZCOG. Guidelines for the use of Rh (D) Immunoglobulin (Anti-D) in obstetrics in Australia; 2019.
- MacKenzie IZ, Bichler J, Mason GC, Lunan CB, Stewart P, Al-Azzawi F, et al. Efficacy and safety of a new, chromatographically purified rhesus (D) immunoglobulin. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):154-161.
[Crossref] [Google Scholar] [PubMed]
- Lo YMD, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CWG, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485-487.
[Crossref] [Google Scholar] [PubMed]
- Lo YMD, Tein MSC, Lau TK, Haines CJ, Leung TN, Poon PMK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for non-invasive prenatal diagnosis. Am J Hum Genet. 1998;62(4):768-775.
[Crossref] [Google Scholar] [PubMed]
- Tiblad E, Taune Wikman A, Ajne G, Blanck A, Jansson Y, Karlsson A, et al. Targeted routine antenatal anti-D prophylaxis in the prevention of RhD immunisation-outcome of a new antenatal screening and prevention program. PLoS One. 2013;8(8):e70984.
[Crossref] [Google Scholar] [PubMed]
- Clausen FB, Christiansen M, Steffensen R, Jørgensen S, Nielsen C, Jakobsen MA, et al. Report of the first nationally implemented clinical routine screening for fetal RHD in D-pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion. 2012;52(4):752-758.
[Crossref] [Google Scholar] [PubMed]
- de Haas M, Thurik FF, van der Ploeg CP, Veldhuisen B, Hirschberg H, Soussan AA, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: Prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:1-8.
[Crossref] [Google Scholar] [PubMed]
- Haimila K, Sulin K, Kuosmanen M, Sareneva I, Korhonen A, Natunen S, et al. Targeted antenatal anti-D prophylaxis program for RhD-negative pregnant women-outcome of the first two years of a national program in Finland. Acta Obstet Gynecol Scand. 2017;96(10):1228-1233.
[Crossref] [Google Scholar] [PubMed]
- Sorensen K, Kjeldsen-Kragh J, Husby H, Akkok CA. Determination of fetal RHD type in plasma of RhD negative pregnant women. Scand J Clin Lab Invest. 2018;78(5):411-416.
[Crossref] [Google Scholar] [PubMed]
- Sorensen K, Stjern HE, Karlsen BAG, Tomter G, Ystad I, Herud I, et al. Following targeted routine antenatal anti-D prophylaxis, almost half of the pregnant women had undetectable anti-D prophylaxis at delivery. Acta Obstet Gynecol Scand. 2022;101(4):431-440.
[Crossref] [Google Scholar] [PubMed]
- Wikman AT, Tiblad E, Karlsson A, Olsson ML, Westgren M, Reilly M. Non-invasive single-exon fetal RHD determination in a routine screening program in early pregnancy. Obstet Gynecol. 2012;120(2):227-234.
[Crossref] [Google Scholar] [PubMed]
- Thyer J, Wong J, Thomson A, Bell B, Hyland C, Challis D. Fifty years of RhD immunoglobulin (anti-D) therapy in Australia: Celebrating a public health success story. Med J Aust. 2018;209(8):336-339.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372: 71.
[Crossref] [Google Scholar] [PubMed]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349.
[Crossref] [Google Scholar] [PubMed]
- Dettori JR, Norvell DC, Chapman JR. Seeing the forest by looking at the trees: How to interpret a meta-analysis forest plot. Global Spine J. 2021;11(4):614-616.
[Crossref] [Google Scholar] [PubMed]
- Bowman JM, Pollock JM. Antenatal prophylaxis of Rh isoimmunization: 28-weeks'-gestation service program. Can Med Assoc J. 1978;118(6):627-630.
[Crossref] [Google Scholar] [PubMed]
- Chaffe B, Ford J, Bills V. Routine antenatal anti-D prophylaxis and patient compliance with the two-dose regimen. Transfus Med. 2007;17(5):399-403.
[Crossref] [Google Scholar] [PubMed]
- Clausen FB, Steffensen R, Christiansen M, Rudby M, Jakobsen MA, Jakobsen TR, et al. Routine non-invasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women-2 years of screening experience from Denmark. Prenat Diagn. 2014;34(10):1000-1005.
[Crossref] [Google Scholar] [PubMed]
- Damkjaer MB, Perslev A, Clausen FB, Dziegiel MH, Jørgensen FS. Study of compliance with a new, targeted antenatal D immunization prevention programme in Denmark. Vox Sang. 2012;103(2):145-149.
[Crossref] [Google Scholar] [PubMed]
- Glazebrook B, Akers C, Bielby L, Bastin K, Von Wielligh K, Daly J. Quality audit of the guidelines for the use of RhD immunoglobulin in obstetrics: Are we getting it right? Aust N Z J Obstet Gynaecol. 2020;60(4):504-508.
[Crossref] [Google Scholar] [PubMed]
- MacKenzie IZ, Bowell P, Gregory H, Pratt G, Guest C, Entwistle CC. Routine antenatal Rhesus D immunoglobulin prophylaxis: The results of a prospective 10 year study. Br J Obstet Gynaecol. 1999;106(5):492-497.
[Crossref] [Google Scholar] [PubMed]
- MacKenzie IZ, Findlay J, Thompson K, Roseman F. Compliance with routine antenatal rhesus D prophylaxis and the impact on sensitisations: Observations over 14 years. Bjog. 2006;113(7):839-843.
[Crossref] [Google Scholar] [PubMed]
- MacKenzie IZ, Dutton S, Roseman F. Evidence to support the single-dose over the two-dose protocol for routine antenatal anti-D Rhesus prophylaxis: A prospective observational study. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):42-46.
[Crossref] [Google Scholar] [PubMed]
- Rowley M, Davies T, Hawkins T, Hibbert J, Grant-Casey J. 2013 national comparative audit of anti-D IG prophylaxis. Transfus Med. 2014;24:18.
[Crossref]
- Rudensky B, Mazaki E, Na'amad M, Samueloff A. Lack of anti-D in women at birth following antepartum immune globulin prophylaxis. Eur J Obstet Gynecol Reprod Biol. 2003;107(1):45-46.
[Crossref] [Google Scholar] [PubMed]
- Trolle B. Prenatal Rh-immune prophylaxis with 300 micrograms immune globulin anti-D in the 28th week of pregnancy. Acta Obstet Gynecol Scand. 1989;68(1):45-47.
[Crossref] [Google Scholar] [PubMed]
- White SW, Cheng JC, Penova-Veselinovic B, Wang C, White M, Ingleby B, et al. Single dose vs. two-dose antenatal anti-D prophylaxis: A randomised controlled trial. Med J Aust. 2019;211(6):261-265.
[Crossref] [Google Scholar] [PubMed]
- Wikman A, Mörtberg A, Jalkesten E, Jansson Y, Karlsson A, Tiblad E, et al. Altered strategy of prophylactic anti-D administration in pregnancy to cover term and post-term - a pilot study. Vox Sang. 2021;116(9):1005-1011.
[Crossref] [Google Scholar] [PubMed]
- Eklund J, Hermann M, Kjellman H, Pohja P. Turnover rate of anti-D IgG injected during pregnancy. Br Med J (Clin Res Ed). 1982;284(6319):854-855.
[Crossref] [Google Scholar] [PubMed]
- Bichler J, Schöndorfer G, Pabst G, Andresen I. Pharmacokinetics of anti-D IgG in pregnant RhD-negative women. BJOG. 2003;110(1):39-45.
[Crossref] [Google Scholar] [PubMed]
- MacKenzie IZ, Roseman F, Findlay J, Thompson K, Jackson E, Scott J, et al. The kinetics of routine antenatal prophylactic intramuscular injections of polyclonal anti-D immunoglobulin. BJOG. 2006;113(1):97-101.
[Crossref] [Google Scholar] [PubMed]
- Prevention of Rh sensitization. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1971;468:3-36.
[PubMed]
- Witter FR, Shirey RS, Nicol SL, Ness PM. Post-injection kinetics of antepartum Rh immune globulin. Am J Obstet Gynecol. 1990;163(3):784-786.
[Crossref] [Google Scholar] [PubMed]
- van der Schoot CE, de Haas M, Clausen FB. Genotyping to prevent Rh disease: Has the time come? Curr Opin Hematol. 2017;24(6):544-550.
[Crossref] [Google Scholar] [PubMed]
- Neovius M, Tiblad E, Westgren M, Kublickas M, Neovius K, Wikman A. Cost-effectiveness of first trimester non-invasive fetal RHD screening for targeted antenatal anti-D prophylaxis in RhD-negative pregnant women: A model-based analysis. BJOG. 2016;123(8):1337-1346.
[Crossref] [Google Scholar] [PubMed]
- Moise KJ, Hashmi SS, Markham K, Argoti PS, Bebbington M. Cell free fetal DNA to triage antenatal rhesus immune globulin: Is it really cost-effective in the United States? Prenat Diagn. 2019;39(3):238-247.
[Crossref] [Google Scholar] [PubMed]
- Gordon LG, Hyland CA, Hyett JA, O'Brien H, Millard G, Flower RL, et al. Noninvasive fetal RHD genotyping of RhD negative pregnant women for targeted anti-D therapy in Australia: A cost-effectiveness analysis. Prenat Diagn. 2017;37(12):1245-1253.
[Crossref] [Google Scholar] [PubMed]
Citation: Tee KM, Jackson DE (2024) The Effectiveness and Compliance of Routine Antenatal RhIG Prophylaxis using Different Strategies: A Systematic Review and Meta-analysis. J Blood Disord Transfus. 15.573.
Copyright: © 2024 Tee KM et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

