Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 0, Issue 0
The Availability of Frozen Cord Blood Mononuclear Cells for Erythrocytes Generation In Vitro
Ju Mi Park1,2, Kyeongwon Jo1,2, Suyeon Kim2,3, Arim Shin1, Neha Kaushik4, Ki Young Ryu5, Ji Myung Kim6* and Eun Jung Baek1,2,3*2Department of Research and Development, ArtBlood Inc., Seoul, South Korea
3Department of Laboratory Medicine, Hanyang University, Seoul, South Korea
4Department of Biotechnology, The University of Suwon, Hwaseong, South Korea
5Department of Obstetrics and Gynecology, Hanyang University, Seoul, South Korea
6Department of Laboratory Medicine, Chungnam National University Hospital, Daejeon, South Korea
Received: 19-Jul-2023, Manuscript No. JBDT-23-22251; Editor assigned: 21-Jul-2023, Pre QC No. JBDT-23-22251 (PQ); Reviewed: 11-Aug-2023, QC No. JBDT-23-22251; Revised: 18-Aug-2023, Manuscript No. JBDT-23-22251 (R); Published: 25-Aug-2023, DOI: 10.4172/2155-9864.23.S1.001
Abstract
Efforts have been made toward the in vitro generation of stem cell–derived Red Blood Cells (RBCs) to overcome the worldwide blood shortage for transfusion. As starting stem cell sources, primary Cluster of Differentiation CD34+ cells derived from umbilical Cord Blood (CB) are well known for its limited proliferation capacity. Blood Mononuclear Cells (MNCs) also can be used and many frozen CB MNCs are banked, but their value as a source for generating RBCs has not been evaluated. Here, we cultured fresh and frozen CB-MNCs to generate RBCs. The viability of frozen-thawed CB-MNCs was approximately 80%, and the proliferation rates were not significantly different from those of fresh MNCs. The presence of macrophages among the MNCs did not significantly increase erythroblast expansion. This suggests that frozen CB-MNCs are comparable to fresh MNCs in terms of RBC generation, but establishing erythroid cell lines would be necessary to overcome the proliferation capacity.
Keywords
Erythropoiesis; Erythrocytes; Blood mononuclear cells; Transfusion; In vitro cell culture
Introduction
Several culture methods are available for the in vitro production of Red Blood Cells (RBCs). The first protocol for pure RBC production was developed using CD34+ cells isolated from umbilical Cord Blood (CB), Bone Marrow (BM), and Peripheral Blood (PB) [1,2]. Cluster of Differentiation CD34+ cells isolated from CB are commonly used for producing RBCs without further genetic manipulation. However, the isolation of CD34+ cells is costly, time-consuming, and more complex concerning (GMP) Good Manufacturing Practice requirements.
Therefore, Mononuclear Cells (MNCs) have been used as an alternative resource for producing RBCs [3-5]. Most importantly, a large number of CB units are stored in CB banks, and these units are discarded after storing for certain duration. Therefore, it would be practical and economical to use MNCs isolated from discarded CB for RBC production [6]. Although many review articles have cited the results of several studies showing large-scale erythroblast cultures using MNCs, the reproducibility of the results has not been verified [7,8].
In our previous experiment, we successfully generated erythrocytes from CB-CD34+ cells using bioreactors and attempted to develop RBCs from MNCs rather than from CD34+ cells because CBMNCs are easily accessible and abundant. In this study, we aimed to determine the appropriate protocol wherein erythroid expansion using fresh or frozen CB-MNCs is evident, reproducible, and optimal and to evaluate whether the expansion ability is comparable between fresh and frozen CB-MNCs [9].
Materials and Methods
CB samples were collected after obtaining written consent from 15 healthy pregnant women. This study was approved by the Institutional Review Board of Hanyang University (IRB No. HYU-2019-07-006). The CB-MNCs were purified by the concentration gradient method using Ficoll-Paque (GE Healthcare, Uppsala, Sweden). Contaminated RBCs in the CB-MNCs were removed using RBC lysis buffer (BioLegend, San Diego). The separated CB-MNCs were either used directly for cultivation or cryopreserved in a liquid nitrogen tank. Before using for cultivation, frozen CB-MNCs were thawed in a 37ºC water bath for 2 min and then centrifuged in Iscove’s modified Dulbecco’s medium (Gibco) Grand Island Biological Company containing 10% fetal bovine serum.
Media compositions are listed in Table 1. In the lab-modified protocol, CB-MNCs isolated from 12 CB samples were seeded in erythroid induction medium (EI medium) at 1 × 106 cells/ml (4 × 106 cells/4 ml in 6-well culture plates); until day 8, half of the medium was changed every 2 days. For the proliferation of CB-MNC-derived erythroblasts, the medium was replaced with Exp or Exp+b medium that is modified from the Bristol Erythroid Line-Adult (BEL-A) cell culture method for the long-term proliferation of erythroblasts. The cells were then cultured for 30 days [10].
| Lab modified protocol | MNC culture protocol A | MNC culture protocol B | ||
|---|---|---|---|---|
| Erythroid induction (EI) medium | Expansion Medium | IMDM (Gibco) | StemSpan (STEM CELL) | |
| IMDM (with 1% P/S) | Exp | Exp+b | P/S 100 µg/ml (Gibco) | P/S 1% (Gibco) |
| FBS 1% (Hyclone) | StemSpan (STEM CELL) | StemSpan (STEM CELL) | holo-transferrin 300 ng/ml (sigma) | IGF-1 40 ng/ml (R&D System) |
| holo-Transferrin 200 µg/ml (sigma) | P/S 1% (Gibco) | P/S 1% (Gibco) | sodium pyruvate 100 µM (sigma) | Cholesterol-rich lipid mix 40 µg/ml (sigma) |
| insulin 10 µg/ml (sigma) | EPO 3 IU/ml (Calbiochem) | EPO 6 IU/ml (Calbiochem) | cholesterol 100 ng/ml (sigma) | EPO 2 U/ml (Calbiochem) |
| heparin 3 IU/ml (sigma) | SCF 50 ng/ml (R&D System) | SCF 10 ug/ml (R&D System) | L-a-phosphatydilcholine 5 µg/ml (sigma) | rhSCF 100 ng/ml (R&D System) |
| EPO 6 IU/ml (Calbiochem) | HC 1 µM (sigma) | IL-3 1 µg/ml (R&D System) | Oleic acid 4 ng/ml (sigma) | Dexamethasone 1 µM (sigma) |
| SCF 10 µg/ml (R&D System) | L-glutamine 2 mM (Gibco) | |||
| IL-3 1 µg/ml (R&D System) | Human Serum Albumin 0.1% (sigma) Insulin 10 µg/ml (sigma) EPO 2 U/ml (Calbiochem) rhSCF 100 ng/ml (R&D System) Dexamethasone 1 µM (sigma) Nucleosides Trace elements |
|||
| Abbreviations: CB- Cord Blood; IMDM- Iscove’s Modified Dulbecco’s Medium; SFEM- StemSpan SFEM II (StemCell technologies); Exp- Expansion medium; Exp+b- Expansion medium + concentration of BEL-A medium cytokine | ||||
Table 1: Components of medium for CB-MNC culture.
Results and Discussion
In two previous experimental protocols, MNCs (5 × 106 cells/ml) were seeded in 24-well plates following MNC culture protocols A and B. MNC culture protocol A was taken from the experiment of Heshusius et al. and MNC culture protocol B was taken from the experiment of Leberbauer et al. Half of the medium was changed every 2 days [3,4]. The different reagents from the original protocols and the reagents used for flow cytometry were shown in the Table 1.
Cell culture experiments were performed using at least three CB samples under each condition. The culture experiments of MNC culture protocols A and B were conducted 4-5 times, similar to the number of iterations in the reference paper, as an experiment for reproducibility evaluation of the previous study results.
As results, during culture days 3-5, proerythroblasts appeared, and from day 7, proerythroblasts and basophilic erythroblasts expanded homogeneously in the EI medium (Figure 1A). During the first 5 days of culture, the cell numbers decreased because of the loss of nonerythroid lineage cells included in the CB-MNCs. The proliferation rate began to recover on day 7. Cells proliferated up to 1.4 × 102-fold in the Exp medium and 2.5 × 102-fold in the Exp+b medium for 30 days. Cell proliferation was significantly higher in the Exp+b medium than in the Exp medium on days 14-21 (Figure 1B). From day 17, erythroblasts in the Exp+b medium gradually decreased, and nonerythroid cells began to proliferate (Figures 1A and 1B).

Figure 1:Optimization of CB-MNC culture protocols for RBC production.
A); Representative morphology of cultured cells. Blue arrows indicate monocyte/macrophages or myeloid cells. Scale bar=50 μm; 400X magnification.
B); Cumulative cell numbers (n=14, Wilcoxon’s signed rank test, paired t-test; p<0.05, Student’s t-test; *p<0.05, mean±SEM). C,D); Percentages per
quadrant of CD71/CD235a (n=11). E,F); Analysis of enucleation rates by flow cytometric analysis of CD235a+/nuclei- cells, RBC in Exp or Exp+b.
(n=8, p>0.05). G,H); Cumulative cell numbers and cell viability in MNC culture protocol A (n=6) and protocol B (n=5). I); Representative images of
cultured cells on day 17, showing more macrophages than erythroid cells. Wright-Giemsa staining. Scale bar=50 μm; 400X magnification. Red arrows
indicate erythroid cells; blue arrows indicate monocyte/macrophages or myeloid cells. J); Comparison of erythroblast percentages at the indicated
culture days (MNC culture protocol A, n=5; protocol B, n=4; Student’s t-test, **p<0.01; mean ± SEM).
CB- Cord Blood; CD- Cluster Of Differentiation; MNC- Mononuclear Cell; RBC- Red Blood Cell; SEM- Standard Error of the Mean.
In flow cytometry analysis, CD71+CD235a- cells (erythroid progenitor cells) were 39% on day 7 and CD71+CD235a+ cells (mature erythroid cells) were approximately 31% on day 17 in both media (Figures 1C and 1D). The purity of erythroid cells was highest as 76.2% on day 17. On day 9, the mean percentage of CD11b+CD13- cells (monocytes/ macrophages) and CD11b-CD13+ cells (myeloid cells) was 3.6% and 8.3%, respectively. However, by day 24, CD11b+CD13- cells increased up to 15% in the Exp medium and up to 5.3% in the Exp+b medium (data not shown), suggesting that myeloid cells and monocytes survived relatively at the terminal culture period. Although macrophages are known to assist erythropoiesis in vivo, conditions with a lower percentage of macrophages in the Exp+b medium showed significantly higher cell proliferation and enucleation rates (CD235a+Nucred- cells) (Figures 1E and 1F). Next, we increased the seed number of CB-MNCs from 1 × 106 cells/ml to 5 × 106 cells/ml following previously reported protocols. However, high proliferation rates, such as 3 × 107-fold as reported previously, was not achieved [3]. Therefore, we could induce and expand erythroid cells from CB-MNCs using the lab-modified protocol; however, the degree of expansion was lower than that previously reported.
Next, we followed the previously reported protocols to evaluate the reproducibility. After starting MNC culture, the cell numbers decreased under both media conditions (Figure 1G). Although we expected erythroblasts to proliferate from day 7, the expansion rate did not recover and steadily decreased; cell cultures were eventually discontinued owing to low cell numbers. In both media conditions, cells (5 × 106 cells/ml) were seeded at the start, but their numbers decreased to 7 × 105 cells/ml and 1 × 106 cells/ml by day 19 when following MNC culture protocols A and B, respectively. In MNC culture protocols A and B, only one and two out of six and five samples, respectively, were cultured for 24 and 26 days (Figure 1G). The cell viability did not exceed 80% during culture in either medium (Figure 1H). Because of the continuous decrease in cell number, the differentiation stage could not be confirmed using flow cytometry. Morphological analyses revealed the formation of pro- to basophilic erythroblasts on day 7. However, the percentage of erythroblasts decreased from days 7 to 14 (Figure 1I), and the cells in protocol B media showed a significantly decreased erythroblast percentage (Figures 1I and 1J). Therefore, in our repeated experiments using different CB cases, CB-MNCs cultured in MNC culture protocol A and B media did not proliferate as much as in previous studies, with increased macrophages derived from MNCs.
In CB banks, many frozen CB MNCs are stored with very low usage. Even though the number of viable CD34+ cells decreases after thawing, the effects of frozen MNCs versus fresh MNCs on erythroblast proliferating potential have not been reported [11,12]. The viability of fresh CB-MNCs decreased to 75% on day 3, whereas that of frozen MNCs decreased rapidly to 52% (Figure 2A). However, viability under both conditions recovered to 84% on day 9. From day 11, the viability of thawed CB-MNCs was lower than that of fresh cells in both media. The cell proliferation rates were higher in the Exp+b medium in both fresh and frozen samples, demonstrating that medium composition is more important than cell freshness (Figure 2B).
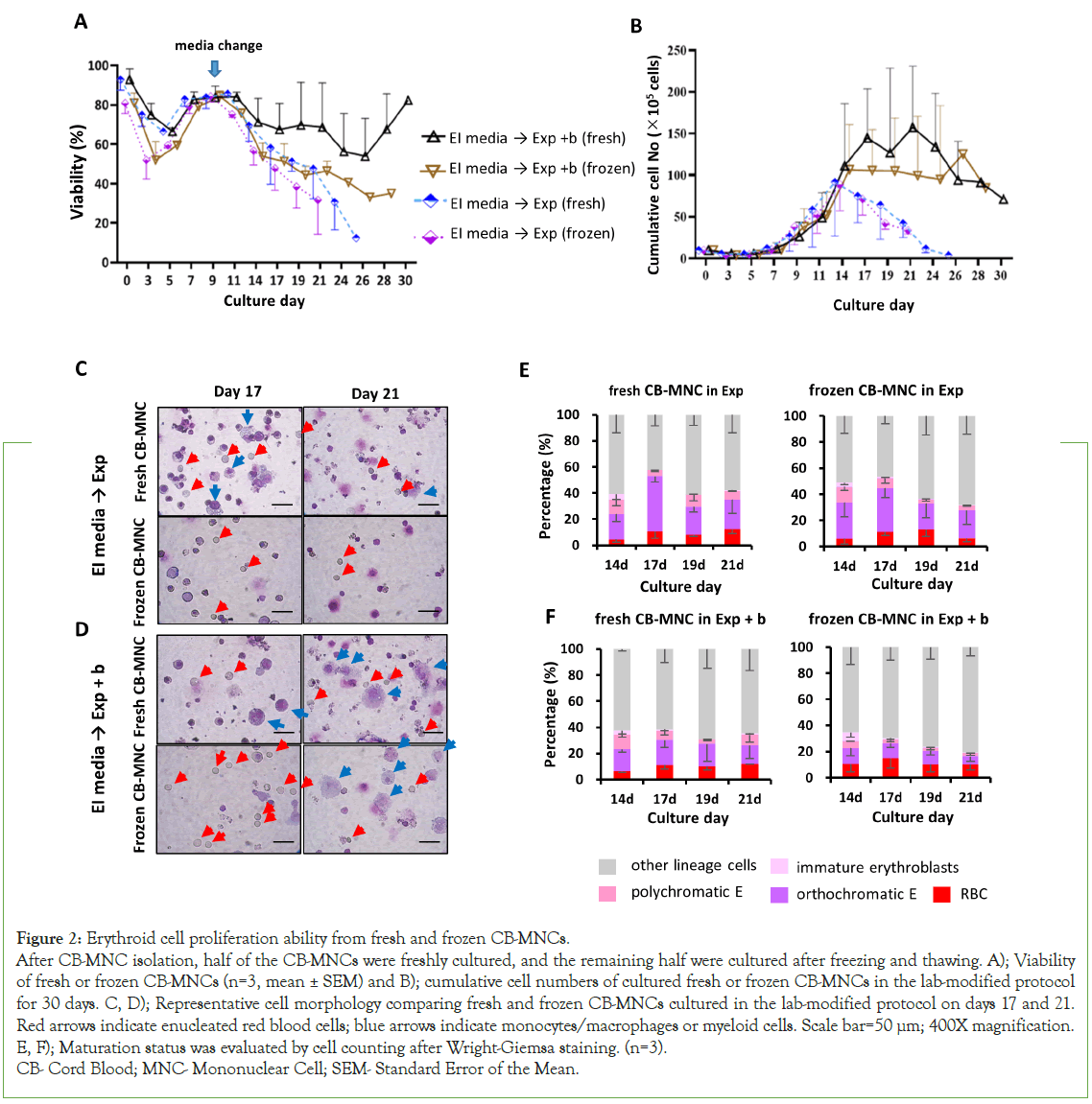
Figure 2:Erythroid cell proliferation ability from fresh and frozen CB-MNCs.
After CB-MNC isolation, half of the CB-MNCs were freshly cultured, and the remaining half were cultured after freezing and thawing. A); Viability
of fresh or frozen CB-MNCs (n=3, mean ± SEM) and B); cumulative cell numbers of cultured fresh or frozen CB-MNCs in the lab-modified protocol
for 30 days. C, D); Representative cell morphology comparing fresh and frozen CB-MNCs cultured in the lab-modified protocol on days 17 and 21.
Red arrows indicate enucleated red blood cells; blue arrows indicate monocytes/macrophages or myeloid cells. Scale bar=50 μm; 400X magnification.
E, F); Maturation status was evaluated by cell counting after Wright-Giemsa staining. (n=3).
CB- Cord Blood; MNC- Mononuclear Cell; SEM- Standard Error of the Mean.
The mean erythroblast proliferation in both samples was 1.6-1.8 × 102- fold on days 21-24, with no significant differences between the fresh and frozen conditions (paired t-test, p>0.05; Figure 2B). Moreover, the morphology of fresh or frozen cells showed no difference (Figures 2C and 2D). Both fresh and frozen cells were similarly differentiated into RBCs, with enucleation rates of approximately 13% in both media (paired t-test, p>0.05) (Figures 2E and 2F). Therefore, both fresh and frozen CB-MNCs are useful for studying erythropoiesis.
When reviewing previous reports, we found that the high proliferation power (3 × 107-fold) was not calculated from MNC numbers but by extrapolation, assuming that the culture started from pure erythroblasts, not MNCs. However, MNCs contain very low percentages of erythroblasts or stem cells, which correspond to 0.2%- 0.5% cells per 1 ml of PB [13]. Therefore, cell numbers during the first five culture days are decreased owing to the death of non-erythroid cells, which contributes to the lower number of final erythroid cells. Therefore, we believe that the recalculated practical proliferation power into erythroid cells of MNCs was 3 × 104-fold over 25 days [14,15].
Conclusion
Even though several factors such as donor source variation and the difference in medium additives including serum can affect the failure to reproduce prior results, we demonstrate that the MNC is not good enough for RBC generation.
However, the MNC cell number is at least 100-fold higher than CD34+ cells and the erythroblast expansion ability of the frozen MNCs was comparable to that of fresh MNCs. The stored CB-MNC sources could be useful for lab scale research.
Author Contributions
Conception and design: Eun Jung Baek. Collection and/or assembly of data: Ju mi Park, Kyeong won Jo and Arim Shin. Data analysis and interpretation: Suyeon Kim, Eun Jung Baek. Provision of study material: Ki Young Ryu, Manuscript review and editing: Neha Kaushik. Manuscript writing: Ju mi Park, Ji Myung Kim and Eun Jung Baek.
Acknowledgments: We would like to thank Dr. Jaehyung Yoo for providing samples.
Funding information: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1A2C2090053).
Conflicts of interest: Eun Jung Baek is the founder and CEO of ArtBlood, Inc. Ju Mi Park, kyeong won Jo and Suyeon Kim are employee of ArtBlood. The other authors declare no conflict of interest.
ORCID
Eun Jung Baek, 0000-0001-5477-6486
Ji Myung Kim, 0000-0003-0530-3425
References
- Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: What is the future in transfusion? Transfus Med Rev. 2007;21(2):91-100.
[Crossref] [Google Scholar] [PubMed]
- Baek EJ, Kim HS, Kim JH, Kim NJ, Kim HO. Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion. 2009;49(11):2285-2295.
[Crossref] [Google Scholar] [PubMed]
- Heshusius S, Heideveld E, Burger P, Thiel-Valkhof M, Sellink E, Varga E, et al. Large-scale in vitro production of red blood cells from human peripheral blood mononuclear cells. Blood Adv. 2019;3(21):3337-3350.
[Crossref] [Google Scholar] [PubMed]
- Leberbauer C, Boulme F, Unfried G, Huber J, Beug H, Mullner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105(1):85-94.
[Crossref] [Google Scholar] [PubMed]
- vanden AE, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34− cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010;95(9):1594.
[Crossref] [Google Scholar] [PubMed]
- Lee MN, Kim K-H, Kim B-G, Goh R-Y, Kim JN. Improving Storage Policy in Korean Public Cord Blood Banks: Comparison of Quality between Long-Term and Short-Term Storage of Cord Blood. Korean J Blood Transfus. 2020;31(2):119-130.
- Severn CE, Toye AM. The challenge of growing enough reticulocytes for transfusion. ISBT Sci Ser. 2018;13(1):80-86.
- Pellegrin S, Severn CE, Toye AM. Towards manufactured red blood cells for the treatment of inherited anemia.Haematologica. 2021;106(9):2304.
[Crossref] [Google Scholar] [PubMed]
- Han SY, Lee EM, Lee J, Lee H, Kwon AM, Ryu KY, et al. Red cell manufacturing using parallel stirred‐tank bioreactors at the final stages of differentiation enhances reticulocyte maturation. Biotechnol Bioeng. 2021;118(5):1763-1778.
[Crossref] [Google Scholar] [PubMed]
- Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M, et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat Commun. 2017;8:14750.
[Crossref] [Google Scholar] [PubMed]
- Beshlawy A, Metwally HG, Khalek K, Hammoud RF, Mousa SM. The effect of freezing on the recovery and expansion of umbilical cord blood hematopoietic stem cells. Exp Clin Transplant. 2009;7(1):50-55.
[Crossref] [Google Scholar] [PubMed]
- Sartor M, Antonenas V, Garvin F, Webb M, Bradstock K. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Transplant. 2005;36(3):199-204.
[Crossref] [Google Scholar] [PubMed]
- Kikuchi-Taura A, Soma T, Matsuyama T, Stern DM, Taguchi A. A new protocol for quantifying CD34+ cells in peripheral blood of patients with cardiovascular disease. Tex Heart Inst J. 2006;33(4):427.
[Crossref] [Google Scholar] [PubMed]
- Baker M. Reproducibility: Respect your cells! Nature. 2016;537(7620):433-435.
[Crossref] [Google Scholar] [PubMed]
- Koh SK, Park J, Kim S-E, Lim Y, Phan M-TT, Kim J, et al. Natural killer cell expansion and cytotoxicity differ depending on the culture medium used. Ann Lab Med. 2022;42(6):638-649.
[Crossref] [Google Scholar] [PubMed]
Citation: Park JM, Jo K, Kim S, Shin A, Kaushik N, Ryu KY, et al. (2023) The Availability of Frozen Cord Blood Mononuclear Cells for Erythrocytes Generation In Vitro. J Blood Disord Transfus. S1.001.
Copyright: © 2023 Park JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

