Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2020) Volume 8, Issue 6
Surgical Treatment for Nutcracker Syndrome associated with Pelvic Congestion: Two Case Reports
Ryo Nakamura, Kentaro Honda*, Mitsuru Yuzaki, Takahiro Fijimoto and Yoshiharu NishimuraReceived: 25-Sep-2020 Published: 25-Oct-2020, DOI: 10.35248/2329-6925.20.8.397
Introduction
NCS is a rare clinical entity caused by compression of the Left Renal Vein (LRV) by the superior mesenteric artery (SMA) and the abdominal aorta, exhibiting various symptoms such as hematuria, back pain, and lower abdominal pain [1]. Additionally, PCS is a chronic pelvic pain condition caused by gonadal venous insufficiency; NCS is one cause for PCS [2]. We report on two cases of NCS with PCS, and performed gonadal vein transposition and/ or renal vein transposition with good results.
Case Report
Case 1 was a 61 year-old female, complaining of chronic left lower abdominal pain during exercise. She was diagnosed with NCS, based on a presentation of hematuria and congestion of the pelvic veins. Balloon dilation of the LRV was performed with little improvement. Although the symptoms recurred and balloon dilatation was performed again one year later, the symptoms recurred within two months, and the patient was referred for cardiovascular surgery. Contrast-Enhanced Computed Tomography (CECT) demonstrated the compression of the LRV between the Superior Mesenteric Artery (SMA) and abdominal aorta, renal vein stenosis caused by repeated endovascular treatment, and dilatation of the left gonadal vein with a diameter of approximately 10 mm (Figure 1a). Venography revealed that most of the LRV blood reflexedinto the left gonadal vein, causing pelvic congestion, and finally the blood flowed from the right gonadal vein into the Inferior Vena Cava (IVC) via intrapelvic communication (Figure 1b). The intravenous pressure difference between the LRV and IVC was 3 mmHg, which was consistent with the NCS diagnosis criteria [3]. The operation was scheduled for transposition of the LRV to the distal IVC and gonadal vein ligation. A partial midline laparotomy was made, revealing dilated LRV and gonadal vein under the retroperitoneum. The LRV appeared to be degenerated, with a white fibrotic change, likely representing inflammation due to the second balloon dilation procedure (Figure 1c). This fibrotic change may restrict the blood flow, so we decided to modify the planned operation to gonadal vein transposition. After the heparinization, the distal side of the 10 mm dilated gonadal vein was ligated and cut, and the proximal side was anastomosed to the IVC with 5-0 polypropylene running suture, with the IVC partially clamped, so that LRV blood could flow into the IVC via the gonadal vein (Figure 1d). Shortly following the operation, her left lower abdominal pain disappeared and renal function did not worsen. Postoperative CECT confirmed the communication between the gonadal vein and IVC, and the dilated collaterals in the pelvis and right gonadal vein disappeared. One year has passed without any recurrence of symptoms.
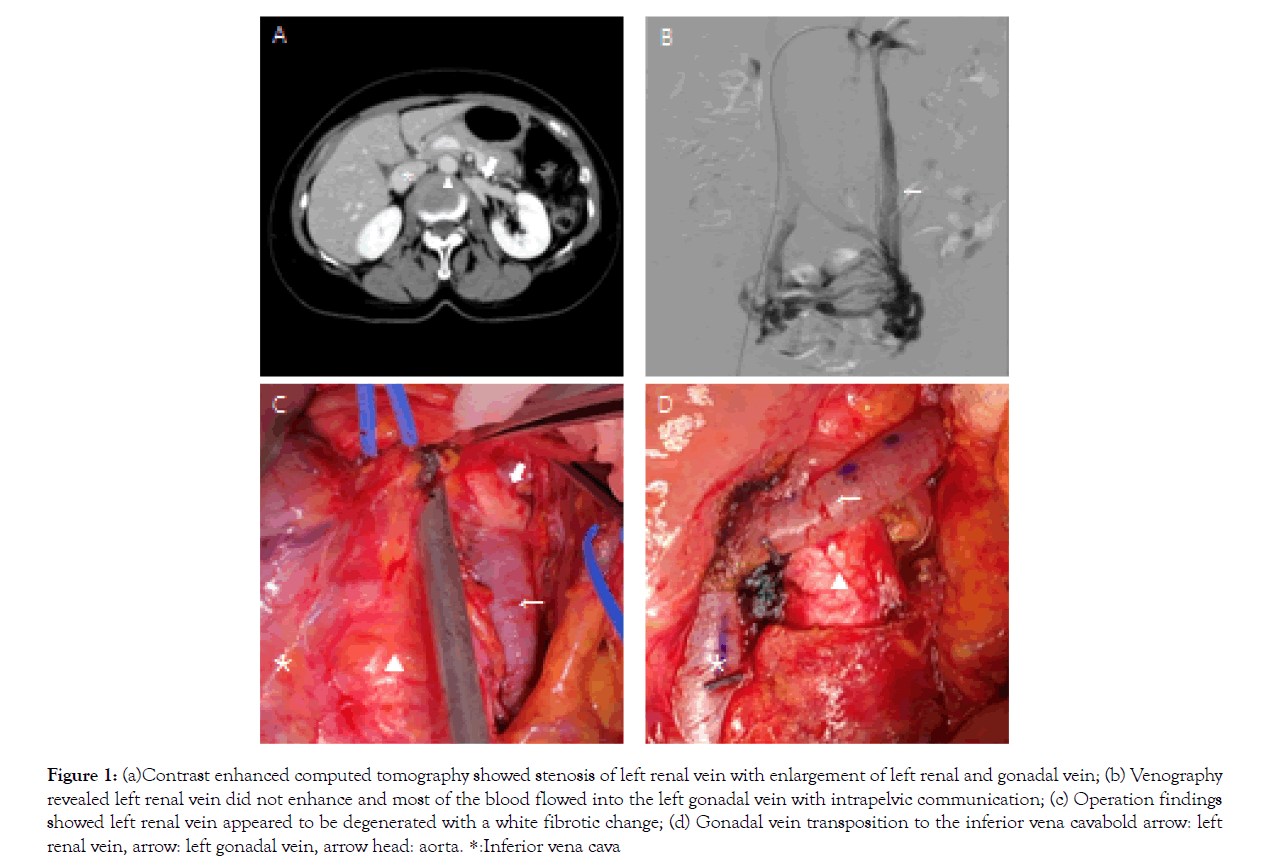
Figure 1: (a)Contrast enhanced computed tomography showed stenosis of left renal vein with enlargement of left renal and gonadal vein; (b) Venography revealed left renal vein did not enhance and most of the blood flowed into the left gonadal vein with intrapelvic communication; (c) Operation findings showed left renal vein appeared to be degenerated with a white fibrotic change; (d) Gonadal vein transposition to the inferior vena cavabold arrow: left renal vein, arrow: left gonadal vein, arrow head: aorta. *:Inferior vena cava
Case 2 was a 46-year-old female with a history of hematuria since childhood. Gross hematuria was observed 3 years ago, and she had back pain and left lower abdominal pain, making it difficult to work. CECT revealed compression of the LRV between the SMA and abdominal aorta, and intrapelvic communication of the left and right gonadal veins, similar to Case 1. Venography showed that the pressure difference between the LRV and IVC was 5 mmHg, with increased pressure on the LRV. We planned LRV transposition and left gonadal vein ligation. A laparotomy was performed, and the left gonadal vein and LRV were found under the retroperitoneum.
The LRV was connected to the cranial side of the IVC than usual (Figure 2a), and was compressed by the SMA and abdominal aorta. The intraoperative pressure gradient was approximately 3 mmHg between the LRV and IVC. Following heparinization, the LRV was resected from the IVC, and was anastomosed 3 cm distal to the original attachment with 5-0 polypropylene running suture (Figure 2b). However, the pressure difference still remained at around 3 mmHg between the LRV and IVC, and we considered that the treatment for NCS might be insufficient, despite the gonadal vein ligation done for PCS. We then added a gonadal vein ligation and gonadal vein transposition to the IVC (Figure 2c), and finally the pressure difference disappeared. Shortly after the operation, the lower abdominal pain and hematuria disappeared, and she was discharged without any complication. Postoperative CECT and MRI confirm the communication between LRV and IVC, and the reduction of the communication of intrapelvic collateral vein. One year has passed without any recurrence of symptoms.
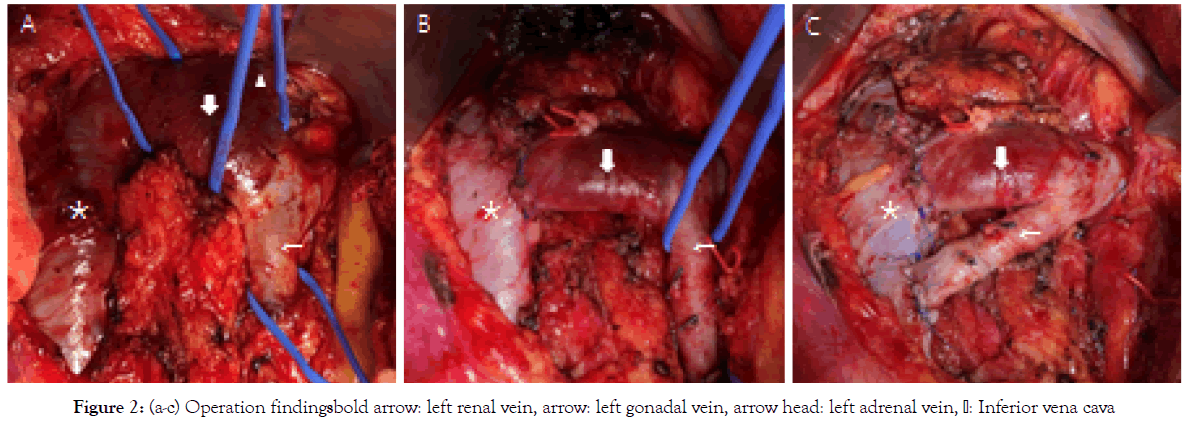
Figure 2: Figure 2: (a-c) Operation findingsbold arrow: left renal vein, arrow: left gonadal vein, arrow head: left adrenal vein, Inferior vena cava
Results and Discussion
There is no evidence that symptoms will be improved by each treatment alone in the case of NCS associated with PCS [4], and it is useful to perform both treatments for NCS and PCS at the same time.
NCS is characterized by impeded outflow from the LRV into the IVC, due to extrinsic LRV compression, often accompanied by demonstrable lateral dilatation and medial narrowing [1], which bring about hematuria and left back pain. The concept was first anatomically reported by Grant in 1937 [5],and was reported by El Sader and Mina in 1950 as a human condition [6]. On the other hand, PCS was first proposed by Taylor in 1950 [7],and is a pathological condition causing regurgitation into the pelvis due to pelvic and gonadal varices. One of the causes is the congestion of LRV located upstream, i.e., NCS.
The fundamental concept of a therapeutic strategy for NCS is to relieve the congestion of the LRV. Surgical options to reduce the LRV pressure include LRV transposition, renocaval or gonadocaval bypass, and gonadal vein transposition, in cases with a dilated gonadal vein4). External stent of the LRV [8], renal auto transplantation and mesoaortic transposition are the other surgical options. Internal stent or balloon angioplasty of the LRV are the endovascular options4). The therapeutic strategy for PCS is to reduce the reflex blood flow in the gonadal vein, and the surgical options include clipping or ligation of the dilated gonadal vein, transposition into the IVC; coiling is the endovascular treatment. In cases of PCS without NCS, treatment for PCS often improves the symptoms; however, in cases of PCS with NCS, treatment for PCS alone leads to increased congestion in the LRV, resulting in worsening the symptoms of the NCS. On the other hand, there is no evidence that NCS treatment alone will improve the patient’s symptoms, as reflex of the gonadal vein still exists.
Both of our cases were patients who had NCS associated with PCS. Case 1 caused narrowing of the LRV via multiple endovascular treatments, resulting in dilatation and regurgitation of the gonadal vein as a drainage vein. The gonadal vein diameter was large enough to anastomose to the IVC, so we performed gonadal vein transposition to serve as a drainage pathway for the renal vein and then ligated the distal side of the gonadal vein in order to eliminate the pelvic congestion. In Case 2, we performed renal vein transposition; however, an intraoperative pressure measurement showed that the pressure gradient between the LRV and IVC still remained. We added the gonadal vein transposition and gonadal vein ligation both as an accessory drainage pathway for the renal vein and to eliminate the pelvic congestion. The symptoms of the NCS and PCS improved immediately following surgery in both cases, and the CECT findings showed the connection of the LRV and the IVC, along with the gonadal vein and the IVC, along with the disappearance of the contralateral collateral pelvic circulation.
As an alternative intervention for NCS with PCS, it may be effective to perform the stenting in the LRV and coiling in the gonadal vein4). Endovascular treatments have some disadvantages, such as stent thrombosis, intrastent stenosis, stent migration and the need for antiplatelet therapy. Additionally, long term results are not clear, so further evidence is required.
Conclusion
We performed surgical treatments for two cases of NCS with PCS, resulting in good results. For a patient presenting with both syndromes, it is useful to perform surgical treatment for each syndrome concurrently.
REFERENCES
- Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552-559.
- Jeanneret C, Beier K, von Weymarn A, Traber J. Pelvic congestion syndrome and left renal compression syndrome-clinical features and therapeutic approaches. Vasa. 2016;45(4):275-282.
- Ananthan K, Onida S, Davies AH. Nutcracker syndrome: An update on current diagnositic criteria and management guideleines. Eur J Vasc Endovasc Surg. 2017;53(6):886-894
- Avgerinos E, MacEnaney R, Chaer R. Surgical and endovascular interventions for nutcracker syndrome. Semin Vasc Surg. 2013;26(4):170-177.
- Grant J. Method of Anatomy. Wilkins BW. 1937:158.
- El Sadr A, Mina A. Anatomical and surgical aspects of the operative management of varicoceles. Urol Cutan Rev. 1950;54:257-262.
- Taylor H. The problem of pelvic pain. In: MeigsJas SH (ed) Progress in Gynecology. New York: Grune & Stratton. 1957:191-208.
- Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34(5):812-819.
Citation: Honda K, Nakamura R, Yuzaki M, Fijimoto T, Nishimura Y (2020) Surgical Treatment for Nutcracker Syndrome associated with Pelvic Congestion: Two Case Reports. J Vasc Med Surg. 8:397.
Copyright: © 2020 Honda K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

