Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Commentary - (2023) Volume 11, Issue 1
Smart Pill Bottle Caps and Pain Pharmacotherapy Regime Post-Operatively
Panagiotis Peitsidis*Received: 17-Jan-2023, Manuscript No. TPMS-23-19590; Editor assigned: 20-Jan-2023, Pre QC No. TPMS-23-19590(PQ); Reviewed: 06-Feb-2023, QC No. TPMS-23-19590; Revised: 12-Feb-2023, Manuscript No. TPMS-23-19590(R); Published: 19-Feb-2023, DOI: 10.35248/2329-9088.23.11.283
Description
The “Pillsy” smart pill bottle cap and app is a “connectable” tool which can be exploited to monitor and manage patient consumption of pharmaceuticals. This device records accession to a pill bottle with a date and time stamp that is archived and can be downloaded/transmitted by wireless. Pill bottle opening can possibly notify other technologies to prompt sending the patient/healthcare provider a questionnaire or other messages through a smart phone [1]. Monitoring and managing post op/ discharge patient pharmaceutical consumption can be the link to close the “last mile” dilemma in global monitoring of the use of pharmaceuticals, whether DEA controlled or not.
Medicines are administered in the hospital and prescribed for post hospital discharge. These medicines are both controlled and non-controlled substances. Among the controlled substances, opioids are most prominent given their potential for abuse and diversion [2]. Opioids have been historic agents of significance in their use for analgesia of severe pain, either from malignancy, during surgery, post-operatively, or for other conditions.
Opioid abuse in the US has been widespread, costly, and catastrophic. It’s root cause have been studied and physician prescribing has been identified as a key element. Alternative prescribing and analgesia strategies have been devised as possible solutions to physician contributions to opioid abuse. Essential to patient comfort and recovery is adherence to pain management regimes [3]. Medicine is prescribed during hospital admission including for surgery. Prescriptions are written for patient discharge. These two elements of pharmacologic consumption are tracked in the patient Electronic Medical Record (EMR) and represent the first element of medication accounting. Prescriptions filled for controlled substances are tracked by state level prescription monitoring programs and represents the second element. Prescription consumption by the patient as an outpatient, the third element or “last mile”, is largely occult to providers and researchers. Assumptions have historically been made based on patient self-reporting or counting pills left in their respective bottles. Smart pill cap technology with wireless connectivity offers the possibility to know pill use, pain regime compliance, and correlate with patient pain levels and other outcomes [4].
A smart pill cap allows for tracking of any pill consumption through bottle opening. Multiple caps can be used on multiple bottles for a single patient, each holding their own prescription. This accommodates for the possibility of multiple medication regimes. Present technology does not account for recording the number of pills taken upon each pill bottle cap opening but this may become an option as the technology develops. Presently, this issue is reconciled by patient reporting, pill counting, or duration of the prescribed supply as indicated by refill requests.
In the case or controlled substances such as opioids, smart pill caps can monitor the number of pills used which can be translated into Morphine Milligram Equivalents (MME) consumed. This information can be used to limit MME consumption per day or in total, which can be referenced to MME guidance related to reported risks of opioid dependence. Upon consumption of opioids, the cap can send a message to the patients’/provider’s smart phone inquiring for a 0 to 10 pain score or other desired data. This data offers clinicians insight to the individual’s need for opioids, opioid effectiveness on the patient’s perception of pain, amount of medication consumed, the schedule of pain perception, compliance with pain regimen instructions, etc. [5].
Data, from our small pilot study, allowed for the creation of normative graphs which may be enlarged in the future and provide clinical guidance for the effectiveness and use of post discharge and specifically postoperative controlled substance use (Figure 1).
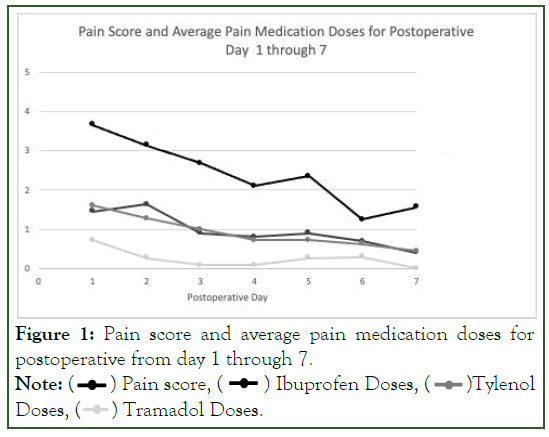
Figure 1: Pain score and average pain medication doses for
postoperative from day 1 through 7.
Note: ( ) Pain score, (
) Pain score, ( ) Ibuprofen Doses, (
) Ibuprofen Doses, ( )Tylenol
Doses, (
)Tylenol
Doses, (  ) Tramadol Doses.
) Tramadol Doses.
This data helps clinicians anticipate when postoperative pain is most severe and the expected duration for postoperative pain. This can alter prescriber behavior by reducing the quantity and duration of their routine post-operative opioid prescribing.
Conclusion
In our study, post-operative opioid prescriptions were written at the discretion of the surgery team and tramadol (0.1 MME/tab) was preferred over stronger opioids and combination formulations. Pain score were recorded in the Post Anesthesia Care Unit (PACU) prior to discharge and longitudinally over the first post-operative week. Patients were given standard postoperative instructions, the plan was explained in written instructions and reviewed with the patient and escort prior to discharge from the PACU: acetaminophen up to 1000 mg every 6 hours with ibuprofen up to 800 mg every 6 hours, with 3 hours separation between acetaminophen and ibuprofen consumption. This could result in up to 4G of acetaminophen and 3200 mg of ibuprofen being consumed daily. Tramadol, when prescribed, was written for 50 mg every 6 hours as needed for severe breakthrough pain. Patients were instructed to wean medications as they were able.
References
- Creighton EW, Dayer L, King D, Vural E, Sunde J, Moreno MA, et al . Remote smart pill cap monitoring of post-surgical pain management in thyroid and parathyroid surgery. Am J Surg. 2022.
[Crossref] [Google Scholar] [PubMed]
- Ferrell JK, Shindo ML, Stack Jr BC, Angelos P, Bloom G, Chen AY, et al. Perioperative pain management and opioid‐reduction in head and neck endocrine surgery: an american head and neck society endocrine surgery section consensus statement. Head Neck.2021;43(8):2281-94.
[Crossref] [Google Scholar] [PubMed]
- Lide RC, Creighton EW,Yeh J, Troughton M, Hollowoa B, Merrill T, et al. Opioid reduction in ambulatory thyroid and parathyroid surgery after implementing enhanced recovery after surgery protocol. Head Neck. 2021;43(5):1545-52.
[Crossref] [Google Scholar] [PubMed]
- Ferrell JK, Singer MC, Farwell DG, Stack Jr BC, Shindo M. Evaluating contemporary pain management practices in thyroid and parathyroid surgery: a national survey of head and neck endocrine surgeons. Head Neck. 2019;41(7):2315-23.
[Crossref] [Google Scholar] [PubMed]
- Rutledge J, Siegel E, Belcher R, Bodenner D, Stack Jr BC. Barriers to same-day discharge of patients undergoing total and completion thyroidectomy. Otolaryngol. Head Neck Surg. 2014;150(5):770-4.
[Crossref] [Google Scholar] [PubMed]
Citation: Peitsidis P (2023) Smart Pill Bottle Caps and Pain Pharmacotherapy Regime Post-Operatively. Trop Med Surg.11:283.
Copyright: © 2023 Peitsidis P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
