Citations : 2345
Dentistry received 2345 citations as per Google Scholar report
Indexed In
- Genamics JournalSeek
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 5
Single-Center Retrospective Study of Flowable Umbilical Cord Tissue Allograft Application in Temporomandibular Joint Defects: An Investigative Study
Ryan Phillip Robinson1, Rachel Arlene Reynolds1, John Jiang Shou2, Naomi Elaine Lambert3*, Ryaan Bennani4, Nicole A Dyer1, Summiah T Laviba5 and Tyler Chad Barrett42Department of Dentistry, Baylor College of Medicine, Houston, United States of America
3Department of Dentistry, Regenative Labs, Florida, United States of America
4Department of Dentistry, University of Delaware, Newark, United States of America
5Department of Dentistry, New York University, New York, United States of America
Received: 31-Aug-2023, Manuscript No. DCR-23-22832; Editor assigned: 04-Sep-2023, Pre QC No. DCR-23-22832 (PQ); Reviewed: 18-Sep-2023, QC No. DCR-23-22832; Revised: 25-Sep-2023, Manuscript No. DCR-23-22832 (R); Published: 03-Oct-2023, DOI: 10.35248/2161-1122.23.13.658
Abstract
Introduction: Temporomandibular Dysfunction (TMD) is a chronic condition affecting bone, muscle, and other tissues of the temporomandibular joint and surrounding areas. The symptoms of temporomandibular dysfunction vary depending on the severity of the case. They can include muscle spasms, restricted jaw and cheek movement, difficulty opening and closing the mouth, tenderness, lockjaw, and pain in the ear and neck. While temporomandibular dysfunction is multifaceted, this study will focus on patients with intraarticular cartilage defects of the temporomandibular joint. Cartilage defects are usually caused by wear and tear, but some patients may develop them as a result of disease, infection, or trauma.
Methods: This study documents observational data from five refractory temporomandibular dysfunction patients at the Pain and Sleep Therapy Center, ages 16-81, whose primary pathology is articular cartilage damage. Each patient received a comprehensive medical exam and history evaluation. All patients gave informed consent. Patients received one Wharton’s Jelly (WJ) tissue allograft application in addition to standard care practices. Progress was tracked over 90 days with Cone-Beam Computed Tomography (CBCT) scans, patient-recorded pain scales, and range of motion evaluations.
Results: There were no adverse events recorded. All patients reported 50%-100% improvement in symptomatic pain. CBCT scans showed marked improvement in two patients and the recorded range of motion measurements displayed improved mobility.
Conclusion: This preliminary data shows positive patient outcomes and provides grounds for future research on Wharton’s jelly tissue allografts in cases of treatment-resistant temporomandibular defects.
Future research: This preliminary data observed through an Institutional Review Board (IRB)-approved retrospective study is the basis for a more extensive and in-depth prospective cohort study that will further analyze patient outcomes with Wharton’s jelly applications to treatment-resistant Temporomandibular Joint (TMJ) defects compared to a control group receiving standard care treatments. The study was approved by the IRB at the Institute of Cellular and Regenerative Medicine on March 29, 2023.
Keywords
Temporomandibular joint defects; Regenerative medicine; Wharton’s jelly
Introduction
Temporomandibular Dysfunction (TMD) is a heterogeneous group of orofacial musculoskeletal and neuromuscular conditions involving the temporomandibular joint and surrounding masticatory muscles [1]. Temporomandibular Dysfunction represents the most common chronic orofacial pain condition [2]. Nearly 10 million patients suffer from TMDs in the United States each year [3]. These patients can suffer from joint arthralgia, displacement, degenerative arthritis, limited and painful mouth opening, painful subluxation of the joint, joint clicking, and referred pain to the periauricular area or head and neck muscles [2,4]. 85% of the cost to treat TMD is associated with patients suffering from chronic pain with or without mechanical joint dysfunction [2]. Social, emotional, and cognitive triggers may both spark the onset or exacerbate the presence of pain [5]. This manifests in the workplace as TMD contributes to almost 18 M lost work days per year for every 100,000,000 working adults [6]. Although there are many treatments for TMD, therapy specific to degenerative pathology of the TMJ disc itself is limited in scope and therapeutic effect. Standard treatment includes simply resting the jaw muscles and avoiding use, massage, physical therapy, medication management, and, when all else fails, surgery [7]. This study aims to report observations of a safe and effective alternative intervention method for refractory temporomandibular joint defects. The observed treatment focuses on utilizing Wharton’s jelly tissue allografts. Wharton’s jelly is a unique structural matrix found in the umbilical cord that serves as a supportive and protective scaffold for arterial and venous blood flow [8]. Properties of Wharton’s jelly have been tested and deemed a highly promising substitute for structural tissue matrixes [9,10]. We hypothesize that when damaged articular cartilage within the temporomandibular joint is supplemented with the homologous structural extracellular matrix tissue found in Wharton’s Jelly, further deterioration and malfunction of the joint could be mitigated, leading to a decrease in patient-reported pain.
Materials and Methods
All methods complied with the Food and Drug Administration (FDA) and American Association of Tissue Banks (AATB) standards. This study was conducted under an Institute of Regenerative and Cellular Medicine IRB-approved protocol (RLUCT- 001), and informed consent was obtained from each study participant. Wharton’s jelly tissue allograft was processed and distributed by Regenative Labs. Patient recruitment, allograft application, and patient tracking were performed at the Pain and Sleep Therapy Center.
Patients were recruited for this study based on medical necessity and homologous use. The selected patients suffered TMD, primarily caused by articular cartilage disk deterioration. All patients had radiographic evidence of cartilage degeneration of the symptomatic temporomandibular joint. All patients had failed prior systemic treatments that may have included in whole or part Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), muscle relaxants, physical therapy, pain medications, and oral appliance use.
Human umbilical cords were obtained from consenting mothers who underwent comprehensive medical, social, and blood testing before delivery. The donations were tested for infectious disease by an independent certified laboratory under regulatory standards (CLIA of 1988, 42 CFR part 493 and FDA regulations). All birth mothers underwent testing for Hepatitis B core antibody, Hepatitis B surface antigen, Hepatitis C antibody, human immunodeficiency virus antibody (HIV-1/HIV-2 plus O), Human T-lymphotropic Virus antibody (HLTV-I/11), syphilis, HIV-1/HCV/HBV NAT, and West Nile virus using FDA-approved testing kits. Negative or non-reactive results were mandatory before processing the umbilical cord tissue.
Wharton’s jelly was aseptically separated from the cleansed umbilical cord and suspended in sterile sodium chloride 0.9% solution (normal saline). Dimethyl sulfoxide was added as a cryoprotectant, calculated to comprise 5% of the total suspension volume. This cryoprotectant aids in preserving the integrity of fibroblasts, pericytes, local cells, and growth factors within the stored allograft at -40°C temperatures. The sample adheres strictly to regulations outlined in 21 CFR Part 1271.10(a), ensuring no combination occurs with other cells, tissues, or articles beyond permissible exceptions for human cells, tissues, and cellular and tissue-based product regulation.
Each patient received a single Wharton’s jelly tissue allograft application in the affected jaw joint (SecreTextTM from Regenative Labs), a pterygoid release, and mandibular range of motion exercises. Under sterile technique and with musculoskeletal guidance, 1 cc of Wharton’s jelly flowable allograft (25 mg of Wharton’s jelly tissue) was applied into the temporomandibular joint in close approximation to the temporomandibular disc after proper identification of the posterior joint space. First, the provider palpated the temporomandibular joint in the patient’s fully occluded position to understand where the condyle’s lateral pole sits in the temporomandibular joint capsule and applied an instant topical skin refrigerant. Then the tragus was identified, and after keeping the patient’s mouth wide open with a bite block if necessary, the vacated joint space just behind the lateral pole of the condyle and anterior to the tragus of the ear was entered approximately 3/8 inch, 8 mm in front of the tragus of the ear, with a 25 gauge by 1-inch needle. The needle was positioned upward and slightly forward to slide beneath the zygoma, along the condyle, towards the posterior uppermost slope of the articular eminence. The Wharton’s jelly was then dispensed into the joint space on each affected side.
All patients were monitored for 30 minutes post-procedure. No patients experienced post-procedure complications and were all discharged home in stable condition. Progress was tracked from the application date to 30 days, 60 days, and 90 days post-application with Computed Tomography (CT) scans, range of motion assessments, and a patient-recorded questionnaire consisting of Numeric Pain Rating Scale (NPRS) for chief complaints. The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (IRCM-2022-311) on 12 January 2022. Written informed consent was obtained prior to enrollment in the study.
Results
Upon initial assessment, the patients had more than five chief complaints that caused significant discomfort and did not show improvement even after receiving standard care. After 90 days of intervention, the patient’s self-reported outcomes indicated a 50% to 100% improvement in all areas related to their chief complaints. Their range of motion measurements displayed improvement in mobility. These results suggest that the intervention may have positively impacted the patient’s health status, and further research could provide additional insight into alternative treatments for conditions that do not respond to conventional therapies.
Discussion
That we are aware, this is the first documented observational data collection reporting safety and efficacy outcomes after WJ allografts were applied to cartilage-based structural tissue defects of the TMJ. All patients had documented objective evidence of temporomandibular joint cartilage degeneration with or without degeneration of the temporomandibular disc at the time of initial evaluation. Significant decreases in jaw pain, pain associated with chewing, joint locking, headaches, dizziness, and improved sleep in five patients were observed. The percentage of improvement for each patient was calculated by the change of initial NPRS scores to the final NPRS scores 90 days after Wharton’s jelly application (Table 1). There was an average of 70% improvement per chief complaint across the sample population. All patients showed slight improvements in the range of motion measurements, bringing their final measurements toward the normal range; the vital notation was a significant decrease in pain during motion (Table 1).
| Patient (P1) | Patient (P2) | Patient (P3) | Patient (P4) | Patient (P5) | |
|---|---|---|---|---|---|
| Gender | F | F | F | F | F |
| Age | 16 | 19 | 51 | 68 | 81 |
| Chief complaints | Percent improvement from initial application to final exam | ||||
| Jaw pain reduction | 60% | 65% | 75% | NA | 100% |
| Jaw noises/cracking | 50% | 15% | 50% | 90% | 100% |
| Pain while chewing | 50% | 80% | 75% | NA | 100% |
| ROM | 50% | 50% | 100% | 75% | 100% |
| Dizziness | NA | NA | NA | 75% | 100% |
| Headaches | NA | NA | 50% | NA | NA |
| ROM w/o Orthotic | Change in mm from initial application to final exam | ||||
| Interincisal opening | 5 mm | 6 mm | 3 mm | 5 mm | 4 mm |
| Lateral excursion Rt | 4 mm | 0 mm | 5 mm | 5 mm | 7 mm |
| Lateral excursion Lt | 1 mm | 1 mm | 7 mm | 0 mm | 9 mm |
| Protrusive | 1 mm | 1 mm | 1 mm | 1 mm | 0 mm |
| Cervical ROM | Change in degrees from initial application to final exam | ||||
| Seated left rotation | 0° | 10° | 5° | 10° | 30° |
| Seated right rotation | 0° | 15° | 0° | 20° | 30° |
| Flexion | 10° | 0° | 0° | 15° | 25° |
| Extension | 15° | 0° | 5° | 0° | 20° |
Note: ROM: Range of motion
Table 1: This table reports the percent change in the patient's pain scales and millimeter or degree difference in range of motion measurements from the initial application visit to the 90-days follow-up visit.
The before and after CT scans did not show major differences in three patients and will be tracked from a larger cohort over more extended periods in future research, but the most notable changes were in patients one and two. The left condyle on patient one demonstrated a continuous and thin corticated border without evidence of active focal erosions from the previous scan, and subcortical sclerosis, which was apparent in historical imaging, has subsided (Figure 1). Patient two had no improvement in degenerative changes and acute vertical aspect volume loss but a marked increase in proliferative bone formation affecting the posterior surface of the condyle (Figure 2).
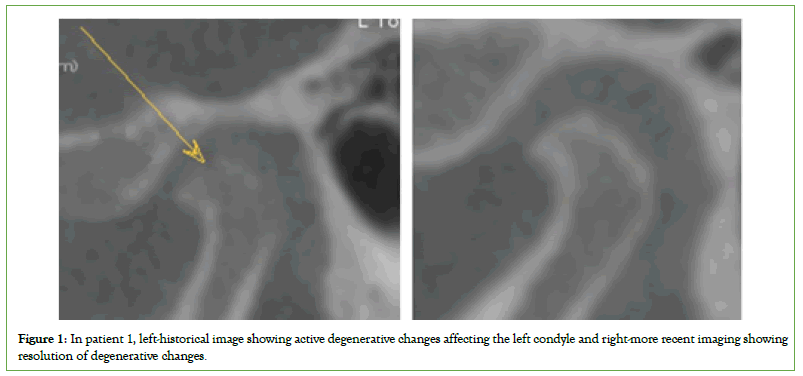
Figure 1: In patient 1, left-historical image showing active degenerative changes affecting the left condyle and right-more recent imaging showing resolution of degenerative changes.
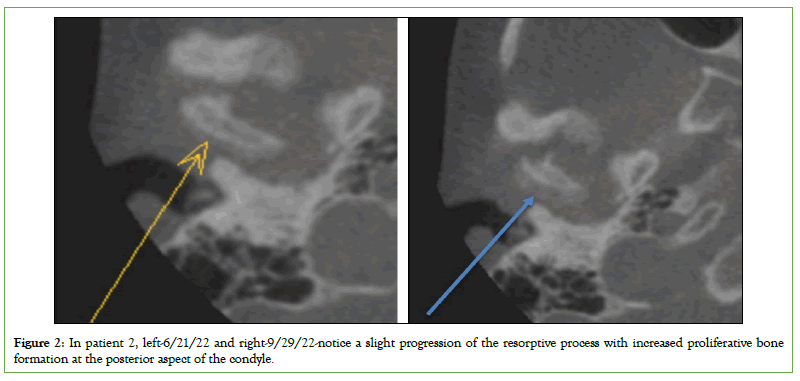
Figure 2: In patient 2, left-6/21/22 and right-9/29/22-notice a slight progression of the resorptive process with increased proliferative bone formation at the posterior aspect of the condyle.
Our study had a few limitations, and while still providing results on a Potential novel intervention for treatment-resistant TMJ defects, concrete determinations of the efficacy can only be concluded with larger cohorts. A larger cohort could provide a more precise representation of the population through statistical analysis. The clinical severity of the patient’s TMD may be more or less severe than the overall population. Future studies could group cohorts of patients by initial severity of tissue degeneration and pain to better understand the level of improvement achieved over time. The age range of this sample population was also highly varied, with patients from 16 to 81. Although it reports significant improvements despite age, further research with specified cohorts of young, middle-aged, and elderly patients should be observed to determine if age affects the efficacy of this alternative intervention. Additionally, this preliminary study did not follow up with patients after 90 days post-application. Yearly visits could be tracked to define the tissue supplementation’s durability further. While the sample size was too small to determine consistent physical differences with imaging, the patient-reported improvements were significant to warrant further studies. The future extension of this research will consist of a prospective single-site cohort study with at least 60 patients over an extended period with CBCT and Magnetic Resonance Imaging (MRI) scans to define further and evaluate the safety and efficacy of WJ allografts applied to structural tissue defects in the articular cartilage bed of TMD.
Conclusion
All observed patients experienced a significant reduction in pain levels where the standard of care practices had failed. No adverse events have been reported to date. These results demonstrate the clinical importance of incorporating regenerative medicine into orofacial practices for outcomes-based alternative care of treatmentresistant temporomandibular defects. The study lays the foundation for further investigation into the proactive use of alternative treatment measures in treatment-resistant TMD. Additional prospective clinical studies may clarify the dose, protocol, and durability of WJ allograft application outcomes compared to corticosteroids, hyaluronic acid, or botox. Future investigations may also evaluate the long-term disability, work productivity, and total joint replacement rates for patients with treatment-resistant TMD after the application of WJ connective tissue allografts.
Declarations
Ethics approval and consent to participate
All participants provided written consent to data collection.
Consent for publication
All patients consented to release their data for publication.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of Interest Statement
John Shou, Naomi Lambert, and Tyler Barrett are associated with Regenative Labs. Regenative Labs was involved in the design of the study, data analysis and writing. An independent physician performed the treatment and data collection at the Pain and Sleep Therapy Center. Regenative Labs influenced the decision to publish.
Funding
This research received no external funding. Regenative Labs is responsible for all APC charges.
Author Contributions
Conceptualization-RPR, RAR, JJS, TCB; Methodology-RPR, RAR, TCB; Software-NEL, TCB, RB, SL, ND; Validation-NEL; Formal analysis-NL, TCB; Investigation-RPR, RAR, ND; Resources-TCB; Data curation-NL, RB, ND, SL; Writing-original draft preparation- NEL; Writing-review and editing-RPR, RAR, NEL, JJS, TCB; Visualization-NEL; Supervision-RPR, TCB; Project administration- TCB; Funding acquisition-TCB. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank the team at Beam Redears for the analysis of CT scans.
References
- Maini K, Dua A. Temporomandibular Syndrome. StatPearls publishing. 2022.
[Google Scholar] [PubMed]
- Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C. Orofacial pain prospective evaluation and risk assessment study-The OPPERA study. J Pain. 2011;12(11):T4-T11.
[Crossref] [Google Scholar] [PubMed]
- National academies of sciences, engineering, and medicine. Health and medicine division; Board on health care services; Board on health sciences policy; Committee on care interventions for individuals with dementia and their caregivers. In meeting the challenge of caring for persons living with dementia and their care partners and caregivers: A way forward. National academies press (US) 2021.
- Valesan LF, Da-Cas CD, Réus JC, Denardin AC, Garanhani RR, Bonotto D. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin Oral Investig. 2021;25:441-453.
[Crossref] [Google Scholar] [PubMed]
- Namvar MA, Afkari BF, Moslemkhani C, Mansoori K, Dadashi M. The relationship between depression and anxiety with temporomandibular disorder symptoms in dental students. Maedica. 2021;16(4):590.
[Crossref] [Google Scholar] [PubMed]
- Dworkin SF. Temporomandibular disorder pain: Epidemiologic data. APS Bull. 1993;3(2):12-13.
- Li DT, Leung YY. Temporomandibular disorders: Current concepts and controversies in diagnosis and management. Diagnostics. 2021;11(3):459.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, El-Amin SF, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res. 2020;15(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Davis JM, Sheinkop MB, Barrett TC. Evaluation of the efficacy of cryopreserved human umbilical cord tissue allografts to augment functional and pain outcome measures in patients with knee osteoarthritis: An observational data collection study. Physiol. 2022;2(3):109-120.
- Converse GL, Li D, Buse EE, Hopkins RA, Aljitawi OS. Wharton’s jelly matrix decellularization for tissue engineering applications. Methods Mol Biol. 2018:25-33.
[Crossref] [Google Scholar] [PubMed]
Citation: Robinson RP, Reynolds RA, Shou JJ, Lambert NE, Bennani R, Dyer NA, et al. (2023) Single-Center Retrospective Study of Flowable Umbilical Cord Tissue Allograft Application in Temporomandibular Joint Defects: An Investigative Study. J Dentistry.13:658.
Copyright: © 2023 Robinson RP, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.