Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 11, Issue 5
Silent Acute Rheumatic Fever Unmasked by Using Handheld Echocardiography for Febrile Children Presenting in an RHD Endemic Area
Sulafa Ali1,2, Andrea Beaton3,4, Emma Ndagire5 and Lamia Elhag6*2Department of Cardiovascular Surgery, University of Khartoum, Khartoum, Sudan
3Department of Cardiovascular Surgery, Cincinnati Children’s Hospital Medical Center, Khartoum, Sudan
4Department of Cardiovascular Surgery, The University of Cincinnati School of Medicine, Khartoum, Sudan
5Department of Cardiovascular Surgery, The Uganda Heart Institute, Khartoum, Sudan
6Department of Cardiovascular Surgery, Sudan Medical Specialization Board, Khartoum, Sudan
Received: 06-Sep-2023, Manuscript No. JVMS-23-22875; Editor assigned: 11-Sep-2023, Pre QC No. JVMS-23-22875 (PQ); Reviewed: 29-Sep-2023, QC No. JVMS-23-22875; Revised: 06-Oct-2023, Manuscript No. JVMS-23-22875 (R); Published: 13-Oct-2023, DOI: 10.35248/2329-6925.23.11.538
Abstract
Background: Rheumatic Heart Disease (RHD) is a preventable sequelae of Acute Rheumatic Fever (ARF), an immune reaction to group A streptococcal infection. ARF is often underdiagnosed leading to late presentation with advanced RHD. Handheld Echocardiography (HHE) proved to be reliable in diagnosis of subclinical RHD. This study aims to assess the utility of HHE in diagnosis of ARF in febrile children.
Methods: A cross sectional, hospital based study was carried out at the Pediatric Hospital in Al Obeid city, North Kordofan, Sudan. Febrile children 3-18 years of age with or without symptoms of ARF and no other identifiable cause for the fever were included. History, examination, blood investigations (complete blood count, erythrocyte sedimentation rate, C reactive protein, anti streptolysin O titre and blood film for malaria) were done. HHE was performed by a trained physician using a single Echocardiographic (echo) view where rheumatic carditis was defined by color Doppler and morphologic criteria of the left sided valves. ARF is diagnosed by the Jones Criteria. Clinical ARF (CARF) was diagnosed if the patient presents with a clinical major Jones criteria and silent ARF (SARF) if the only major Jones criteria is HHE-diagnosed carditis. Patients with positive HHE were sent for confirmation by a standard echo performed by a cardiologist.
Results: The study was conducted between September 2022 and January 2023. It included 400 children (55% males) with a mean age of 9 years. Clinical ARF was diagnosed in 95 patients (24% of 400). The most common clinical manifestation was joint symptoms in 88 patients (92%). Among the 281 children who did not present with a clinical manifestation of ARF, HHE revealed carditis in 44 patients (16%). Of these 44 children, 18 (40%) met criteria for definite silent ARF, 13 (30%) for possible silent ARF while 13 (30%) had isolated subclinical RHD. The total frequency of silent ARF in this population of was 8%. Most children with positive HHE (41,63%), had mild subclinical carditis. Moderate or severe carditis was present in 25, 38%. Mitral regurgitation was the most common lesion (97%). HHE had a reliability of 76%, both sensitivity and specificity of HHE versus standard echo were 88%.
Conclusion: There is a significant burden of silent ARF in Sudan revealed by the use of HHE. RHD prevention policies in endemic areas should prioritize decentralization of echo to improve ARF detection. Further implementation research is needed to evaluate the impact of these findings on outcomes of ARF and RHD in similar low-resource settings.
Keywords
Acute rheumatic fever; Handheld echocardiography; Rheumatic heart disease
Introduction
Untreated, superficial Group A Streptococcal (GAS) infections can trigger the immune reaction Acute Rheumatic Fever (ARF), which results in permanent, incurable heart valve destruction, or Rheumatic Heart Disease (RHD). RHD remains endemic in low and middle-income countries and is considered the most common cause of acquired heart disease in children and young adults, affecting over 38 million individuals worldwide [1]. Improved strategies are desperately needed to both prevent RHD and to diagnose this disease earlier, when effective interventions can reduce disease severity.
The efficacy of echocardiography (echo) in screening for RHD has been well established. Studies from nearly every continent have found a high burden of screen detected RHD in classically endemic settings [2-4]. Portable Hand-Held Echocardiography (HHE) machines have shown excellent correlation with Standard Echocardiography (SE) using simplified protocols [5,6]. More recently, a randomized clinical trial has shown that outcomes can be improved for children with latent RHD through the prescription of Benzathine Penicillin G (BPG) secondary prophylaxis, showing for screening as a tool for early diagnosis and treatment of RHD in endemic areas [7].
In contrast, early diagnosis of ARF has lagged [8]. Very few children in low-income countries are diagnosed when they have ARF, with the most common clinical presentation being advanced RHD. One primary driver of missed ARF diagnosis is the complexity of making this diagnosis in low-resource settings. There is no single diagnostic test for ARF. Instead, clinicians must implement clinical, laboratory and imaging testing to fulfill major and minor Jones Criteria, the historical gold standard [9]. Yet, few low-resource countries have ready access to the diagnostic tools needed to make this complex assessment [1].
A recent modelling study from Uganda examined which elements of the Jones Criteria would result in the highest ARF case detection. These investigators showed that the addition of echo, as compared to building other diagnostic resources such as streptococcal antibody titers, would contribute most to improving ARF diagnosis in the community [10]. Building on this concept and given that many children with ARF have carditis as their only major Jones criteria, we hypothesized that there may be a proportion of children with febrile illness in RHD endemic regions who have no other major features of ARF other than subclinical carditis. For these children, echo would be the only way to uncover ARF, when it would not otherwise be clinically suspected.
To understand if there was a burden of silent ARF, we implemented echocardiographic evaluation with a portable Handheld Echo (HHE) device along with standard laboratory investigations for ARF evaluation for all febrile children without a clear alternative diagnosis, presenting to our pediatric emergency room between September 2022 and January 2023.
Materials and Methods
This was a prospective cross-sectional hospital-based study conducted in Al Obeid, the capital of North Kordofan state, western Sudan from September 2022 to January 2023. The primary outcome of interest was the number of febrile children with silent ARF, defined as those without obvious signs/symptoms of ARF but with echocardiographic carditis and otherwise met the 2015 Jones Criteria for ARF in a moderate/high risk setting.
Enrollment and diagnostic work-up
Children (3-18 years) presenting to the pediatric emergency room were included if they had fever of unknown etiology within the last 2 days either in isolation or associated with clinical signs/symptoms of ARF (joint complaints, murmur or heart failure, chorea, erythema marginatum, or skin nodules). Children were excluded if they had known RHD or congenital heart disease, viral symptoms (rhinorrhea, pharyngitis, cough), suspicion of meningitis (convulsion, neck stiffness), urinary tract infection or gastroenteritis, or a known cause of fever and joint pain (sickle cell crisis most commonly).
All enrolled children underwent a complete history and physical examination. A single view HHE study (General Electric V scan) including a parasternal long access view in black and white and color Doppler imaging was obtained by a pediatric resident who had undergone study-specific training. All recorded echo studies were further reviewed by a pediatric cardiologist. Investigations including a complete blood count, Erythrocyte Sedimentation Rate (ESR), C Reactive Protein (CRP), Anti-Streptolysin O Titer (ASOT), and malaria blood smear were completed in order to apply the 2015 Jones Criteria. Antideoxyribonuclease B antibody titers and routine electrocardiograms were not available in our setting and could not be included in case evaluation.
Handheld echocardiogram was considered positive for rheumatic carditis if it showed at least one of the following: Mitral regurgitation measuring 1.5 cm or more, any aortic regurgitation, or mitral or aortic valve morphology consistent with ARF/RHD. When possible, children with a positive HHE were sent for a standard complete echocardiogram performed by a cardiologist for confirmation.
Diagnostic determination
We applied the 2015 Jones Criteria for high-risk settings to confirm definite ARF. Children with definite ARF were further classified as either definite clinical (joint complaints, a typical murmur, chorea, erythema marginatum, or skin nodules) ARF or definite silent ARF if their only major criteria was echo-diagnosed carditis (Table 1).
| Category | Operational Definition |
|---|---|
| Definite ARF | Fulfilment of the 2015 Jones Criteria for high-risk settings |
| Definite Clinical ARF | Presented with clinical signs/symptoms of ARF that would have prompted diagnostic consideration including joint complaints, murmur, chorea, erythema marginatum, or skin nodules and fulfilled the 2015 Jones Criteria. |
| Definite Silent ARF | Presented with no clinical signs/symptoms of ARF but found to have sub-clinical carditis by screening echocardiogram and otherwise fulfilled the 2015 Jones Criteria. |
| Possible ARF | |
| Possible Clinical ARF | Presented with clinical signs/symptoms of ARF that would have prompted diagnostic consideration including joint complaints, murmur, chorea, erythema marginatum, or skin nodules and positive inflammatory markers but there was negative ASOT. |
| Possible Silent ARF | Positive screening echocardiogram with either elevated ASOT or elevated markers of inflammation, but not both (incomplete fulfilment of the Jones Criteria) |
| Isolated Silent RHD | Positive screening echocardiogram with normal ASOT and markers of inflammation, unable to differentiate ARF from pre-existing subclinical RHD during febrile illness |
| Alternate Diagnosis | Screening echocardiogram negative and did not otherwise fulfil the Jones Criteria |
Table 1: Operational definitions for febrile children evaluated for ARF.
Additionally, as we did not have Antideoxyribonuclease B antibody titers in our setting and many children self-prescribe with anti-inflammatory medications for fever prior to coming to the emergency department, we used the 2015 Jones Classification of Possible ARF for those who only partially fulfilled the Jones Criteria. Children with 2 major or 1 major and 2 minor criteria, but a normal ASOT were labeled Possible Clinical ARF, if at least one major criteria was joint involvement. Children who had a positive screening echocardiogram but were missing either an elevated ASOT or elevated markers of inflammation were labeled Possible Silent ARF.
Finally, febrile children with a positive HHE without any additional major or minor criteria other than fever were labeled as isolated silent RHD. These children remained suspicious for ARF or for subclinical RHD in the setting of another febrile illness.
Treatment
Those who were diagnosed with definite or possible ARF were started on standard treatment as well as secondary BPG prophylaxis and follow up visit to the cardiologist was arranged. The patient’s flow chart is shown in Figure 1.
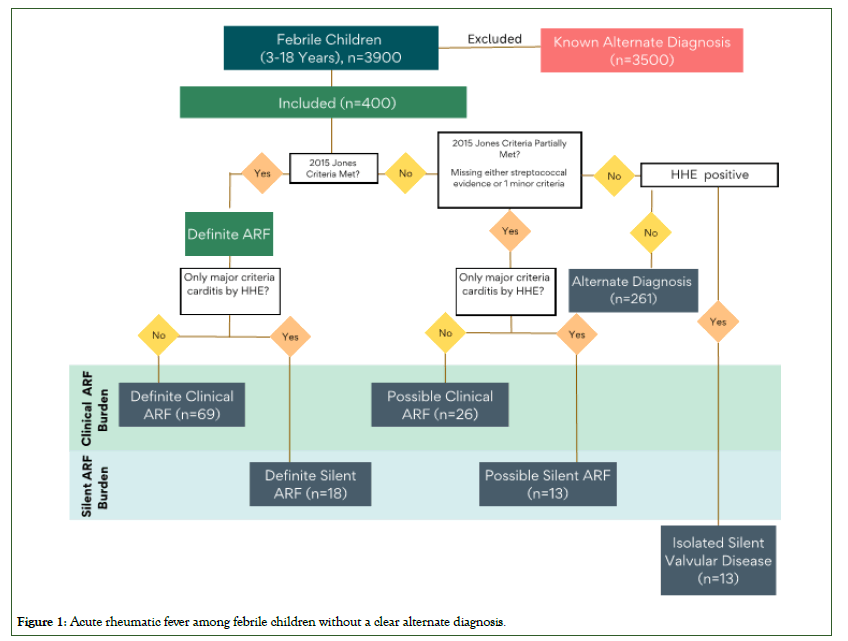
Figure 1: Acute rheumatic fever among febrile children without a clear alternate diagnosis.
Data analysis
Descriptive analysis was performed, frequency tables for all categorical variables in addition to mean (+SD) for numeric variable. Sensitivity, specificity and reliability analysis was generated to compare HHE with SE.
Results
During the study period 3900 febrile children (3-18 years) presented to the emergency department. Of these, 3500 were excluded for known alternate diagnosis. This left 400 febrile children without a clear alternate diagnosis for study participation. Of these, 55% (220) were males and the mean age was 9.1 years SD ± 3.6. A breakdown of demographics and clinical features by final diagnostic group is presented in Table 2.
| Definite Clinical ARF | Possible Clinical ARF | Definite Silent ARF | Possible Silent ARF | Isolated Silent Valvular Disease | Alternate Diagnosis | |
|---|---|---|---|---|---|---|
| n | 69 | 26 | 18 | 13 | 13 | 261 |
| Age (median, IQR) | 9 (6.0-12.0) | 10 (6.0-13.0) | 6.5 (5.0-8.0) | 10 (8.0-13.0) | 5 (4.0-8.0) | 8 (6.0-12.0) |
| Sex (female n, %) | 32 (53%) | 14 (54%) | 10 (56%) | 4 (31%) | 6 (46%) | 113 (43%) |
| History of sore throat in the last 14 days | 53 (8%) | 12 (46%) | 11 (61%) | 8 (62%) | 3 (23%) | 124 (48%) |
| Joint symptoms | 63 (91%) | 25 (96%) | 0 | 0 | 0 | 24 (9%) |
| Chorea | 6 (7%) | 0 | 0 | 0 | 0 | 0 |
| Heart murmur | 11 (9%) | 0 | 0 | 0 | 0 | 2 (<1%) |
| Erythema marginatum | 1 (<1%) | 1 (4%) | 0 | 0 | 0 | 0 |
| Skin nodule | 1 (<1%) | 0 | 0 | 0 | 0 | 0 |
| Symptoms of heart failure | 4 (6%) | 0 | 0 | 0 | 0 | 0 |
| Positive Blood Film for Malaria | 38 (55%) | 9 (53%) | 8 (44%) | 5 (38%) | 6 (46%) | 143 (55%) |
| High ESR >30 mm/hour | 62 (90%) | 17 (100%) | 16 (89%) | 5 (38%) | 1 (8%) | 142 (54%) |
| Positive CRP | 63 (91%) | 10 (59%) | 14 (78%) | 2 (15%) | 0 | 84 (32%) |
| High ASO >200 | 69 (100%) | 0 | 18 (100%) | 6 (46%) | 0 | 92 (35%) |
Table 2: A comparison of presenting features suggestive of ARF between diagnostic categories.
Diagnostic evaluation
Out of 400 children, 119 (30%) presented with at least one obvious major Jones Criteria, suggestive of clinical ARF. Of these, 112 (94%) presented with a joint manifestation, of which 62 children (55%) were confirmed to have definite clinical ARF, 26 children (23%) possible clinical ARF, and 24 (22%) with alternate diagnoses. Chorea was the primary presentation for an additional 6 children (5%) and a single child presented with rash consistent with erythema marginatum (<1%); all 7 of these children met criteria for definite clinical ARF. The total frequency of clinical ARF in this population of 400 febrile children without known alternate diagnosis was 24% (95 out of 400).
Among the 281 children who did not present with a clinical manifestation suggestive of ARF, HHE revealed carditis in 44 patients (16%). Of these 44 children, 18 (40%) met criteria for definite silent ARF, 13 (30%) for possible silent ARF and 13 (30%) for isolated silent RHD. The total frequency of silent ARF in this population of 400 febrile children without known alternate diagnosis was 8% (31 out of 400).
Echocardiographic findings
Of the 66 children who had a positive HHE evaluation, only 11 (16.7%) had a notable cardiac murmur. Most children with positive HHE (41, 63%), had mild subclinical carditis. Moderate or severe carditis was present in the remaining third (25, 38%). Mitral regurgitation was the most common lesion (97%). Those with severe carditis most commonly had combined mitral and aortic regurgitation (9 patients, 14%). Isolated moderate to severe MR was found in 6 children (9%).
HHE compared to standard echocardiography
Of those with positive echo findings (66 patients), 43 (65%) underwent Standard Echo (SE) study performed by cardiologist and could be included in this comparison. HHE had a reliability of 76%, both sensitivity and specificity of HHE versus SE were 88%. Positive and negative predictive value for HHE were 91% and 84% respectively.
Discussion
In this study, we have described the role of integration of echo in clinical practice to improve ARF detection among children who present with fever and unknown alternative diagnosis in areas where the disease is prevalent. Our data demonstrate that a high number of these children (17%) have definite clinical ARF with an additional 5% meeting criteria for possible clinical ARF. Additionally, up to 8% of children without obvious symptoms or signs of ARF were diagnosed with definite or possible silent ARF, with their only major criteria being evidence of rheumatic carditis on HHE. These data suggest more investment is needed in testing to evaluate children for ARF in endemic settings, in particular highlighting the critical need for echo evaluation to capture the burden of both clinical and otherwise silent ARF.
In the recent era of HHE, there is clear evidence that the rate of subclinical RHD is high among children in endemic areas indicating a high level of previous ARF exposure. However, it has been observed that a history of ARF is not reported by many patients with established RHD indicating that the episode of ARF passed unnoticed [8]. This low ARF detection rate could be attributed to many factors. Firstly, joint affection which is the commonest manifestation as found in this study, is typically transient and often resolves without treatment therefore it is expected to be under reported. Secondly, implementation of the Jones Criteria requires laboratory investigations that are not readily available in most RHD endemic. Thirdly, carditis, a major Jones Criterion, can be subclinical and missed by clinical examination.
In this study HHE revealed a substantial burden of otherwise silent ARF, or ARF where the only major Jones Criteria is sub-clinical carditis. The addition of echo screening improved ARF diagnosis by 30%. This of critical importance, as these children with silent ARF had the earliest forms of rheumatic valvular disease, with the most to gain from initiation of secondary prophylaxis. Furthermore, the patients diagnosed with fever and subclinical RHD in our cohort could well have ARF which is not confirmed due to the prior use of anti inflammatory medications or the lack of other tests such as anti-DNase B titers and electrocardiogram. In contrast, among children who were diagnosed with clinical RHD, or at least one other major clinical feature of ARF which could prompt evaluation, more than a quarter already had moderate/severe valvular heart disease where the use of prophylactic penicillin is of unclear benefit.
Overall, when we added active case finding for ARF among febrile children in this context, a very high burden was revealed. In this context, where laboratory testing for ARF, including makers of inflammation and streptococcal exposure, is not routinely available, more education is needed to increase the index of suspicion among clinicians. This is particularly important when diagnostic overlap exists, such as in the case of malaria positivity, which was found in nearly 50% of patients in our context and in 41% of children in a community-based study in Uganda [11,12]. Ultimately, an improved diagnostic test is of urgent importance to simplify and clarify the diagnosis of ARF in low-resource settings.
Until a better diagnostic test is available, these data continue to support that investment in echo screening equipment and training may have the greatest impact on improving not only RHD diagnosis but also ARF. Handheld echo, which has been shown to have high sensitivity and specificity compared to standard echo for RHD, performed similarly well for ARF. Standardized training approaches to both skills acquisition and maintenance remain a challenge, with more research and testing needed to develop scalable strategies.
This study has several limitations. A single ASO titer as compared to serial titers and lack of anti-DNase B titers reduced our sensitivity for ARF diagnosis. High rates of medication usage in the community, which we could not measure in this study, could have blunted the inflammatory reaction prior to evaluation. Together, these missing elements led to high rates of possible ARF, both clinical and silent. However, even focusing on only definite ARF, rates were substantial for both clinical and silent ARF. Finally, not all children could undergo a confirmatory echocardiogram, which could have overestimated the number of children with subclinical carditis. However, we believe this impact was also minimal based on our performance data comparing HHE to standard echocardiography.
Conclusion
In conclusion, there is a significant and underestimated burden of ARF in Sudan. Even with a higher index of suspicion and access to standard diagnostic testing for ARF, many children present with sub-clinical carditis as their only major finding. RHD prevention policies in these settings should prioritize decentralization of echocardiography to improve ARF detection. Future research should include implementation and dissemination research of these types of integrated services, to improve outcomes of ARF and RHD in low-resource settings.
Ethical Considerations
The research protocol was approved by the Ethics Board of the Sudan Medical Specialization Board. A written permission was obtained from the State Ministry of Health in North Kordofan as well as the Pediatric Hospital Administration. Written informed consent was obtained from the care takers of all children included in the study.
Funding
None
Acknowledgement
To Drs. Khalid Al Talib and Ibrahim Adam, consultant cardiologists for performing standard echocardiograms for the patients.
References
- Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722.
[Crossref] [Google Scholar] [PubMed]
- Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–476.
[Crossref] [Google Scholar] [PubMed]
- Saxena A. Rheumatic heart disease screening by “point-of-care” echocardiography: An acceptable alternative in resource limited settings? Transl Pediatr. 2015;4:254-255.
[Crossref] [Google Scholar] [PubMed]
- Sulafa A, Sara D, Bahja A, Rabab A, Tajudeen B, Al Awad K, et al. Echocardiographic screening for rheumatic heart disease in 4 515 Sudanese school children: Marked disparity between two communities. Cardiovasc J Afr. 2018;29.
[Crossref] [Google Scholar] [PubMed]
- Beaton A, Aliku T, Okello E, Lubega S, McCarter R, Lwabi P, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr. 2014;27(1):42-49.
[Crossref] [Google Scholar] [PubMed]
- Francis JR, Whalley GA, Kaethner A, Fairhurst H, Hardefeldt H, Reeves B, et al. Single-view echocardiography by non-expert practitioners to detect rheumatic heart disease: A prospective study of diagnostic accuracy. Circ Cardiovasc Imaging. 2021;14(8):e011790.
[Crossref] [Google Scholar] [PubMed]
- Beaton A, Okello E, Rwebembera J, Med M, Grobler A, Engelman D, et al. Secondary antibiotic prophylaxis for latent rheumatic heart disease. N Engl J Med. 2022;386:230-240.
[Crossref] [Google Scholar] [PubMed]
- Parks T, Kado J, Colquhoun S, Carapetis J, Steer A. Underdiagnosis of acute rheumatic fever in primary care settings in a developing country. Trop Med Int Health. 2009;14:1407–1413.
[Crossref] [Google Scholar] [PubMed]
- Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, et al. Revision of the jones criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: A scientific statement from the American Heart Association. Circulation. 2015;131(20):1806-1818.
[Crossref] [Google Scholar] [PubMed]
- Okello E, Ndagire E, Atala J, Bowen AC, di Fazio MP, Harik NS, et al. Active case finding for rheumatic fever in an endemic country. J Am Heart Assoc. 2020;9(15):e016053.
[Crossref] [Google Scholar] [PubMed]
- Okello E, Ndagire E, Muhamed B, Sarnacki R, Murali M, Pulle J, et al. Incidence of acute rheumatic fever in northern and western Uganda: A prospective, population-based study. The Lancet. 2021;9:e1423-1430.
[Crossref] [Google Scholar] [PubMed]
- Okello E, Ndagire E, Atala J, Bowen AC, DiFazio MP, Harik NS, et al. Active case finding for rheumatic fever in an endemic country. J Am Heart Assoc. 2020;9:e016053.
[Crossref] [Google Scholar] [PubMed]
Citation: Ali S, Beaton A, Ndagire E, Elhag L (2023) Silent Acute Rheumatic Fever Unmasked by Using Handheld Echocardiography for Febrile Children Presenting in an RHD Endemic Area. J Vasc Surg. 11:538.
Copyright: © 2023 Ali S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

