Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 10, Issue 2
Selective Permeation of CO2 through Amine Bearing Facilitated Transport Membranes
Panchali Bharali, Somiron Borthakur and Swapnali Hazarika*Received: 19-May-2020 Published: 11-Jun-2020, DOI: 10.35248/2155-9589.2020.10.203
Abstract
The membranes containing facilitated transport groups for Carbon dioxide had been prepared by immobilizing PAMAM (Polyamidoamine) (Generations 0, 1, 2, 3, 4) dendrimer into the polymeric membranes. The dendrimer incorporated membranes were prepared by the phase inversion method. The permeation abilities of the membranes for pure CO2 and binary mixture of CO2/N2 were calculated. The effects of feed gas pressure on the permeability of the membranes were studied. The results of the permeation experiments showed that PAMAM dendrimer (Generation 4) composite membrane possessed a better CO2 permeability and selectivity over N2 than the any other membranes composite with other generations of dendrimer (Generations 0, 1, 2, 3).
Keywords
Facilitated transport; Membranes; PAMAM dendrimer; Phase Inversion
Introduction
The increase in the concentration of various hazardous gases in the atmosphere results in numerous environmental problems like global warming, greenhouse effect etc. A large number of research studies have been carried out on the process of capturing and storage of CO2 from gaseous mixture [1,2]. Membranes and membrane processes are not a recent invention. The preparation of synthetic membrane and their utilization on a large industrial scale however are a more recent development which has rapidly gained a substantial importance due to the large number of practical application. Now a days; membranes are used to produce potable water from sea to clean industrial effluent and recover valuable constituent, purify or fractionate macromolecular mixture in food and drug industries and to separate gas and vapours. They are also key component in energy conversion system and in artificial organs and drug delivery devices. Membrane technology is one of the most interesting technologies for its applications in various fields including Biological applications [3-6]. Lin et al. [5] in his research study reported the substrate selectivity of Lysophospholipid Transporter (LplT) involved in Membrane Phospholipids remodelling in Escherichia coli. Tong et al. [6] carried out their research study on the structural insight into substrate selection and catalysis of Lipid Phosphate Phosphates in the cell membrane. Besides its applications in different fields membrane separation technology can be used for the gas separation purpose as it is one of the cost effective process and easy to operate. Because of high permeability and selectivity, the facilitated transport membranes are very useful for gas separation applications. There are various kinds of membranes containing facilitated transport groups, such as ion-exchange membrane, fixed carrier membrane and liquid membranes etc. [1]. Because of the higher permeability and selectivity fixed carrier membranes are more suitable for gas separation. A few researchers have been reported about the fixed carrier membranes for CO2 separation [2,7]. Works on gas separation applications using membranes composite with PAMAM dendrimer are limited. However, PAMAM dendrimer immobilized liquid membranes are reported by distinguished researchers [8,9]. In this research paper, we have been emphasized on gas separation behaviour of PAMAM dendrimer (G-0, G-1, G-2, G-3, G-4) composite solid membranes due to its stability and regeneration. The dendrimer composite membranes selectively permeated CO2 because of the reaction between –NH2 and CO2 producing a carbamate ion and a protonated base [8].
Thus for separation of CO2 molecules the reactions occurred as follows:
Bicarbamate: CO2+H2O+R-NH2=HCO3 - (Bicarbonate)+R-NH+
Carbamate: CO2+2(R-NH2)=R-NHCOO-(Carbamate)+R-NH3+
Kovvali AS [9] reported the performance of Poly (amidoamine) (PAMAM G-0) dendrimer and ionic liquid composite membranes which provide a challenging way for CO2 separation performances. Belmabkhout et al. [10] reported that amine bearing pore expanded MCM-41 (Mobil Composition of Matter, No 41) Silica can be used for gas separation applications for CO2/CH4. Jin Huang and Donohue et al. [8,11] reported the concept of facilitated transport mechanism for capture of CO2 from a gaseous mixture CO2/H2. Duan et al. [12] reported the CO2 separation performance of a poly (amidoamine) (PAMAM) dendrimer composite membrane containing hyaluronic acid (HA) in a chitosan (CTS) gutter layer. The novelty of our research work lies in the performance of membrane containing Amine compound (PAMAM dendrimer, G-0, G-1, G-2, G-3, G-4) for separation of CO2 from CO2/N2 gaseous mixture, describing facilitated transport mechanism for separation of CO2 and a structure activity relationship study of dendrimers with gas permeability.
Materials and Methods
Materials
Polysulfone (average molecular weight 22000) was obtained from Aldrich Chemicals was used as the membrane material. Polyethylene Glycol (PEG) (average molecular weight 400) used as additive was obtained from Central Drug House (P) Ltd. Bombay. N-Methyl Pyrollidine (NMP) obtained from Rankem, India. Commercial Dendrimer (0, 1, 2, 3, 4 generations) were purchased from Sigma Aldrich Chemical Ltd was used as membrane material. Water was obtained from Millipore water system (Type 1). Carbon di oxide (99.99%) and Nitrogen (99.99%) was supplied by M.S Gas Centre, Jorhat, India.
Methods
Preparation and characterization of polymeric membrane: The flat sheet membranes were prepared by dissolving Polysulfone (PSF) in NMP at room temperature (28°-30°C). To this solution, a definite amount of PEG-400 was added and the solution was kept on stirring to prepare the casting solution. When the solutions become clear a definite amount of Dendrimer (0, 1, 2, 3, 4 generations) was added individually for the preparation of different membranes and kept it on stirring until it became a clear solution. Then the solutions were casted on glass films and the glass films were placed in water (coagulation) bath for about 24 hours. Then the flat sheet membranes were dried at room temperature (28°C). Then the finally prepared membranes were analyzed experimentally by IR analysis (PERKIN Elmer system 2000), X-ray Diffraction analysis (JDX-11P-3A, JEOL, Japan), TGADTA analysis (PERKIN Elmer PC series, DSC 7). The morphology of the membranes were studied by Scanning Electron Microscope (LEO 1427 VP, UK) analysis etc. For each type of membrane we tested three numbers of membrane samples to get the maximum accuracy. The schematic diagram of membrane fabrication system and gas separation experiments shown in the Figures 1 and 2.
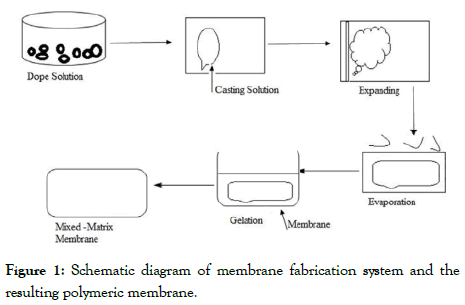
Figure 1: Schematic diagram of membrane fabrication system and the resulting polymeric membrane.
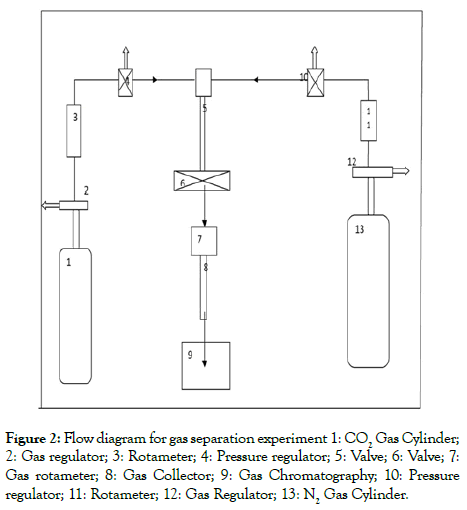
Figure 2: Flow diagram for gas separation experiment 1: CO2 Gas Cylinder; 2: Gas regulator; 3: Rotameter; 4: Pressure regulator; 5: Valve; 6: Valve; 7: Gas rotameter; 8: Gas Collector; 9: Gas Chromatography; 10: Pressure regulator; 11: Rotameter; 12: Gas Regulator; 13: N2 Gas Cylinder.
Gas permeation experiment: The gas permeation experiments were carried out in a membrane cell. The permeation cell was consisting of cylinders, membrane cell, rotameter. The membrane was fitted into the Plate and Frame module. Area of the membrane in the cell was 30 cm2. The permeance i.e the pressure normalized flux, through the membranes can be obtained by equation (1).
(P/I)=Q/ (A.ΔP) (1)
Where Q is the measured volumetric flow rate (at standard pressure and temperature), P is permeability, l is membrane skin layer thickness, A is effective membrane area and ΔP is the Pressure difference across the membrane. The common unit of Permeance is GPU (1 GPU=10-6 cm3 (STP)/cm2 s cm Hg). The ideal gas separation factor or Selectivity is given by equation (2)
Selectivity (α)=Pi/Pj (2)
Where Pi is permeation of i th component (CO2) and Pj is permeation of j th (N2) component.
Results and Discussion
Characterization of membrane
IR analysis: The FTIR analysis of the membranes were performed in order to confirm the immobilization of the PAMAM dendrimer (G0-G4) molecule into the membrane as well as the occurrence of facilitated transport mechanism in the membranes during the permeation of CO2 through the membranes. The prepared membranes were analysed experimentally by IR analysis (PERKIN Elmer system 2000). In FTIR analysis, 12 numbers of scans were used to obtain each spectrum. The FTIR spectrum of the Polysulfone membrane (without PAMAM dendrimer) is shown in Figure 3. The FTIR spectra obtained for polysulfone, dendrimer (Generations 0, 1, 2, 3, 4) composite membranes before and after permeation process are shown in Figure 4. For each membrane (Figures 3 and 4) Polysulfone gives two broad peaks in the range 3474.98 cm-1 and 2531.13 cm-1 attributed to O-H group and C-H (-CH3) stretching respectively. Broad peaks at 3428.76 cm-1, 3468.11 cm-1, 3476.89 cm-1, 3445.38 cm-1 and 3470.10 cm-1 were observed for N-H stretching in case of dendrimer G0, G1, G2, G3 and G4 membranes. Other IR peaks for dendrimer membranes were found at 2912.04 cm-1 for G0, 2948.60 cm-1 for G1, 2948.60 cm-1 for G2, 3139.90 cm-1 for G3, 2942.93 cm-1 for G4 for C-H stretching (-CH2-). For C-O-C stretching vibration peaks were observed at 1412.04 cm-1 for G0, 1451.48 cm-1 for G1, 1444.46 cm-1 for G2 and G3, 1464.67 cm-1 for G4. For C-N stretching peaks were observed at 1092.64 cm-1 for G0, 1139.07 cm-1 for G1, 1146.09 cm-1 for G2, 1084.66 cm-1 for G3 and 1157.60 cm-1 for G4. As there was no –NH2 linkages in the membrane prepared without adding PAMAM dendrimer, it did not facilitate the transportation of CO2 through it. After the permeation process, all membranes showed a broad peak in the range 3422.48 cm-1-3497.55 cm-1 which was due to the formation of amide linkage of the dendrimer molecule with CO2 as a result of facilitated transport mechanism of dendrimer for CO2 separation.

Figure 3: IR analysis of polysulfone membrane.
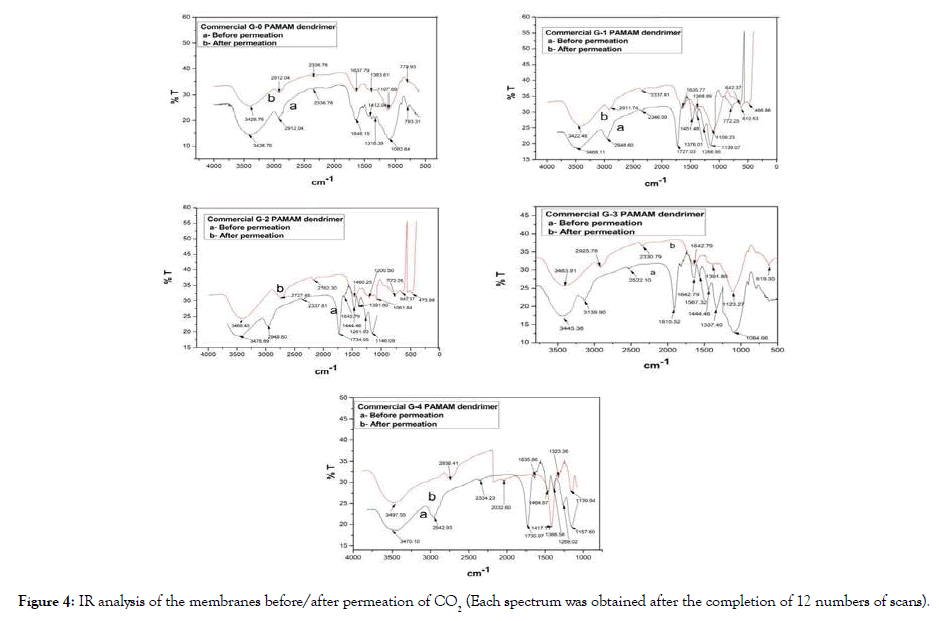
Figure 4: IR analysis of the membranes before/after permeation of CO2 (Each spectrum was obtained after the completion of 12 numbers of scans).
XRD analysis: X-Ray Diffraction (XRD) study of the various membranes was done for the structural analysis of all the membranes. Figure 5 shows the XRD patterns of the membranes prepared with various generations of dendrimer molecule (Generation- 0, 1, 2, 3, 4). All the membranes showed a broad peak in the range of 13.0-36.9. From the patterns of the membranes it was seen that the peak position does not depend on the casting solutions. The broad peaks of the membranes were observed due to complete homogeneity and compatibility among the components of the membrane. However, compared to other membranes, the pattern of G-4 dendrimer composite membrane showed a broadband peak with a decrease in the peak intensity. The broad peak of the composite membrane indicated the amorphous state of the membranes. The value of Full Width Half Maximum value (FWHM) given in Table 1 indicates the value of relative degree of the phase. The FWHM value was maximum in the membranes prepared by using G-4 dendrimer composite membrane and minimum for the G-0 dendrimer composite membrane.
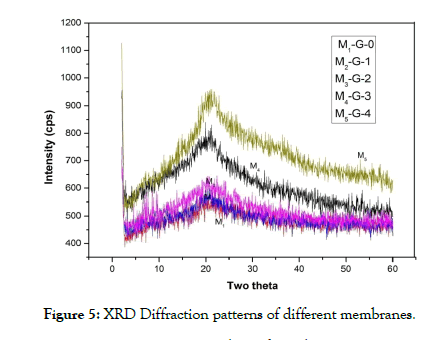
Figure 5: XRD Diffraction patterns of different membranes.
| Dendrimer- Membranes | 2ϴ (0) | d (spacing) | Wavelength (A0) | FWHM (cm) |
|---|---|---|---|---|
| G-4 | 28 | 5.15 | 1.79 | 9.2 |
| G-3 | 26 | 4.3 | 1.79 | 8.5 |
| G-2 | 24 | 3.87 | 1.79 | 7.3 |
| G-1 | 20 | 3.24 | 1.79 | 4.6 |
| G-0 | 18 | 2.76 | 1.79 | 2.51 |
Table 1: XRD analysis of membrane.
Thermal properties: Thermogravimetric analysis (TGA) and Differential Thermal Analysis (DTA) of the membranes prepared with Dendrimer (G-0, 1, 2, 3, 4) are shown in the Figure 6a and 6b. TGA DTA studies of the membranes were done under the atmosphere of N2 at a heating rate of 100°C min-1. The thermal decomposition curves of the membranes are shown in Figure 6a and the derivative curves of the membranes are shown in Figure 6b. From the TGA curves it was observed that all the membranes are stable up to a temperature of 480°C-500°C. Thus; it was observed from the TGA curves that all the membranes prepared by various generations of dendrimer possessed almost similar extent of thermal stabilities. From the DTA curves of the membranes it was observed that all the membranes possessed an exotherm which was obtained around 530°C-560°C.
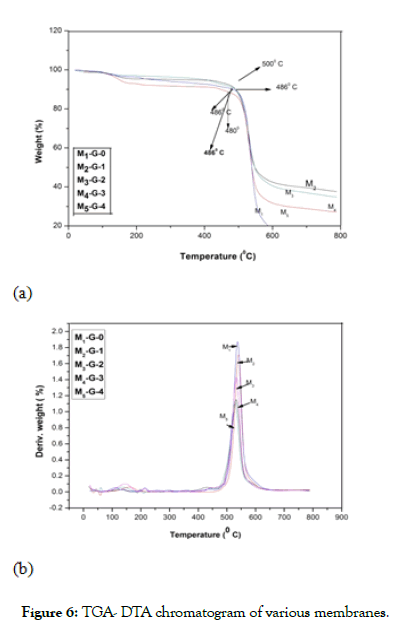
Figure 6: TGA- DTA chromatogram of various membranes.
Morphological study of membranes: The morphological studies of the membranes were carried out by Scanning Electron Microscopic (SEM) analysis. From the SEM images it was observed that the membranes prepared with various generations of Dendrimer undergo morphological changes before and after permeation process. From Figure 7, it was seen that the morphology of the membranes differ before and after permeation process. This can be attributed to the formation of some gates on the surface of the membranes due to the reaction between CO2 and dendrimer molecule during the permeation process. The membranes prepared before the permeation process had the dense uniform morphological structure, whereas the membranes after the permeation process possess some pinholes which were observed as a consequence of the facilitated transport mechanism of the membranes.
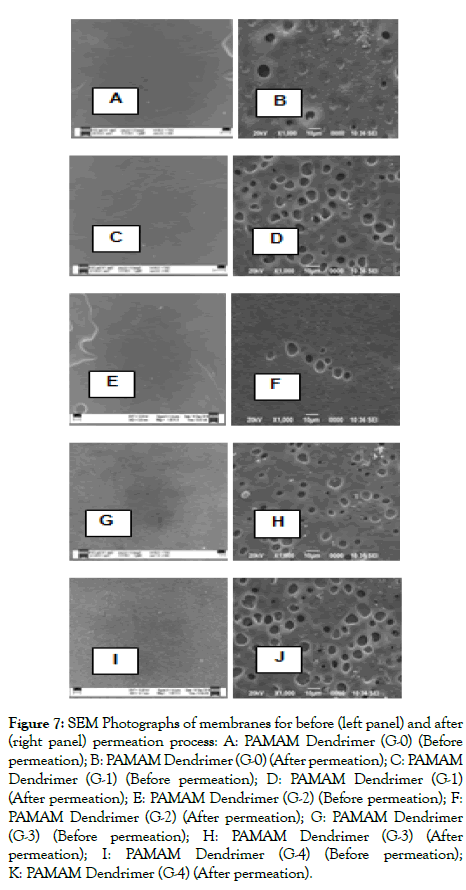
Figure 7: SEM Photographs of membranes for before (left panel) and after (right panel) permeation process: A: PAMAM Dendrimer (G-0) (Before permeation); B: PAMAM Dendrimer (G-0) (After permeation); C: PAMAM Dendrimer (G-1) (Before permeation); D: PAMAM Dendrimer (G-1) (After permeation); E: PAMAM Dendrimer (G-2) (Before permeation); F: PAMAM Dendrimer (G-2) (After permeation); G: PAMAM Dendrimer (G-3) (Before permeation); H: PAMAM Dendrimer (G-3) (After permeation); I: PAMAM Dendrimer (G-4) (Before permeation); K: PAMAM Dendrimer (G-4) (After permeation).
Effect of feed gas pressure on permeability: For the transportation of gases through the fixed-carrier solid membranes, the flux can be expressed in terms of Fick’s law with an effective diffusion coefficient Deff(p) that is dependent on pressure:
J=-Deff(p)dp/dx (3)
The pressure dependence of the diffusion coefficient for gas transport through the membranes can be evaluated from the steady-state permeability. Koros, Chan and Paul in 1977, expressed the equation as zero at the downstream face. Then by applying the Leibenitz rule, the equation becomes
Deff (P0)=P0[dp/ dc]c0+P[ dp/ dc]c0 (4)
From the permeability vs pressure variation plots it is observed that the permeability of the membranes for CO2 gas decreased with the increase in the feed gas pressure. The permeability vs feed gas pressure plot obtained here followed the gas diffusion model for the permeability of CO2 gas through the membranes. The permeabilities of the membranes for CO2 decreased with the increase in feed gas pressure. The membranes showed a continuous decrease in CO2 permeabilities up to a pressure of 50 cm Hg. Figures 8 and 9 showed the effects of feed gas pressure on the CO2 permeabilities of the membranes from pure CO2 and a mixture of CO2 and N2. The decrease in the Permeance of the membranes was observed as a consequence of the facilitated transport mechanism of dendrimer molecules incorporated into the membranes. The facilitated transport mechanism is schematically represented in Figure 10. According to the facilitated transport mechanism CO2 was transformed into small and mobile ion HCO3 - ion. Thus, it enhances the transportation of CO2 through the membrane. Similar variations were observed for Polysulfone and Cellulose Acetate composite membranes and PSF/PDMS composite membrane for separation of CO2 as reported by Mansoori et al. in previous literature [13].
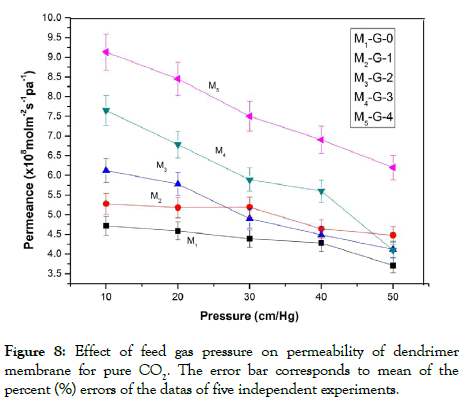
Figure 8: Effect of feed gas pressure on permeability of dendrimer membrane for pure CO2. The error bar corresponds to mean of the percent (%) errors of the datas of five independent experiments.
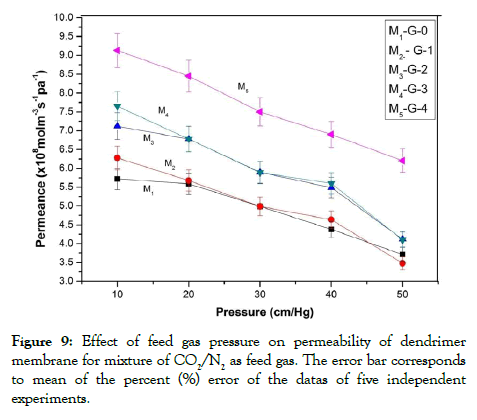
Figure 9: Effect of feed gas pressure on permeability of dendrimer
membrane for mixture of CO2/N2 as feed gas. The error bar corresponds
to mean of the percent (%) error of the datas of five independent
experiments.
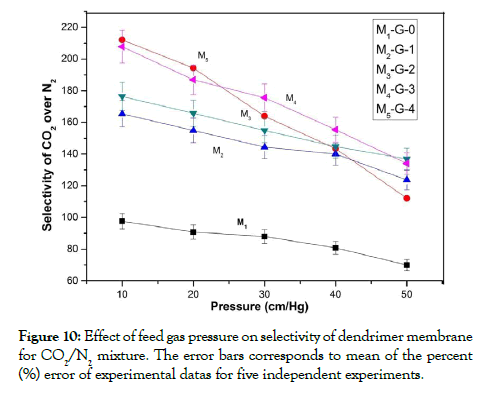
Figure 10: Effect of feed gas pressure on selectivity of dendrimer membrane for CO2/N2 mixture. The error bars corresponds to mean of the percent (%) error of experimental datas for five independent experiments.
Effect of feed gas pressure on selectivity: The selectivity of membranes for CO2 decreased with the increase in feed gas pressure. The effects of cross-linking of different generations dendrimer on membrane selectivities for CO2 with the feed gas pressure are shown in the Figure 11. It may happen due to increase in cross-linking of higher generations dendrimer molecules which form more numbers of gates for CO2 permeation. The selectivity order of the Silica and Zeolite composite membrane also follow the similar variation as reported by Shehu et al. [14] . The effect of pressure on selectivity of CO2 over CH4 also shows the similar behaviour as reported by Zhang et al. [1]. The selectivity of CO2/CH4 is comparable with that of Cellulose Acetate membranes reported in literature [11]. The effect of pressure on selectivity of CO2 over N2 also shows the similar behaviour as reported by Gil et al. [15]. In Table 2, literature datas of CO2 permeation by different membranes are given.
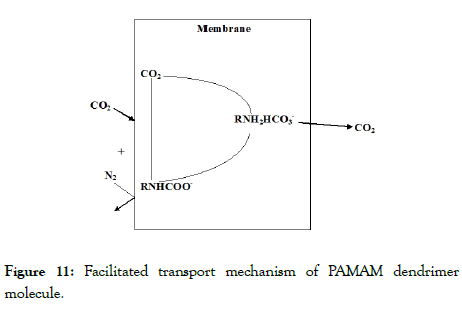
Figure 11: Facilitated transport mechanism of PAMAM dendrimer molecule.
| Sl. No. | Types of Membranes | CO2 permeance PCO2 ( × 108 molm-2s-1pa-1) | Separation factor (α) | References |
|---|---|---|---|---|
| 1 | DDR-Type membranes | 30 | 200 | [16] |
| 2 | Zeolite-T membranes | 4.6 | 400 | [17] |
| 3 | SAPO-34 membranes | 20 | 36 | [18] |
| 4 | Polyvinylalcohol membrane | 6.196 | 493 | [8] |
| 5 | PAMAM (G-0) | 4.1 | 700 | [9] |
| 6 | Polysulfone-dendrimer membrane | |||
| a. G-0 | 1.5 | 98 | Current work | |
| b. G-1 | 5.4 | 165 | ||
| c. G-2 | 5.6 | 178 | ||
| d. G-3 | 6.5 | 210 | ||
| e. G-4 | 8 | 215 |
Table 2: CO2 separation performances of reported membranes.
Structure-activity relationship of dendrimers with permeability: The permeabilities of dendrimer membranes are expected to correlate with their molecular structure. Accordingly the estimated values of permeabilities were plotted against the number of NH2 group present in the dendrimers of different generations and are shown in Figure 12 which indicates increase in permeabilities with amine groups present in the dendrimer. More number of amine groups presents in the dendrimer increases the number of molecular gates for CO2 permeation via facilitated transportation of CO2 gas through the membrane. Thus the membrane prepared by G-4 dendrimer showed maximum permeability for CO2 gas. In order to analyse the effect of dendrimer polysulfone molar ratio in the composite membrane the permeability values were calculated for the membranes with different dendrimer polysulfone molar ratio and are shown in Table 3. From the table it is seen that the values of permeabilities increases with increase in dendrimer polysulfone molar ratio. However, increase in concentration of dendrimer in the casting solution decreases the membrane forming capacity due to high saturation of dendrimer in the solution. Hence, for better performance of the membrane for CO2 permeation the dendrimer polysulfone molar ratio is observed as 0.5 to 10.
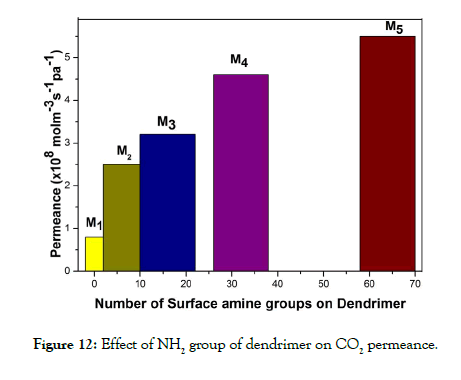
Figure 12: Effect of NH2 group of dendrimer on CO2 permeance.
| Dendrimer | Dendrimer Polysulfone molar ratio | CO2 permeance PCO2 ( × 108 molm-2s-1pa-1) |
|---|---|---|
| G0 | 0.1:10 | 0.67 |
| 0.2:10 | 0.73 | |
| 0.3:10 | 0.8 | |
| 0.4:10 | 1.23 | |
| 0.5:10 | 1.5 | |
| G1 | 0.1:10 | 1.76 |
| 0.2:10 | 1.82 | |
| 0.3:10 | 2.91 | |
| 0.4:10 | 3.56 | |
| 0.5:10 | 5.4 | |
| G2 | 0.1:10 | 3.12 |
| 0.2:10 | 4.21 | |
| 0.3:10 | 4.67 | |
| 0.4:10 | 4.98 | |
| 0.5:10 | 5.6 | |
| G3 | 0.1:10 | 4.54 |
| 0.2:10 | 4.89 | |
| 0.3:10 | 5.12 | |
| 0.4:10 | 6.12 | |
| 0.5:10 | 6.5 | |
| G4 | 0.1:10 | 2.56 |
| 0.2:10 | 3.23 | |
| 0.3:10 | 4.13 | |
| 0.4:10 | 7.4 | |
| 0.5:10 | 8 |
Table 3: Effect of dendrimer polysulfone molar ratio on CO2 permeation.
Conclusion
Membranes for CO2 separation had been prepared and were characterized by IR, XRD, TGA-DTA, SEM Analysis. The permeability of the membranes for CO2 had been studied using the membranes. The facilitated transport mechanism of dendrimer molecule (G-0-G-4) incorporated into the membranes which facilitated the transportation of CO2 through the membranes had been discussed in this paper. The effect of feed gas pressure on the permeabilities and selectivities of the membranes had been also observed. It was observed that permeability and selectivity of the membrane increased with increase in the amine groups present in the dendrimer molecule due to the formation of CO2 molecular gates by facilitated transport mechanism.
REFERENCES
- Zhang Y, Wang Z, Wang SC. Selective permeation of CO2 through new facilitated transport membranes. Desalination. 2002;145:385-388.
- Zhang Y, Wang Z, Wang S. A study on facilitated transport membranes for removal of CO2 from CH4. Fuel Chem Preprint. 2002;47:73-74.
- Fuerst O, Lin Y, Granell M, Leblanc G, Padrós E, Lórenz-Fonfría VA, et al. The melibiose transporter of Escherichia coli critical contribution of lys-377 to the structural organization of the interacting substrate binding sites. J Biol Chem. 2015;290:16261-16271.
- Lin Y, Fuerst O, Granell M, Leblanc G, Lórenz-Fonfría V, Padrós E. The substitution of Arg149 with Cys fixes the melibiose transporter in an inward-open conformation. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2013;1828:1690-1699.
- Lin Y, Bogdanov M, Tong S, Guan Z, Zheng L. Substrate selectivity of lysophospholipid transporter LplT involved in membrane phospholipid remodeling in Escherichia coli. J Biol Chem. 2016;291:2136-2149.
- Tong S, Lin Y, Lu S, Wang M, Bogdanov M, Zheng L. Structural insight into substrate selection and catalysis of lipid phosphate phosphatase PgpB in the cell membrane. J Biol Chem. 2016;291:18342-18352.
- Tong Z, Winston Ho WS. Facilitated transport membranes for CO2 separation Capture. Sep Sci Technol. 2019;6:1-12.
- Huang J, Zou J, Ho WW. Carbon dioxide capture using a CO2-selective facilitated transport membrane. Ind Eng Chem Res. 2008;47:1261-1267.
- Kovvali AS, Chen H, Sirkar KK. Dendrimer membranes: a CO2-selective molecular gate. J Am Chem Soc. 2000;122:7594-7595.
- Belmabkhout Y, Serna-Guerrero R, Sayari A. Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: application for gas purification. Ind Eng Chem Res. 2010;49:359-365.
- Donohue MD, Minhas BS, Lee SY. Permeation behavior of carbon dioxide-methane mixtures in cellulose acetate membranes. J Membrane Sci. 1989;42:197-214.
- Duan S, Chowdhury FA, Kai T, Kazama S, Fujioka Y. PAMAM dendrimer composite membrane for CO2 separation: addition of hyaluronic acid in gutter layer and application of novel hydroxyl PAMAM dendrimer. Desalination. 2008;234:278-285.
- Mansoori S, Pakizeh M, Jomekian A. CO2 selectivity of a new PDMS/PSF membrane prepared at different conditions. J Membr Sci Technol. 2011;1.
- Shehu H, Okon E, Orakwe I, Gobina E. Study of the selectivity of methane over carbon dioxide using composite inorganic membranes for natural gas processing. J Adv Chem Eng. 2016;6:150.
- Francisco Gil J. Separation of carbon dioxide from nitrogen using poly vinyl alcohol)-amine blend membranes. Int J Mol Sci. 2011;46:1245-1250.
- Himeno S, Tomita T, Suzuki K, Nakayama K, Yajima K, Yoshida S. Synthesis and permeation properties of a DDR-type zeolite membrane for separation of CO2/CH4 gaseous mixtures. Ind Eng Chem Res. 2007;46:6989-6997.
- Cui Y, Kita H, Okamoto KI. Preparation and gas separation performance of zeolite T membrane. J Mater Chem. 2004;14:924-932.
- Poshusta JC, Tuan VA, Pape EA, Noble RD, Falconer JL. Separation of light gas mixtures using SAPO‐34 membranes. AIChE J. 2000;46:779-789.
Citation: Bharali P, Borthakur S, Hazarika S (2020) Selective Permeation of CO2 through Amine Bearing Facilitated Transport Membranes. J Membra Sci Technol 10:203. doi: 10.35248/2155-9589.2020.10.203
Copyright: © 2020 Bharali P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

