Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2018) Volume 9, Issue 4
Safety and Immunogenicity of Rift Valley Fever MP-12 and a Novel arMP-12?NSm21/384 Recombinant Vaccine Candidate in Native Breed of Black Head Sheep (Ovis aries) from Tanzania
Abstract
Objective: Rift Valley fever (RVF) is a mosquito-borne zoonotic viral disease that affects humans and ruminants in Africa and the Arabian Peninsula. Efforts to develop effective vaccines have had limited success. Therefore, the aim of this study was to evaluate the safety and immunogenicity of RVF MP-12, and arMP-12ΔNSm21/384 vaccine candidates in sheep.
Methods: One group of 6 sheep was vaccinated intramuscularly (IM) each with one ml of 1×105 PFU/ml of the RVF MP-12 and 9 sheep were inoculated IM each with one ml of the arMP-12ΔNSm21/384 vaccine candidate, and two control sheep received one ml each of only Eagle's Minimum Essential Medium. Blood samples obtained on days 14 and 0 before vaccination and on days 3, 4, and 5 post vaccination (PV) were tested for RVFV in Vero cells and by RT PCR assay, and samples collected at interval PV through day 87 and on days 7, 14 and 21 following revaccination on day 87 PV to test for RVFV neutralizing antibody response by the plaque reduction neutralization test.
Results: All animals, including the controls remained in good health during the PV period as supported by normal body temperature, and the absence of clinical manifestations throughout this study. A viremia was not detected in any of the animals. Six of 6 animals that received the RVF MP-12 and 8 of 9 animals that received the arMP-12ΔNSm21/384 had antibody titers that ranged from 1:10 on day 5 PV to as high as 1:40 to 1:160 through day 87 PV. The antibody titers for these 15 animals following revaccination on day 87 PV with 1×104 PFU/ml of the MP-12 vaccine increased rapidly and by day 21 PV the titers for most animals ranged from 1:160 to 1:640.
Conclusion: Overall, these findings based on a limited number of sheep indicated that both the MP-12 and the arMP-12ΔNSm21/384 are promising vaccine candidates for the prevention of RVF in sheep in Africa.
Keywords: Rift Valley Fever (RVF); RVF virus; RVF antibody; RVFMP-12 vaccine; arMP-12ÎNSm21/384 Vaccine; Sheep; Tanzania
Introduction
Rift valley fever (RVF) is an arthropod-borne viral disease that has a significant social, health and economic impact on ruminants and humans throughout much of Africa and the Arabian Peninsula [1,2]. RVF is caused by Rift valley fever virus (RVFV), a negative singlestranded RNA virus that belongs to the genus Phlebovirus, family Bunyaviridae RVFV is mainly transmitted by mosquitoes of the genus Aedes and Culex, but other important routes of transmission to humans include the consumption of uncooked meat of infected animals and contact with excretions and bodily tissue of infected animals. The disease is characterized by fever, ocular and nasal discharge, bloody diarrhoea, abortion storms in gestating ewes and 90-100% mortality in new-born lambs. In humans, the disease causes self-limiting febrile illness which can lead to recovery and in rare cases progress to neurological disorder, vision loss, haemorrhagic fever and sometimes death [3-5].
RVF is among the most important zoonotic diseases throughout Africa because of the continuing occurrence of outbreaks with more recent ones occurring during 2013-2014 in Senegal, 2015 in Mauritania, 2016 in Uganda and Niger, 2018 in South Sudan involving morbidity and mortality in livestock and humans [6-8]. In Tanzania, mortality among domestic ruminants during the last outbreak of RVF in 2006 and 2007 resulted in decreased food security and malnutrition in affected communities. Economical losses due primarily to high mortality of sheep was approximately USD 2.2 million and USD 3.84 million were used in efforts to control the disease [9,10]. As a result of the devastating impact of RVF on human and animal health, there is a critical need for effective surveillance and control strategies, especially the availability of safe and efficacious vaccines to prevent human and animal disease [11,12].
RFV vaccines are considered to be effective strategies for the prevention of RVF in animals and humans [13-15]. Several live attenuated RVF vaccines are available, but the Smith Burn and Clone 13 NSs-deletion are the more commonly used vaccines [16]. However, the Smith Burn vaccine causes abortion in gestating ewes and fetal malformation [17-19]. The Clone 13 vaccine affords protection to domestic ruminants, but experimental studies indicated that the vaccine has potential teratogenic effect among pregnant sheep [16,20]. Among the candidate vaccine, the live attenuated, RVF Mutagenesis Passage 12 (MP-12) vaccine candidate is a promising human and veterinary vaccine [21-25] and the recombinant derivative arMP-12ΔNSm21/384 is also a safe and efficacious vaccine candidate for sheep and calves [26-28].
RVF MP-12 is a live-attenuated mutagenized vaccine that was developed by 12 serial passages of the wild type RVFV ZH 548 strain in human diploid lung (MRC-5) cells in the presence of the chemical mutagen 5-fluorouracil [29]. Although studies have demonstrated that RVF MP-12 was safe and efficacious in ruminants and humans, the vaccine is not designed to elicit antibody that can differentiate natural infected animals from vaccinated animals (DIVA). Therefore, RVF arMP-12ΔNSm21/384 was developed from the parent recombinant arRVF MP-12 virus using reverse genetic technology to delete the non-structural nucleotides of the viral RNA medium segment to produce a biomarker that could possibly serve as a serological marker for use as a DIVA vaccine [30-32]. While the RVF MP-12 and RVF arMP-12ΔNSm21/384 candidate vaccines were found to be safe and efficacious for cattle and sheep in the United States and the arMP-12ΔNSm21/384 for sheep in Canada, these candidates have not been tested in Africa livestock where this disease is enzootic [21-29]. Therefore, the purpose of this study was to test safety and immunogenicity of MP-12 and arMP-12ΔNSm21/384 in local breeds of sheep in Tanzania with the aim of generating data required to contribute to the long term goal of developing a commercial veterinary vaccine for use to prevent RVF in Africa.
Materials and Methods
Study site
The study was conducted at the Sokoine University of Agriculture (SUA) in Morogoro, Tanzania (6°49′S 37°40′E/ 6.817°S 37.667°E) in an insect proof and secured Animal Biosafety Level 2 (ABSL-2) facility and a Biosafety Level 2 (BSL-2) virology laboratory. The animal facility is designed to prevent the entry of arthropods and to provide sanitation measures and is well ventilated with a water supply and a nearby incinerator for disposing animal carcasses and animal waste. All work with the RVF MP-12 and arMP-12ΔNSm21/384 vaccine virus was done at the Biosafety Level 2 (BSL 2) and Animal Biosafety Level 2 (ABSL 2) because these viruses are classified as BSL 2 agents. Although challenge studies of the vaccinated animals to assess possible vaccine induced protective immunity were strongly desired, such studies could not be conducted because appropriate BSL 3 agricultural plus containment facilities were not available.
Experimental animals
Seventeen healthy black head fat tailed sheep (Ovis aries) 6-9- months-old were purchased from local vendors in the Mvomero district of Morogoro, Tanzania. The animals were ear tagged with individual identification numbers and treated with ®Steladone 300 EC Acaricide and 4 ml 2.5% Albendazole orally for ectoparasites and endoparasite, respectively. After ear tagging and treatment, the animals were allowed to acclimatize for two weeks in the ABSL 2 facility. Throughout the experiment, all animals were fed ad libitum with fresh grasses, water, and mineral blocks and monitored daily for elevated body temperature as a possible indication of illness. The protocol for the use and care of animals in this study was approved by the University of Texas at El Paso and the SUA Institutional Animal Care and Use Committees.
Vero E6 cells and vaccine viruses
The Vero E6 cells used in this study were provided by the University of Texas at El Paso (UTEP), Texas. Aliquots of 1.0 ml in freeze dried form of the arMP-12ΔNSm21/384 vaccine (Lot No 15/3/2017) were provided by the Multi-chemical industry (MCI) Sante Animal Biopharmaceutical Company in Mohammedia, Morocco. The identity of arMP-12ΔNSm21/384 virus was confirmed at MCI by qualitative real time polymerase chain reaction assay (QPCR) [33] that targeted the L and M viral RNA segments of the virus and then sequenced in Genewiz laboratories (GENEWIZ Global Headquarters; USA), using Next Generation Sequencing technology (NGS) Illumina method 1 × 50 bp SR, HiSeq2500, High Output, per lane (V4 chemistry). The infectivity titer of the arMP-12ΔNSm21/384 vaccine virus was 105.5TCID50/ml in Vero E6 cells. The MP-12 virus was originally obtained by UTEP from the World Reference Centre for Emerging Viruses and Arboviruses, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston Texas. At UTEP, the identity of the MP-12 vaccine virus was confirmed by plaque reduction neutralization test using RVF MP-12 specific monoclonal antibody. The Mab neutralized the infectivity titer of the MP-12 virus from 106 plaque forming units (PFU)/ml to 102 PFU/ml, but did not neutralize the infectivity titer of Sindbis and/or West Nile viruses. A stock virus of RVF MP-12 with an infectivity titer of 1.4 × 107 PFU/ml was prepared in Vero E6 cells and stored in 0.5 ml aliquots at -80°C. Of this stock, 10 aliquots of 0.5 ml each were provided to the SUA virology laboratory for used to prepare working virus stocks to support this study. At SUA, a working stock of the MP-12 virus was prepared in Vero E6 cells that had an infectivity titer of 1.0 × 107 PFU/ml.
Vaccination of sheep
RVF vaccines inoculum doses of 1×105 PFU/ml were prepared 2 hours before vaccination for the RVF arMP-12ΔNSm21/384 and MP-12 vaccines. Each vial of lyophilized arMP-12ΔNSm21/384 vaccine was reconstituted in 2 ml of Eagle's Minimum Essential Medium (EMEM) containing 4% fetal bovine serum (FBS) to yield a dose of 1×105 PFU/ml. The MP-12 vaccine stock (1.0 × 107 PFU/ml) was diluted 1:100 in EMEM to yield a final concentration of 1 × 105 PFU/ml. After preparation, one ml doses of each vaccine were loaded into 5 ml syringes in a class II A2 biological safety cabinet. EMEM medium supplemented with 4% FBS was prepared likewise to administer to the control animals. The loaded syringes were kept at 4°C and transported to the ABSL2 facility in a refrigerated container.
On day 14 before vaccination and on day 0 immediately prior to administering the RVF arMP-12ΔNSm21/384 and MP-12 vaccines, a 3 ml venous blood sample was collected from the jugular vein of each sheep with an 18 gauge needle attached to 5 ml syringe. Each of 9 sheep was then vaccinated intramuscularly (IM) with one ml in the neck area with 1 × 105 PFU/ml of the arMP-12ΔNSm21/384 vaccine candidate, and each of 6 sheep were inoculated IM with one ml in the same area with 1 × 105 PFU/ml of the MP-12 vaccine, and 2 sheep were vaccinated IM each in the neck area with one ml of EMEM media supplemented with 4% FBS to serve as controls. Information was recorded for each animal, including the date of inoculation, vaccine dose and route, identification numbers, sex, and the animal pen number. All 17 animals were housed in the same room of the ABSL 2 facility. Blood samples were obtained from each animal on days-14, 0, 3, 4, 5, 7, 14, 21, 28, 35, 70, 84, and 87 PV.
One to 2 ml of sera were obtained from each of the blood samples after leaving the samples overnight at 4°C followed by centrifugation at 1200 × G for 10 min. Aliquots of 0.5 ml of each serum sample was stored at -80°C for antibody testing. Also, rectal temperatures were recorded for each animal at weekly interval PV. On day 87 PV, all sheep including the 2 EMEM control animals received a booster dose of one ml of 1 × 104 PFU/ml of the MP-12 vaccine. All animals were observed for sign of illness and rectal temperatures were recorded once a week. Blood samples were obtained 14 days before vaccination and on day 0 immediately before vaccination to test for RVFV and RVFV antibody, and after inoculation on days 0, 3, 4, and 5 to test for RVFV, and thereafter on days 7, 14, 21, 28, 35, 70, 84, and 87 following the initial vaccination and on days 7, 14 and 21 following revaccination on day 87 PV to determine the neutralizing antibody response by the plaque reduction neutralization test. The purpose for conducting the study for 87 days was to obtain a preliminary estimate of the duration of antibody response that was based on the availability of the ABSL 2 facility.
Reverse transcription polymerase chain reaction assay
The RNA was extracted from sera samples obtained from sheep following the manufacturer’s instructions using the QIAamp® Viral RNA Mini kit (Qiagen, Hilden, Germany) and stored at -80°C. Sera samples collected from the animals on days-14 and 0 and on days 3, 4, and 5 PV were tested as pools of 2 samples for RVFV RNA. The RNA samples were tested for RVFV RNA with a one-step RT-PCR assay using the following set of primers partially targeting the M segment (551 bp):RVF forward 5’-TGT GAA CAA TAG GCA TTG G’-3 and RVF reverse 3’-GAC TAC CAG TCA GCT CAT TAC-5’ with a concentration of 0.1 μM. Reverse transcription of the viral RNA into cDNA was done at 50ºC for 30 min [34]. Denaturation of double stranded cDNA into two single stranded was done at 95°C for 30 min followed by primer annealing and attachment to the cDNA template for 1 min with annealing temperature of 58°C. Polymerization was done at 72°C for 2 min. Final extension was done for 10 min at 72°C. A total of 40 cycles were run during PCR. The PCR amplicons were run in 10 ul of gel red stain and 1.5% agarose at 120 voltages for 45 min and visualized under UV-transilluminator. MP-12 vaccine virus as RVFV viral RNA as a known positive control and master mix only as negative controls were included in the RT-PCR assay.
Viral isolation
Viral isolation assay were performed in Vero cells to test sheep sera samples for RVFV that were collected 14 days and on day 0 prior to vaccination and on days 3, 4, and 5 PV. Briefly, Vero E6 cells were seeded in 24 well plates and incubated at 37°C with 5% CO2 until the monolayer was 80% confluent. Then 50 ul of each sera sample was diluted 1:2 in EMEM medium and inoculated onto medium free cell monolayers in duplicate wells. RVF MP-12 vaccine virus and EMEM medium only were used as positive and negative control, respectively. After one hour of incubation at 37°C and 5% CO2, 0.5mL of Eagle's Minimum Essential Medium (EMEM) containing 4% fetal bovine serum (FBS) (maintenance media) was added to each culture and incubated at 37°C, 5% CO2 for 10 days. Cells were observed once daily for cytopathic effect (CPE). After incubation, the 24 well plates containing the cells and inoculum were stored at -80°C for a subsequent blind passage. Cultures were thawed at a room temperature and 50 uL of the diluted supernatant (1:2) was inoculated into Vero E6 cells and the procedures were repeated as described above. Any cultures that developed CPE were harvested and stored in aliquots of 1.0 ml for further study using RT-PCR to determine if the CPE was caused by RVFV. If there was evidence of RVFV, all aliquots and any remaining cultures were destroyed by heat in an autoclave at 112°F because of biosafety requirements that RVFV as a select agent must not be kept in a BSL 3 plus laboratory. The specific animals were isolated and quarantined in a holding facility separate from the ABSL 2 facility and were not used in this study.
Plaque reduction neutralization test
Sera samples obtained from sheep on day 14 before vaccination and on day 0 with the MP-12 and arMP-12ΔNSm21/384 vaccines, and on days 5, 7, 14, 21, 28, 35, 70, 84, and 87 PI were tested by the PRNT for RVFV neutralizing antibody. Also, sera samples obtained from the same animals on days 7, 14 and 21 PI after revaccination on day 87 with MP-12 were tested by the same technique for RVFV neutralizing antibody. Each PRNT assay included the test sera, and a known RVFV antibody positive serum sample and a RVFV antibody negative serum sample from sheep. Each sheep test serum samples was diluted in Hanks’ Balanced Salt Solution (HBSS) supplemented with one % each of HEPES, penicillin and streptomycin and heat-inactivated FBS. The dilutions sera samples were made in 96 well plates beginning with a 1:5 dilution in the first wells followed by 4-fold serial dilutions of 1:20, 1:80, 1:320, 1:1280, and 1:5120 in each of subsequent wells. Each diluted serum sample was then mixed with an equal volume of 60 to 80 PFU of MP-12 vaccine virus. The number of PFU was confirmed by plaque assay based on testing a mixture of equal volumes of the 60-80 PFU and HBSS to confirm that the final virus dose ranged from 30-40 PFUs. The antibody positive control consisted of a mixture of equal volume of 60-80 PFU and a 1:10 dilution of antibody positive sheep serum. The antibody negative control consisted of a mixture of equal volume of 60-80 PFU a 1:10 dilution of RVFV antibody negative sheep serum. The virus dose-serum dilution mixtures were incubated at 37°C in the absence of CO2 for one hour. Next, 50 ul of the virus dose-serum dilution mixtures were inoculated onto each of 2 Vero E6 cell cultures propagated in 24-well tissue culture plates and incubated for one hour at 37°C and 5% CO2. The mixture of the virus dose and the antibody positive control serum mixture were inoculated onto each of 20 cultures and the virus dose-antibody negative control serum mixture was inoculated onto 4 cultures. After the cultures and inoculum were incubated for one hour at 37°C with 5% CO2, each culture was overlaid with 0.5 ml of a Seakem agarose (1%) with an equal volume of 2X Eagle’s Basal Medium with Earle’s salts (EBME) supplemented with 8% FBS and one % penicillin/streptomycin, and Glutamine+8 g/l HEPES. After 2 more days incubation at 37°C with 5% CO2, each culture was overlaid with 0.5 ml of a mixture of an equal volume of agarose (1%) and 2X EBME supplemented with 5% neutral red, 8% FBS, and penicillin and streptomycin (1%) and Glutamine+8 g/l HEPES, and incubated overnight at 37°C with 5% CO2. The PFU were counted and recorded for both the controls and sheep sera test samples, and the antibody positive and negative controls. The neutralizing antibody titer of the dilution of each serum sample that reduced the number of PFU by 80% based on the number of PFU observed for the virus dose and antibody negative serum sample.
Statistical analysis
All serological data were analysed by using R software 3.4.1. Welch two-sample t-test to compare antibody responses in animals that received the MP-12 versus animals that received the arMP-12ΔNSm21/384 vaccine for possible statistical significance. Values of P<0.05 were considered significant.
Results
Screening animals
Sera samples obtained from all sheep 14 days before vaccination and on day 0 immediately prior to vaccination with the MP-12 and arMP-12ΔNSm21/384 were negative for RVFV by RT-PCR and isolation attempts in Vero cells. In addition, these sera samples from the sheep were negative for RVFV neutralizing antibody.
Animal health
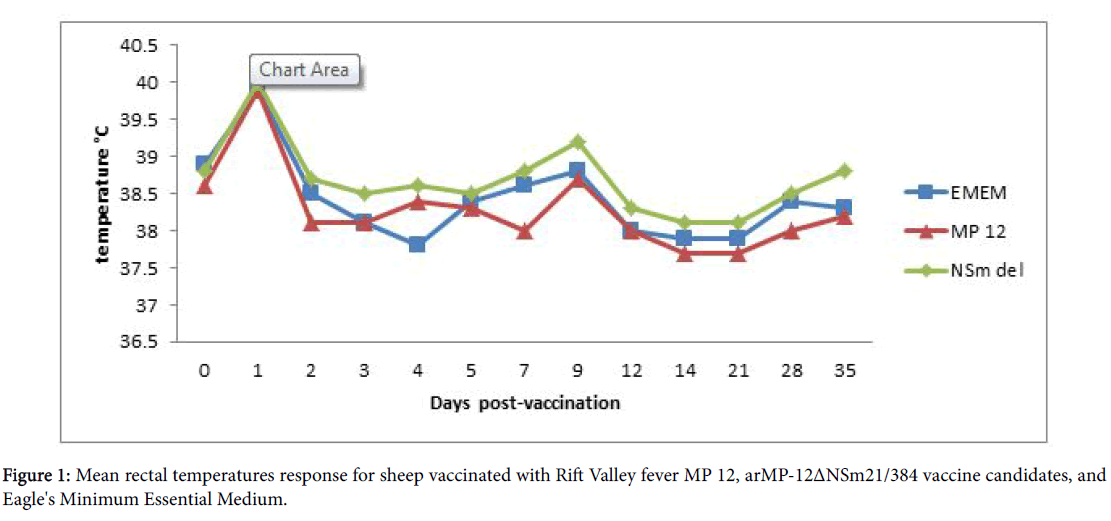
All sheep remained healthy based on the absence of any clinical signs of fever, nasal and ocular discharge, weakness and death throughout the study. The body temperature of the animals did not exceed 41°C throughout the study (Figure 1) and no changes were observed in eating, movement and drinking water.
Viremia
The MP-12 and arMP-12ΔNSm21/384 vaccine viruses were not detected in sera collected on days 3, 4 and 5 PV based on the absence of CPE in Vero cells, thus indicating that the animals did not develop a detectable viremia following vaccination. The 2 control animals remained antibody negative throughout the study period suggesting that the vaccinated animals did not shed the virus during the study.
Immunogenicity
All sheep vaccinated with MP-12 developed antibody with titers of 1:10 on day 5 PV, followed by titers that ranged from 1:10 to 1:160 through day 87, or before the animals were revaccinated with the MP-12 vaccine (Table 1). The antibody titer for sheep #51 was 1:160 for days 7-35 PV, which was higher than titers observed for other sheep that received the same vaccine dose. On revaccination of the sheep with the MP-12 vaccine, including the 2 EMEM control animals, the antibody titers increased rapidly in all animals, reaching maximum titers of 1:640 in 3 sheep, 1:160 in 3 sheep and 1:40 in two sheep by day 7 PV. The titers for some animals increased, especially for 2 animals that had titers of 1:2560 on days 14 and 21 PV (Table 1). The antibody response by the 2 EMEM control sheep was more comparable to that observed for the initially vaccinated animals, with titers of 1:40 and 1:160 on day 7 PV, followed by titers of 1:160 for each of the 2 animals on days 14 and 21 PV (Table 1).
| Days Post Vaccination | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Vaccine | -14 | 0 | 5 | 7 | 14 | 21 | 28 | 35 | 70 | 84 | 87 | 7 | 14 | 21 |
| 52 | EMEM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 160 | 160 | 160 |
| 61 | EMEM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 160 | 160 |
| 51 | MP 12 | 0 | 0 | 10 | 160 | 160 | 160 | 160 | 160 | 40 | 40 | 40 | 40 | 640 | 640 |
| 53 | MP 12 | 0 | 0 | 10 | 10 | 40 | 40 | 40 | 160 | 160 | 40 | 160 | 640 | 640 | 2560 |
| 54 | MP 12 | 0 | 0 | 10 | 40 | 40 | 40 | 40 | 40 | 40 | 160 | 160 | 640 | 2560 | 640 |
| 55 | MP 12 | 0 | 0 | 10 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 640 | 640 | 640 |
| 62 | MP 12 | 0 | 0 | 10 | 40 | 10 | 10 | 40 | 160 | 160 | 40 | 40 | 160 | 640 | 640 |
| 118 | MP 12 | 0 | 0 | 10 | 40 | 40 | 40 | 10 | 40 | 40 | 40 | 40 | 160 | 640 | ND |
| 63 | arMP-12 | 0 | 0 | 10 | 40 | 40 | 40 | 40 | 160 | 40 | 40 | 40 | 40 | 160 | 160 |
| 64 | arMP-12 | 0 | 0 | 0 | 0 | 10 | 10 | 40 | 10 | 10 | 10 | 10 | 160 | 640 | 640 |
| 65 | arMP-12 | 0 | 0 | 10 | 40 | 160 | 640 | 160 | 160 | 40 | 40 | 40 | 640 | 640 | 640 |
| 113 | arMP-12 | 0 | 0 | 10 | 40 | 40 | 40 | 160 | 160 | 160 | 40 | 40 | 160 | 40 | 640 |
| 115 | arMP-12 | 0 | 0 | 10 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 160 | 640 | 640 |
| 116 | arMP-12 | 0 | 0 | 10 | 40 | 160 | 160 | 160 | 40 | 40 | 40 | 40 | 160 | 2560 | 640 |
| 117 | arMP-12 | 0 | 0 | 10 | 40 | 40 | 10 | 10 | 10 | 10 | 160 | 160 | 160 | 2560 | 2560 |
| 119 | arMP-12 | 0 | 0 | 10 | 40 | 160 | 160 | 40 | 40 | 10 | 10 | 10 | 40 | 160 | 160 |
| 72 | arMP-12 | 0 | 0 | 10 | 40 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 40 | 160 | 160 |
*The antibody titer are expressed as the reciprocal dilution of the sera samples that neutralized 80% of the virus dose
Table 1: Rift Valley fever virus neutralizing antibody titers* for individual sheep following vaccination with MP-12 and arMP-12ΔNSm21/384 and a booster vaccination on day 87 post vaccination with the MP-12 vaccine.
RVFV neutralizing antibody for sheep vaccinated with arMP-12ΔNSm21/384 was detected on day 5 in 8 of the 9 animals with titers of 1:10. One of the animals # 64 did not have detectable antibody on day 5 PV, but had a titer of 1:10 on day 14 which was sustained until day 87 except for a 1:40 titer on day 28 PV. The antibody titers for the other 8 animals increased, reaching peak titers of 1:40 to 1:160 by day 14 and 28 PV. On revaccination of the animals, including the vaccination of the EMEM control animals with the MP-12 vaccine on day 87 PV, all revaccinated animals showed a robust increase in antibody titer by day 7 PV, with titers of 1:160 in 5 sheep, 1:640 in one sheep, and 1:40 in 3 sheep. The subsequent antibody response was characterized by an increasing pattern with titers ranging from 1:160-1:2560 on day 21 PV (Table 1). The antibody titers for the EMEM control animals ranged from 1:40 to 1:160, thus resembling the titers observed for the initial vaccinated animals with MP-12 and arMP-12ΔNSm21/384 vaccines.
Statistical results comparing MP12 and arMP-12ΔNSm21/384
There was no significance difference in antibody titers between sheep vaccinated with the MP-12 and arMP-12ΔNSm21/384 vaccines p=0.75 during first vaccination. Also there was no significance difference in antibody titers for these sheep following revaccination with the MP12 vaccine (p=0.02).
Discussion
The results of this study indicated that the RVF MP-12 and arMP-12ΔNSm21/384 vaccine candidates were safe in sheep vaccinated via the IM route in that all animals maintained normal body parameters such as gait, appetite, and normal rectal temperature (Figure 1). Other clinical manifestations associated with infection by wild type RVFV, such as hemorrhage, diarrhea, nasal and ocular discharge were not observed during this study [2,34,35]. Although only 2 control animals were used due to the limited availability of sheep at the time of the study, there was no evidence that the vaccine viruses were shed by the vaccinated animals as the control animals remained negative for virus while being confined in the same pens with vaccinated animals. In addition, that both the RVF MP-12 and arMP-12ΔNSm21/3 vaccine candidates are promising for the prevention of RVF was further supported by the observations that none of the animals experienced any adverse effects and none developed a detectable viremia.
The rapid immune response in the group of sheep vaccinated with a single dose MP-12 vaccine by day 5 demonstrated that the vaccines could possibly protect animals even if administered after the onset of a RVF outbreak [36]. Also, an overall sustained, as well as an increase in the pattern of neutralizing antibody titers PV showed that the vaccine activated antibody producing B cells with the highest antibody titers on days 14-35 in most animals The antibody response following revaccination with MP 12 vaccine virus was characterized by a rapid increase and high antibody titers on day 7 PV, followed by an increasing pattern to maximum titers of 1:2560 on day 14 and 21 PV thus, demonstrating that the animals were likely to be protected if exposed to a virulent RVFV. According to a previous study done using the arMP-12ΔNSm21/384 vaccine in sheep, an antibody titer of 1:100 or less affords protection against challenge with wild type RVFV [28]. Hence, the results of this study indicated that African breed of sheep vaccinated with MP-12 and arMP-12ΔNSm21/3 vaccines are likely to be protected if exposed to wild type RVFV.
Among the sheep vaccinated with the arMP-12ΔNSm21/384, 8 of 9 (88%) had detectable neutralizing antibodies by day 5 PV, thus demonstrating the potential of the vaccine to elicit a rapid immune response which was consistent with results reported previously [26,28]. Also, antibody titers increased in all sheep with peak titers on day 14 PV which were sustained through day 87 PV in most animals. These findings demonstrated the potential of the vaccine to induce high antibody titers within short period of time, and therefore increased the likelihood of vaccinated animals to be protected almost immediately during epizootics. Also, the results of studies reported by others in sheep, including gestating animals that received the arMP-12ΔNSm21/384 revealed that the vaccine elicited an antibody response that afforded protection and did not cause abortions, thus providing promising evidence in support of this RVFV vaccine candidate for the prevention of RVF among sheep in Africa [26].
The validity of the immune response to the initial vaccination of sheep with the arMP-12ΔNSm21/384 was supported by the pattern of the secondary immune response to the booster vaccination with the MP-12 vaccine. The results showed that the antibody titers increased rapidly in all sheep and were 12 fold higher than titers observed among the animals that received the initial vaccination. These findings show the potential of these animals to elicit a strong and likely protective immune response if exposed to wild type RVFV in the field. That the humoral immune system was primed by the initial vaccination is further supported by the observation that the antibody response in sheep #117 and 64 was poor during first vaccination but the response to revaccination with MP-12 was similar to that of the other animals that had a much stronger antibody response to the initial vaccination. When an animal is exposed to a virus, plasma cells start to differentiate and produce antibodies, and as they multiply the more the antibody are produced, but because these cells have a short life span they differentiate into antibody producing plasma B cells and into memory cells where by the antibody producing cells eventually dies while memory cells remain and serve to afford protection following secondary exposure to a similar pathogen [37]. Hence, if animals are vaccinated with these RVF vaccines they are likely to be protected when they are exposed to the wild type RVFV under field conditions.
There was no statistical difference between the immune response based on antibody titers for sheep vaccinated with the MP-12 and the arMP-12ΔNSm21/384 vaccines, thus indicating that either the MP-12- NSm-del or the MP-12 vaccine could be used to vaccinate sheep. Furthermore, the antibody titers were similar to results reported for the immune response of sheep to these vaccines in the United States with antibody being first detectable on day 5 PV [24-26]. Other data that support the use of either vaccine was generated by studies that showed MP 12 vaccinated sheep with antibody titer of ≥ 1:40 were protected from clinical disease following challenge by wild-type virulent ZH501 RVF virus [38,39]. Also, calves (Bos taurus) vaccinated with the arMP-12NSm21/384 vaccine had antibody titers ≥ 40 by day 14 PV, suggesting that domestic livestock vaccinated with MP-12 and arMP-12ΔNSm21/384 vaccines would not experience any adverse effect and would be protected against infection by the wild type RVFV [27]. However, the DIVA potential of the arMP-12ΔNSm21/384 vaccine candidate, if shown to be effective could provide an advantage over using the MP-12 vaccine candidate.
Other studies involving the vaccination of sheep in the United States and Canada with MP-12 and arMP-12ΔNSm21/384 reported antibody titers as high as ≥ 1:10240, which were substantially higher than peak titers observed for sheep in this study [24-26,28]. However, the titers in sheep in this study exceeded the protective immunity antibody titer reported for the sheep in Canada. According to the results of studies involving arMP-12ΔNSm21/384 vaccinated sheep challenge with a wild type RVFV, the clinical and pathological response to experimental RVFV infection in ruminants was dependent on the strain of RVFV, the species, breed and age of host animals [38-40]. Therefore, the lower antibody titers observed in our study may have in part been due to the fact that the species and age of sheep used in our study differed from sheep used in the previous studies [24-26,28]. Also, difference in the nutritional background of the animals and possible differences in health status might have affected the immune response status of the animals. The sheep used in our study were free ranging animals that were at greater risk to infestation of endo- and ecto-parasites as well as other pathogens making them prone to various infections that might have interfered with their immune response as compared to sheep held in feed lots in the United States and Canada. However, the use of locally bred and reared sheep in Africa is likely to provide a more realistic understanding of the immune response to the MP-12 and arMP-12ΔNSm21/384 vaccine candidates.
Our observations that the antibody response to MP-12 and arMP-12ΔNSm21/384 among local Tanzanian species sheep (Ovis aries) was more comparable to the response of sheep vaccinated with the RVF Clone 13 vaccine. The antibody titers reported for sheep vaccinated with clone 13 in Kenya ranged from a low of ≥ 40 to ≥ 480 [41]. The vaccination of sheep with Clone 13 in Senegal showed that 70% of the animals started seroconverting on day 60 PV with titers ≥ 1:80, however, antibody data before day 60, and titers above 1:80 were not presented, and therefore not possible to consider comparison of the observations [42-46]. Although the exact date of seroconversion and the antibody titers were not readily discernible, sheep vaccinated with Clone 13 vaccine were protected against challenge with wild type RVFV. While these finding suggested that the immune response of African species of sheep based on antibody titers was not as robust as that reported for sheep in the United States and Canada, the lower titers did not interfere with the protective efficacy of the Clone 13 vaccine, nor the safety based on the absence of clinical manifestations and abortions.
The use of antibody response in this study as an immunological marker of protective immunity was based on the results of several studies in the USA and Africa that showed antibody to be crucial for affording protection of humans and several species of animals against Rift Valley fever virus (RVFV) infection [2,21-23,25,26,28,36,41,46-51]. Among animals, antibody mediated protection has been demonstrated more conclusively in RVFV vaccinated domestic ruminants following by challenge with virulent RVFV. While we were aware of the importance of challenge studies in this study, such studies could not be performed because the appropriate biosafety facilities were not available to assess antibody as a correlate of protective efficacy. However, the development of neutralizing antibody in all sheep vaccinated with MP-12 and arMP-12ΔNSm21/384 vaccine candidates is likely to correlate with protection. This observation was further supported by the antibody titers observed in sheep in this study that were comparable or higher than the 1:100 titers observed in sheep following vaccination with the same vaccine and were protected against challenge with virulent RVFV in Canada [28]. Although both the innate and adaptive cellular immune responses may also be involved in protective immunity against RVFV infection [40,52,53], neutralizing antibody titers are the most commonly used correlates of RVFV protective immunity among both human and animals.
Although the findings of this study show the MP-12 and arMP-12ΔNSm21/384 vaccines to be promising for preventing RVF in African species of sheep, further studies using more animals and studies in pregnant sheep are needed to assess the safety and immunogenicity of these vaccines. Also, consideration needs to be given to the evaluation of other routes of vaccination, such as intradermal and intranasal using needle free devices as the two routes are believed to have a more immunogenic advantage [43-46] as compared to the standard route used in this study which have less antigen presenting cells and therefore, a lowered immune response.
Acknowledgments
The authors convey their sincere gratitude to USAID for providing the support required to implement this study. and to the research team of the Feed the Future Innovation Laboratory for Rift Valley Fever Control in Agriculture, including SUA, UTEP and MCI Sante Animale for their invaluable support throughout this study. Also, thanks to the laboratory attendants, Mhoni Peter Marwa and Shida Mkuya Hussein, and Dr. Anthony Mhando, the veterinary technician for assisting with animals experiment.
Source of Support
This study was funded under a subcontract from the University of Texas at El Paso (UTEP) under a Cooperative Agreement (AID-OAAA- 13-00084) from the United States Agency for International Development.
Disclaimer
The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government.
Conflict of Interest
The contents of this manuscript are original and all authors declare no conflict of interest.
Author Contributions
The authors, EA, DMW, JCM, GEB, PW, MKM, JR and SN conceived and designed the experiments. The experiments were performed by EA, SN, JR, and MKM, and the same authors participated in the acquisition, analysis, and interpretation of the data for the work. The authors, SN, EA, PMP, DMW, PW, GEB, MKM, and JCM prepared the manuscript. All authors participated in the revision of the manuscript, and agreed to be accountable for all aspects of the work and approved the final version this manuscript.
References
- Bird BH, Albarino CG, Hartman AL, Erickson BR, Ksiazek TG, et al. (2008) Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol 82: 2681-2691.
- Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J (2010) Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res 41: 61.
- Balkhy HH, Memish ZA (2003) Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int J Antimicrob Agents 21: 153-157.
- Baskerville A, Hubbard KA, Stephenson JR (1992) Comparison of the pathogenicity for pregnant sheep of Rift Valley fever virus and a live attenuated vaccine. Research in Veterinary Science 52: 307-311.
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, et al. (2011) Molecular Epidemiology of Rift Valley Fever Virus. Emerg Infect Dis 17: 2270-2276.
- Boushab BM, Fall-Malick FZ, Baba O, El Wafi S, Ould Salem ML, et al. (2016) Severe human illness caused by Rift Valley Fever virus in Mauritania, 2015. Open forum infect dis 3.
- Sow A, Faye O, Ba Y, Diallo D, Fall G, et al. (2016) Widespread Rift Valley fever emergence in Senegal in 2013–2014. Open forum infect dis 3.
- Stevens G, Mascarenhas M, Mathers C (2009) WHO brochure». Bulletin of the World Health Organization 87: 646–646.
- Sindato C, Karimuribo E, Mboera LEG (2011) The epidemiology and socio-economic impact of rift valley fever in Tanzania: a review. Tanzania J Health Res 13: 305-318.
- Sindato C, Karimuribo ED, Pfeiffer DU, Mboera LE, Kivaria F, et al. (2014) Spatial and temporal pattern of Rift Valley fever outbreaks in Tanzania; 1930 to 2007. PLoS One 2014 9: e88897.
- Rich KM, Wanyoike F (2010) An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am J Trop Med Hyg 83: 52-57.
- Sayed-Ahmed MZ, Nomier Y, Shoeib S (2015) Epidemic situation of Rift valley fever in Egypt and Saudi Arabia. J Dairy Vet Anim Res 2: 23-25.
- Sindato C, Karimuribo E, Mboera LE (2011) The epidemiology and socio-economic impact of Rift Valley fever in Tanzania: a review. Tanzan J Health Res 13: 305-318.
- Faburay B, LaBeaud AD, McVey DS, Wilson WC, Richt JA (2017) Current status of Rift Valley fever vaccine development. Vaccines 5: 29.
- Bird BH, Nichol ST (2012) Breaking the chain: Rift Valley fever virus control via livestock vaccination. Curr Opin Virol 2: 315-323.
- von Teichman B, Engelbrecht A, Zulu G, Dungu B, Pardini A, et al. (2011) Safety and efficacy of Rift Valley fever Smithburn and Clone 13 vaccines in calves. Vaccine 29: 5771-5777.
- Smithburn KC (1949) Rift Valley fever; the neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine. Br J Exp Pathol 1: 1-16.
- Coetzer JA, Barnard BJ (1977) Hydrops amnii in sheep associated with hydranencephaly and arthrogryposis with wesselsbron disease and rift valley fever viruses as aetiological agents. Onderstepoort J Vet Res 44: 119-126.
- Botros B, Omar A, Elian K, Mohamed G, Soliman A, et al. (2006) Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol 78: 787-791.
- Makoschey B, van Kilsdonk E, Hubers WR, Vrijenhoek MP, Smit M, et al. (2016) Rift Valley fever vaccine virus clone 13 is able to cross the ovine placental barrier associated with foetal infections, malformations, and stillbirths. PLoS Neglected Tropical Diseases 10: e0004550.
- Pittman PR, Norris SL, Brown ES, Ranadive MV, Schibly BA, et al. (2016) Rift Valley fever MP-12 vaccine Phase 2 clinical trial: Safety, immunogenicity, and genetic characterization of virus isolates. Vaccine 34: 523-530.
- Pittman PR, McClain D, Quinn X, Coonan KM, Mangiafico J, et al. (2016) Safety and immunogenicity of a mutagenized, live attenuated Rift Valley fever vaccine, MP-12, in a Phase 1 dose escalation and route comparison study in humans. Vaccine 34: 424-429
- Morrill JC, Mebus CA, Peters CJ (1997) Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am J Vet Res 58: 1104-1109.
- Morrill JC, Jennings GB, Caplen H, Turell MJ, Johnson AJ, et al. (1987) Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am J Vet Res 48: 1042-1047.
- Morrill JC, Carpenter L, Taylor D, Ramsburg HH, Quance J, et al. (1991) Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine 9: 35-41.
- Morrill JC, Laughlin RC, Lokugamage N, Pugh R, Sbrana E, et al. (2013) Safety and immunogenicity of recombinant Rift Valley fever MP-12 vaccine candidates in sheep. Vaccine 31: 559-565.
- Morrill JC, Laughlin RC, Lokugamage N, Wu J, Pugh R, et al. (2013) Immunogenicity of a recombinant Rift Valley fever MP-12-NSm deletion vaccine candidate in calves. Vaccine 31: 4988-4994.
- Weingartl HM, Nfon CK, Zhang S, Marszal P, Wilson WC, et al. (2014) Efficacy of a recombinant Rift Valley fever virus MP-12 with NSm deletion as a vaccine candidate in sheep. Vaccine 32: 2345-2349.
- Caplen H, Peters CJ, Bishop DH (1985) Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol 66: 2271-2277
- Won S, Ikegami T, Peters CJ, Makino S (2007) NSm Protein of Rift Valley Fever Virus Suppresses Virus-Induced Apoptosis. J Virol 81: 13335-13345.
- Kalveram B, Lihoradova O, Indran S V, Ikegami T (2011) Using Reverse Genetics to Manipulate the NSs Gene of the Rift Valley Fever Virus MP-12 Strain to Improve Vaccine Safety and Efficacy. J Vis Exp 1: e3400
- Ikegami T, Hill TE, Smith JK, Zhang L, Juelich TL, et al. (2015) Rift Valley Fever Virus MP-12 Vaccine Is Fully Attenuated by a Combination of Partial Attenuations in the S, M, and L Segments. J Virol 89: 7262-7276.
- Garcia S, Crance JM, Billecocq A, Peinnequin A, Jouan A, et al. (2001) Quantitative Real-Time PCR Detection of Rift Valley Fever Virus and Its Application to Evaluation of Antiviral Compounds. J Clin Microbiol 39: 4456-4461.
- Daubney R, Hudson JR, Garnham PC (1931) Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. The Journal of Pathology and Bacteriology 34: 545-579.
- Mackenzie RD (1936) Findlay GM. Studies on neurotropic Rift Valley fever virus the susceptibility of rodents. Br J Exp Pathol 17: 352-361.
- Dungu B, Louw I, Lubisi A, Hunter P, von Teichman BF, et al. (2010) Evaluation of the efficacy and safety of the Rift Valley Fever Clone 13 vaccine in sheep. Vaccine 28: 4581-4587.
- Flehmig B, Staedele H, Xueref C, Vidor E, Zuckerman J, et al. (1997) Early appearance of neutralizing antibodies after vaccination with an inactivated hepatitis A vaccine. J Infect 35: 37-40.
- Weingartl HM, Miller M, Nfon C, Wilson WC (2014) Development of a Rift Valley fever virus viremia challenge model in sheep and goats. Vaccine 32: 2337-2344.
- Faburay B, Gaudreault NN, Liu Q, Davis AS, Shivanna V, et al. (2016) Development of a sheep challenge model for Rift Valley fever. Virology 2016; 489: 128-140.
- Busquets N, Xavier F, Martín-Folgar R, Lorenzo G, Galindo-Cardiel I, et al. (2010) Experimental infection of young adult European breed sheep with Rift Valley fever virus field isolates. Vector Borne and Zoonotic Diseases (Larchmont, NY) 10: 689-696.
- Njenga MK, Njagi L, Thumbi SM, Kahariri S, Githinji J, et al. (2015) Randomized controlled field trial to assess the immunogenicity and safety of rift valley fever clone 13 vaccine in livestock. PLoS Neglected Tropical Diseases: 9: e0003550.
- Lo MM, Mbao V, Sierra P, Thiongane Y, Diop M, et al. (2015) Safety and immunogenicity of Onderstepoort Biological Products’ Rift Valley fever Clone 13 vaccine in sheep and goats under field conditions in Senegal. Onderstepoort J Vet Res 82: 01-06.
- Hickling J, Jones R (2009) Intradermal delivery of vaccines: a review of the literature and the potential for development for use in low-and middle-income countries. Program for Appropriate Technology in Health (PATH), Ferney Voltaire.
- Giudice EL, Campbell JD (2006) Needle-free vaccine delivery. Adv Drug Deliv Rev 58: 68-89.
- Mousel MR, Leeds TD, White SN, Herrmann-Hoesing LM (2008) Comparison of traditional needle vaccination with pneumatic, needle-free vaccination for sheep. J Anim Sci 86: 1468-1471.
- Niklasson BS, Meadors GF, Peters CJ (1984) Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathol Microbiol Immunol Scand C 92: 197-200.
- Meadors GF 3rd, Gibbs PH, Peters CJ (1986) Evaluation of a new Rift Valley fever vaccine: safety and immunogenity trials. Vaccine 4: 179-184.
- Anderson GWJr, Slone TWJr, Peters CJ (1987) Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb Pathog 2: 283-293.
- Peters CJ, Jones D, Trotter R, Donaldson J, White J, et al. (1988) Experimental Rift Valley fever in rhesus macaques. Arch Virol 99: 31- 44.
- Pittman PR, Liu CT, Cannon TL, Makuch RS, Mangiafico JA, et al. (2000) Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine 18: 181-189.
- Morrill JC, Peters CJ (2003) Pathogenicity and neurovirulence of a mutagen-attenuated Rift Valley fever vaccine in rhesus monkeys. Vaccine 21: 2994-3002.
- Boshra H, Lorenzo G, Rodriguez F, Brun A ( 2011) A DNA vaccine encoding ubiquitinated Rift Valley fever virus nucleoprotein provides consistent immunity and protects IFNAR(−/−) mice upon lethal virus challenge. Vaccine 29: 4469-4475.
- Nfon CK, Marszal P, Zhang S, Weingartl HM (2012) Innate Immune Response to Rift Valley Virus in Goats. PLoS Negl Trop Dis 6: e1623.
Copyright: © 2018 Adamson EK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.