Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 8, Issue 4
Safety and Effectiveness of Inactivated Streptococcus thermophilus Atcc 19258 (Neoimmuno Hilus Gb®) Lotion on Atopic Dermatitis: A Randomized Placebo-Controlled Clinical Trial
Marina Piola Rossetto1, Gabriel Fernandes Alves Jesus1, Ana Paula Voytena2, Silvia Dal-Bó3, Zoé Feuser3, Heloisa de Medeiros Borges3 and Monique Michels1*2Gabbia Biotechnology, Itajaí, Santa Catarina, Brazil
3Department of Pharmacy, University of Southern Santa Catarina (UNESC), Criciúma, Brazil
Received: 14-Jun-2023, Manuscript No. JOD-23-21858; Editor assigned: 16-Jun-2023, Pre QC No. JOD-23-21858 (PQ); Reviewed: 03-Jul-2023, QC No. JOD-23-21858; Revised: 10-Jul-2023, Manuscript No. JOD-23-21858 (R); Published: 17-Jul-2023, DOI: 10.35248/2684-1436.23.8.202
Abstract
Aim: Our aim was evaluated the safety and clinical efficacy of a posbiotic lotion in the treatment of atopic dermatitis.
Methods: Randomized, double-blind, placebo-controlled clinical trial was performed in patients who had any degree of atopic dermatitis. A total of 24 volunteers were recruited and randomly divided into two groups: Placebo or Treated. (1% NEOIMUNO HILUS GB®). The ingredient used was NEOIMUNO HILUS GB® (Streptococcus thermophilus strain ATCC 19258). Physical analysis to determine the dermatitis degree and perception questionnaire was applied before and after treatment. The questionnaire included questions about perception of itching, oiliness, humidity and product quality. Statistical analyses were performed.
Results: The volunteers in the Treated Group showed a significant improvement in skin balance, moisture and oiliness after 28 days of using the NEOIMUNO HILUS GB® posbiotic lotion. The perception of the variable “skin irritation”, “flaking” and “itching” by the Treated Group showed a significant increase in the number of people who showed improvement in their condition (“Improved”).
Conclusion: Finally, the posbiotic lotion 1% (NEOIMUNO HILUS GB®) showed safety and efficacy in the treatment of the skin of people with atopic dermatitis.
Keywords
Atopic dermatitis; Posbiotic; Lotion; Cosmetic; Skin barrier; Skin microbiota
Introduction
Our skin is home to millions of bacteria, fungi and other microorganisms (about 102 cells/cm2–107 cells/cm2), which make up the cutaneous microbiota. Similar to the microorganisms in our gut, the microorganisms in our skin play a key role in protecting against invading pathogens and modulating our immune system. As the largest organ in the human body, the skin is colonized by beneficial microorganisms and serves as a physical barrier to prevent the invasion of pathogens. In circumstances where the skin barrier is broken or when the balance between beneficial and pathogenic microorganisms is disturbed (cutaneous dysbiosis), diseases associated with the skin may occur at different stages of life, such as atopic dermatitis [1-3].
Atopic Dermatitis (AD) is a chronic inflammatory dermatosis of multifactorial etiology, characterized by moderate to intense itching. This condition evolves in outbreaks and has a hereditary allergic character. In another form of conceptualization, atopic dermatitis would be defined as a chronic inflammatory cutaneous disease, of a genetic nature, characterized by the presence of recurrent episodes of eczema associated with pruritus, often intense, presenting as a substrate cutaneous immunological alteration that produce inflammation, which may eventually be associated with respiratory diseases such as asthma and allergic rhinitis.
One mechanism involved in the pathogenesis of AD is the decrease in regulatory T cells (Treg), which are crucial regulators of the immune response. It is already known that an imbalance in the activation of cytokines produced by Th1/Th2 are implicated in the development of AD, and increasing evidence suggests that the intestinal and skin microbiota play an important role in the regulation of the immune system. Another mechanism involved in AD is the dysfunction of the skin barrier, where damage to the skin barrier and microbiota can directly affect the mucosa and intestinal microbiome, due to the already proven bidirectional intestine-skin axis [4].
The 4 predominant groups in the skin microbiota are: Firmicutes (Staphylococcus, Streptococcus, Anaerococcus, Finegoldia, Veillonella, Lactobacillus, Peptoniphilus), Actinobacteria (Propionibacterium, Corynebacterium, Micrococcus, Kocuria, Actinomyces, Rothia), Proteobacteria (Acinetobacter, Haemophilus, Enhydrobacter, Neisseria, Microvirgula) and Bacteriodetes (Prevotella, Chryseobacterium, Fusobacteria, Leptotrichia). The most common species found on healthy skin is Staphylococcus epidermidis, which is used as an indicator of skin health [5,6].
Another factor that seems to be decisive in the severity of AD is the diversity of the skin microbiota. A reduction in the diversity of the skin microbiome has been observed in patients with AD, with AD being associated with early colonization by Staphylococcus aureus [7]. Another study found an association between the severity of AD and the abundance of the genus Corynebacterium and the phylum Proteobacteria. The presence and chronicity of eczema appear to be the most important determinants of the configuration of the skin microbiome [6].
In addition, dermatological diseases are a source of negative impact on the emotional state, social relationships and daily activities, to the stigma due to the appearance of the lesions. Chronic pruritus is often untreatable, thus having a major impact on the patient's quality of life [8].
There is growing interest in the effects of probiotics and paraprobiotics/posbiotics in the prevention and treatment of skin diseases due to their immunomodulatory and antiinflammatory properties.
Taverniti, et al. defined paraprobiotics (Ghost biotics) as nonviable, intact or ruptured containing cell components such as peptidoglycans, teichoic acids, surface proteins, etc., as well as crude cellular extracts (complex chemical composition), which, when taken in oral or topical quantities, provides benefit to the consumer human or animal [9]. A number of different terms can be found in the published research to address these preparations: Non-viable probiotics, heat-killed probiotics, tyndallized probiotics, cell lysates, paraprobiotics, ghostbiotics and postbiotics [10-12]. In 2021, a new definition of postbiotics was proposed by the International Scientific Association of Probiotics and Prebiotics (ISAPP), defining postbiotics as “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” The use of this inactivated probiotics (posbiotics) has a number of advantages, such as: Availability in its pure form, ease of production and storage, availability of a production process to increase industrial scale, specific mechanism of action, better accessibility of Molecular Pattern Associated with Microorganismos (MAMP) during recognition and interaction with Pattern Recognition Receptors (PRR) and, more likely to trigger only responses directed by specific ligand-receptor interactions [13]. The use of probiotics and postbiotics has been researched for the treatment of several diseases, including dermatology. Evidences suggest that the intestinal microbiota plays an important role in the pathogenesis of atopic dermatitis, inducing immunosuppression [4]. In AD, the barrier is impaired, not only in the skin, but also in the intestinal mucosa, causing the transfer of exogenous antigens.
The literature suggests that different probiotic and/or paraprobiotic and/or postbiotic strains can contribute to the preservation of skin integrity and homeostasis, since their use can be beneficial in dermatological conditions such as AD [10,14,15]. Di Marzio, et al. reported beneficial effects of Streptococcus thermophilus on atopic dermatitis [3].
Given its immunomodulatory and anti-inflammatory potential previously evaluated in “in vitro” tests, using dermal cells, the ingredient Neoimmuno HILUS GB® (Gabbia Biotecnologia e desenvolvimento Ltda.) produced from the thermal inactivation of the probiotic strain Streptococcus thermophilus ATCC 19258, was tested on volunteers diagnosed with atopic dermatitis. The aim of the present study was to evaluate the safety and clinical efficacy of a posbiotic lotion (Streptococcus thermophilus ATCC 19258) in the treatment of atopic dermatitis [16].
Materials and Methods
Ingredient
The ingredient used was NEOIMUNO HILUS GB®, produced from thermal inactivation the probiotic strain Streptococcus thermophilus ATCC 19258. In vitro assays with a fibroblast cell line (NIH-3T3) were previously performed to assess safety and determine the concentration of inclusion in the lotion (1%). The composition was: NEOIMUNO HILUS GB®-1%, Water qsp.; Cetyl alcohol-4.0%; Ceteareth 20-1.3%; Glyceryl stearate-5.0%; Dissodium EDTA (Ethylenediaminetetraacetic acid disodium)-0.1%; BHT(Butylated hydroxytoluene)-0.05%; Glycerin-2.0%; Ethylhexyl stearate-2.0%; Parafum-0.3%; Phenoxyethanol-0.9%; Caprylyl glycol 0.1%.
Ethics
The Ethics Committees of Universidade do Extremo Sul Catarinense approved the study (Number: 5.182.155), and all patients gave written informed consent, without any refusal.
Experimental design
A randomized, double-blind, placebo-controlled clinical trial was carried out in Universidade do Extremo Sul Catarinense-Brazil, in the pharmacy department. Briefly, during the study period (October 2021 to December 2021), a total of twenty-four adult volunteers (18-65 years old) with atopic dermatitis diagnostic were randomly divided into two groups: Placebo or treated (1% Neoimuno HILUS GB®).
Intervention
The volunteers underwent a careful physical evaluation, aiming to evaluate the atopic dermatitis degree (mild, moderate or severe), in addition to answering the screening questionnaire and perception questionnaire. After, volunteers were randomized into two groups. In the treated group, NEOIMUNO HILUS GB® was included at a concentration of 1% in the lotion, while the volunteers in the control group received a lotion with the same composition, but without the inclusion of the paraprobiotic. Both groups administered the respective lotions on the skin every day for 28 days. The amount of lotion administered was 4 grams (4 “pumps”) and was gently massaging the injury skin. The order and time of application of individual cosmetics have been explained. During the study protocol, the volunteers discontinued the use of any other lotions.
The volunteers were followed up for 28 days and the interventions were carried out in three moments.
Day 0–inclusion day–The volunteer underwent a screening with physical assessment to determine dermatitis degree (mild, moderate or severe), in addition to a screening and perceived efficacy questionnaire. Dermatitis degree was measured by the intensity of the lesions and how much of the body surface was affected. Based on the inflammation degree and proliferation in the skin, the dermatites degree was diagnosed as: Mild, moderate or severe.
Day 14–Physical analysis to determine the dermatites degree in this moment and application of the perceived efficacy questionnaire.
Day 28–Finalization-Physical analysis to determine the dermatites degree in this moment and application of the perceived efficacy questionnaire.
Perception questionnaire
Perception questionnaire was applied before and after treatment. The questionnaire included questions about perception of itching, oiliness, humidity and product quality.
Inclusion and exclusion criteria
Inclusion criteria: Both sexes; age 18-60 years; agree to suspend the use of other treatments during the 28 days of the study and exclusively use the one proposed by the project; volunteers with mild, moderate or severe dermatitis.
Exclusion criteria: Pregnant women, lactating women; comorbidities such as hypothyroidism or other metabolic diseases; use of corticosteroids, immunosuppressants and antihistamines; skin disease: dermatoses, vitiligo, psoriasis; history of allergic reactions, irritation or intense sensations of discomfort to topical products: Cosmetics and medicines.
Demographic variables were collected and qualitative and quantitative analyzes were carried out, through clinical evaluation, using the Skin Analyzer Digital equipment, evaluating the balance, humidity, oiliness and elasticity of the skin. For qualitative evaluation, a questionnaire of perceived effectiveness was applied after 14 and 28 days of product use. At the end of the trial, patients returned empty lotion bottles, ensuring product compliance and use
Statistical analysis
All analyzes were performed in SPSS v.21 software and graphs in GraphPad Prism. For qualitative analysis of perception, a likelihood ratio test was applied. For quantitative analysis, Student's t test was applied, p<0.05 was considered statistically significant.
Results
Data were evaluated after 14 and 28 days of using the paraprobiotic lotion. The results of the qualitative and quantitative assessments are presented below. No adverse effects were verified throughout the study, guaranteeing the safety of the NEOIMUNO HILUS GB® product.
Demographic and clinical data were collected using standardized forms, which included: gender, age, race, degree of dermatitis, site of lesion, use of sunscreen, use of medication for atopic dermatitis (Table 1).
| Total n=24 |
Group, n(%) | ||
|---|---|---|---|
| A n=12 |
B n=12 |
||
| Gender | |||
| Male | 6 (25) | 4 (33,33) | 2 (16,66) |
| Female | 18 (75) | 8 (66,66) | 10 (83,33) |
| Age (years) | |||
| 18-30 | 18 (75) | 9 (75,0) | 9 (75,0) |
| 31-45 | 5 (20,8) | 3 (25,0) | 2 (16,66) |
| 46-60 | 1 (4,2) | 0 (0,0) | 1 (8,33) |
| Race | |||
| White | 23 (95,8) | 12 (100,0) | 11 (91,66) |
| Black | 1 (4,2) | 0 (0,0) | 1 (8,33) |
| Dermatitis grade | |||
| Lite | 13 (54,2) | 6 (50,0) | 7 (58,33) |
| Moderate | 5 (20,8) | 3 (25,0) | 2 (16,66) |
| Severe | 6 (25) | 3 (25,0) | 3 (25,0) |
| Injury site | |||
| Superior members | 8 (33,3) | 4 (33,33) | 4 (33,33) |
| Inferior members | 2 (8,3) | 2 (16,66) | 0 (0,0) |
| Head | 1 (4,2) | 0 (0,0) | 1 (8,33) |
| All body | 13 (54,2) | 6 (50,0) | 7 (58,33) |
| Sunscreen | |||
| Yes | 11 (45,8) | 4 (33,33) | 7 (58,33) |
| No | 13 (54,2) | 8 (66,66) | 5 (41,66) |
| Dermocosmetics | |||
| Yes | 20 (83,3) | 11 (91,66) | 9 (75,0) |
| No | 4 (16,7) | 1 (8,33) | 3 (25,0) |
Table 1: Clinical and demographic aspects of the patients included in the study.
Oiliness
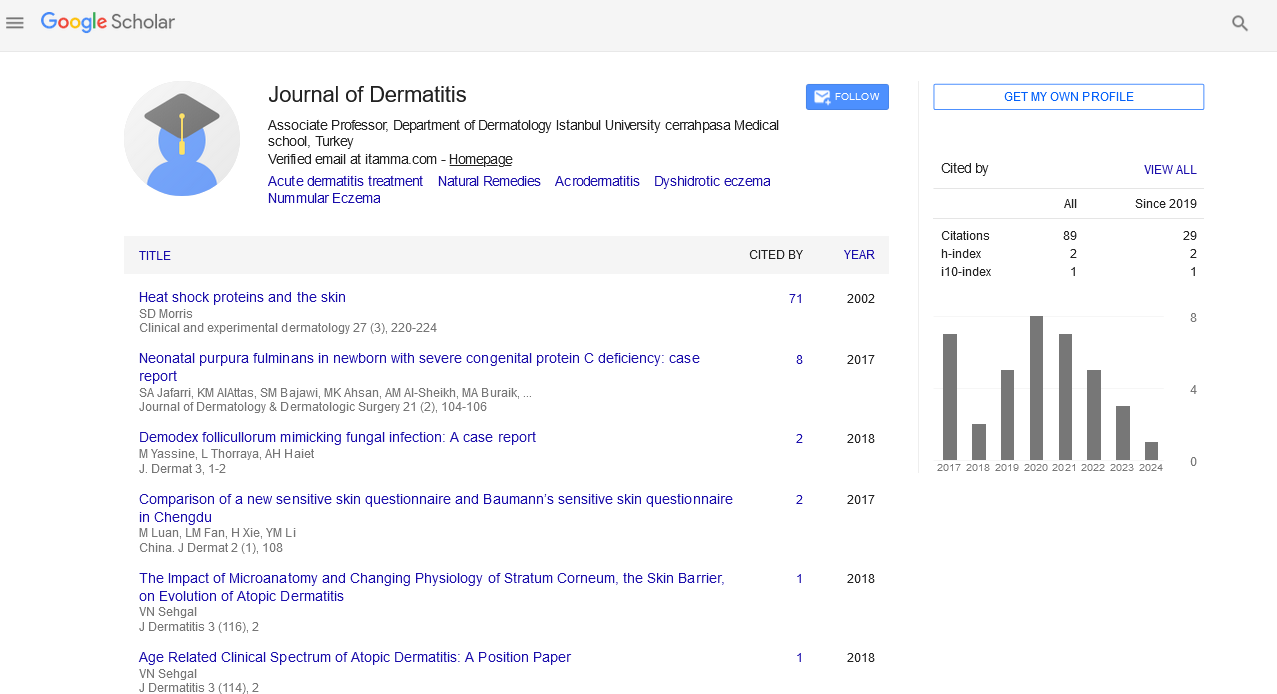
In the assessment of oiliness in relation to the use of the posbiotic lotion, there was a statistically significant improvement in the skin oiliness of people in the Treated Group, after 28 days of using the lotion, with an increase in people with skin oiliness between 15%-22%, values not found in the Placebo Group (Figure 1).
Figure 1: Assessment (before and after) of skin oiliness in patients with atopic dermatitis who used paraprobiotic lotion (NEOIMUNO HILUS GB®), or not, for 28 days. *Indicates statistically significant difference. A)Oiliness before, B) Oiliness 14 days, C) Oiliness 30 days.
Note: ( ) 15%-20%, (
) 15%-20%, ( ) 22%-35%, (
) 22%-35%, ( )>35%
)>35%
Moisture
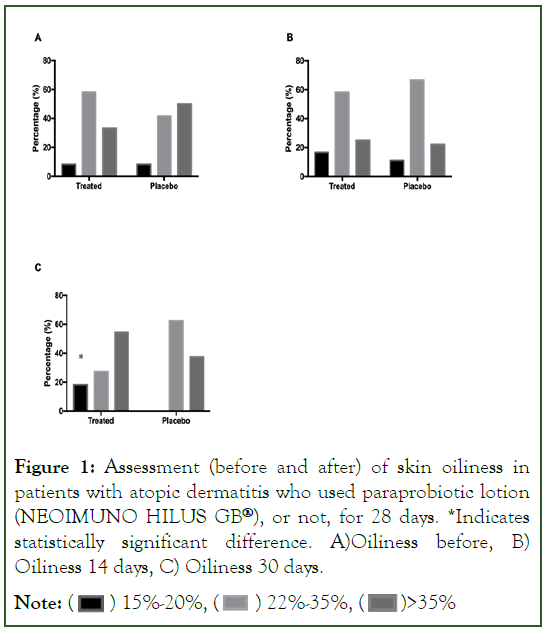
In the evaluation of the skin moisture of the volunteers, there is a statistically significant improvement on the part of the patients after 14 days of using the posbiotic lotion (NEOIMUNO HILUS GB®), with an increase in the number of volunteers with greater skin moisture, between 40%-60%. After 30 days, there is a numerical reduction, in the Treated Group, of volunteers with humidity <20%, when compared to a value greater than 60% of volunteers in the Placebo Group (Figure 2).
Figure 2: Evaluation (before and after) of skin moisture in patients with atopic dermatitis who used paraprobiotic lotion (NEOIMUNO HILUS GB®), or not, for 28 days. *Indicates statistically significant difference. A) Humidity before B) Humidity 14 days, C) Humidity 30 days
Note: ( ) <20%, (
) <20%, ( ) 21%-39%, (
) 21%-39%, ( ) 40%-60%
) 40%-60%
Elasticity
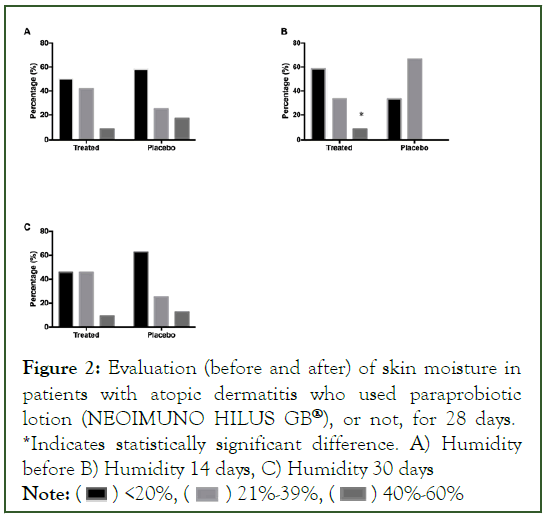
In the assessment of skin elasticity, no statistical changes were found between the groups (Figure 3).
Figure 3: Evaluation (before and after) of skin elasticity in patients with atopic dermatitis who used paraprobiotic lotion (NEOIMUNO HILUS GB®), or not, for 28 days. *Indicates statistically significant difference. A) Elasticity before, B) Electricity 14 days, C) Electricity 30 days.
Note: ( ) Happy face, (
) Happy face, ( ) Sad face
) Sad face
Perceived effectiveness
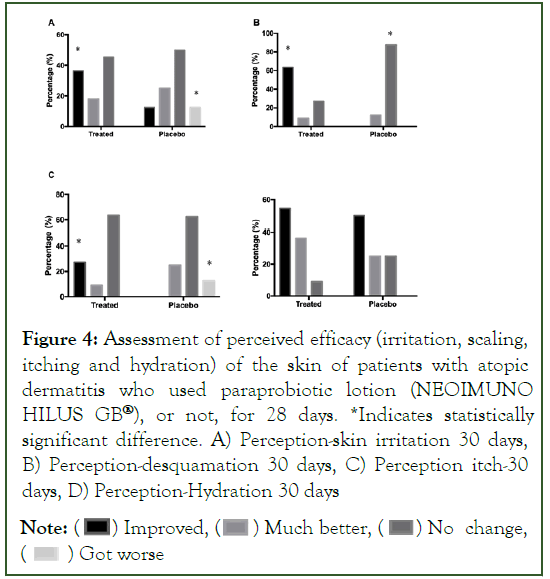
In the assessment of perceived efficacy, there was an improvement on the part of the Group Treated with the posbiotic lotion (NEOIMUNO HILUS GB®). For the perception of skin irritation, after 28 days of treatment, there was a significant increase in people who noticed improvement in skin irritation, while in the Placebo Group, people with worsening skin irritation conditions. For the assessment of desquamation, there is a higher percentage of people in the Treated Group who noticed improvements, while for the Placebo Group, there was a significant increase in people who verified “no change” in skin desquamation. With regard to the perception of itching, there was a significant improvement in the number of people in the Treated Group with the perception of “improvement” of itching, while in the Placebo Group, there was an increase in the number of people with “worsening” of the condition. As for skin hydration, no significant changes were observed (Figures 4 and 5).
Figure 4: Assessment of perceived efficacy (irritation, scaling, itching and hydration) of the skin of patients with atopic dermatitis who used paraprobiotic lotion (NEOIMUNO HILUS GB®), or not, for 28 days. *Indicates statistically significant difference. A) Perception-skin irritation 30 days, B) Perception-desquamation 30 days, C) Perception itch-30 days, D) Perception-Hydration 30 days
Note: (  ) Improved, (
) Improved, ( ) Much better, (
) Much better, ( ) No change, (
) No change, ( ) Got worse
) Got worse
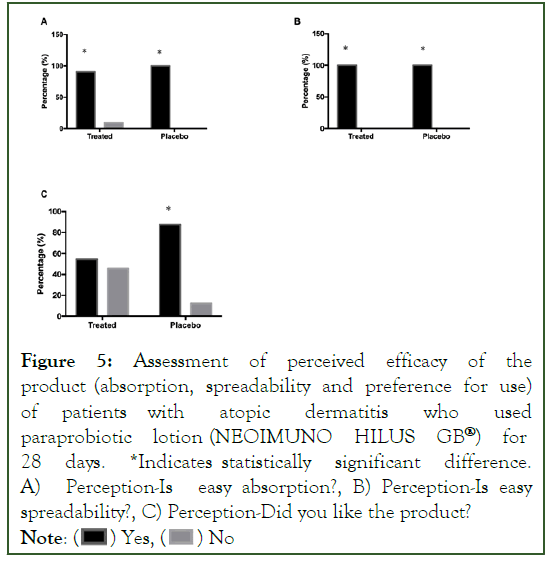
Figure 5:Assessment of perceived efficacy of the product (absorption, spreadability and preference for use) of patients with atopic dermatitis who used paraprobiotic lotion (NEOIMUNO HILUS GB®) for 28 days. *Indicates statistically significant difference. A) Perception-Is easy absorption?, B) Perception-Is easy spreadability?, C) Perception-Did you like the product?
Note: ( ) Yes, (
) Yes, ( ) No
) No
In the evaluation by images (Figure S1), there is an improvement in most of the people in the Treated Group, mainly in relation to the improvement of skin conditions (inflammation, irritation, desquamation, presence of wounds) and healing. It is verified that, after 28 days using the posbiotic lotion (Treated Group), the volunteer had a more hydrated, less inflamed and irritated skin, without flaking and wounds. In general, the representative figure in the supplementary material well represents the clinically verified improvement on the part of the Treated Group volunteers.
Discussion
Unbalanced skin (dysbiosis), as is the case of patients with atopic dermatitis, have a compromised barrier function, which makes this skin much more prone to colonization by pathogenic microorganisms, such as S. aureus, capable of forming biofilm and produce a series of compounds harmful to skin health, such as proteases and toxins, capable of further degrading the skin barrier, activating immune cells and triggering the expression of inflammatory mediators [5].
In view of this problem, the use of postbiotics (or paraprobiotics) has been gaining strength in the market over the last few decades, with a large number of advantages, such as: Availability in its pure form, ease of production and storage, availability of a production process to increase industrial scale, specific mechanism of action, better accessibility of Molecular Pattern Associated with Microorganismos (MAMP) during recognition and interaction with pattern recognition receptors (PRR) and, more likely to trigger only responses directed by specific ligand-receptor interactions [13]. Researchs involving the use of a topical application of probiotics in dermatology with benefits in atopic dermatitis, acne, seborrheic dermatitis, and wound healing has been related.
The skin of atopic people is characterized by dryness and impaired barrier function, resulting from an increase in transepidermal water loss. Studies indicate that the impairment of the barrier associated with atopic dermatitis may be related to the amount and composition of ceramides present in the stratum corneum, regulated by the balance between ceramideproducing enzymes [17,18]. Thus, an altered metabolism in the composition of sphingomyelin in the skin of atopic people has been attributed to a deficiency of ceramides, and a consequent vulnerability of atopic skin to irritating and allergenic compounds. In addition, studies show that the skin of atopic patients is more susceptible to the colonization of ceramidasesecreting bacteria, further compromising skin permeability and moisture/oiliness [19].
The present study found that the group treated with posbiotic lotion (Treated group) showed a significant increase in the number of people with increased skin moisture in 14 days; as well as people with oiliness between 15%-22%, in 30 days. Such benefits may be related to the ability of the posbiotic lotion to increase stratum corneum lipid levels, mainly ceramides, which can improve the lipid barrier function and increase skin moisture by reducing transepithelial water loss.
Corroborating with the present study, Di Marzio, et al. evaluated the effects of topical administration of a lotion containing inactivated S. thermophilus on stratum corneum ceramide levels in patients with atopic dermatitis [3]. For this, the lotion was applied to the skin of the forearms of 11 patients for 2 weeks. There was a significant increase in the amounts of ceramide in the skin, due to the hydrolysis of sphingomyelin by bacterial sphingomyelinase, in addition to an improvement in the characteristic signs and symptoms of atopic dermatitis in all patients, such as scaling, itching and erythema.
The perceived efficacy benefits verified in the present study, such as improvement in skin irritation, flaking and itching, may be associated with the direct effect that the posbiotic lotion can have on the skin. With topical application, the active NEOIMUNO HILUS GB® is capable of modulating the cutaneous immune responses, bringing benefits and restoring the health and commensal microbiota of the skin, which modulate the inflammatory response and are capable of eliminating pathogens, such as, for example, through the production of antimicrobial peptides [20]. With the modulation of the immune response, NEOIMMUNO HILUS GB® may be responsible for modulating inflammation and allergic response, often determining the severity of atopic dermatitis, since atopic dermatitis involves Th2 response, the postbiotic active can modulate the Th2-mediated response, reducing the production of pro-inflammatory cytokines and improving the Th1/Th2 ratio, suppressing Th2 responses, in addition to stimulating phagocytosis, increasing IgA levels, modulating the integrity of the epithelial cell barrier, and regulating the function and expression of proteins and mucus secretion. On the other hand, with the balance and restoration of the commensal (healthy) microbiota, there may be stimulation for the production of short-chain fatty acids, which have significant anti-inflammatory and epithelial activity [21,22].
Corroborating the results of the present study, through a topical application, for 3 weeks, of a lotion containing inactivated Lactobacillus johnsonii (0.3%) was able to reduce the concentrations of S. aureus in the skin of atopic adult patients, as well as the clinical improvement of symptoms of atopic dermatitis.
In another double-blind, placebo-controlled study, 75 atopic volunteers (6-70 years old) randomly received a lotion containing 5% lysed Vitreoscilla filiformis or a placebo lotion (Control) for 30 days. The researchers also found a significant clinical improvement in AD symptoms, possibly due to the immunomodulatory effect of the lysate [23].
Conclusion
With the application of the posbiotic lotion (NEOIMUNO HILUS GB®), the volunteers in the Treated Group showed a significant improvement in skin balance, moisture and oiliness after 28 days of use. The perception of the variable "skin irritation", "flaking" and "itching" by the Treated Group showed a significant increase in the number of people who showed improvement in their condition ("Improved"). The NEOIMUNO HILUS GB® has showed safety and efficacy in the treatment for atopic dermatitis.
Declarations
Ethical approval and consent to participate: The Ethics Committees of Universidade do Extremo Sul Catarinense approved the study (Number: 5.182.155), and all patients gave written informed consent, without any refusal.
Consent for publication: All authors had full access to all data. All authors contributed and approved the final version.
Availability of supporting data: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgements: United Nations Educational, Scientific and Cultural Organization (UNESC), Gabbia Biotechnology and Biohall Research and Innovation.
Authors' information: MM, GFAJ, MPR, ZPF and SDB were responsible for the study design and protocol. MR and APV is member of Gabbia Biotechnology. MM and GFAJ are members of Biohall Research and Innovation. MM, HMB and ZPF performed the experiments. ZPF manipulated the lotion and placebo formula. All authors were involved in interpreting the data and wrote the manuscript and all authors commented and approved the final version. All authors had full access to all data.
Funding: This work was supported by Gabbia Biotechnology, Biohall Research and Innovation and Universidade do Extremo Sul Catarinense (UNESC).
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Baurecht H, Rühlemann MC, Rodríguez E, Thielking F, Harder I, Erkens AS, et al. Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J Allergy Clin Immunol. 2018;141(5):1668-1676.
[Crossref] [Google Scholar] [PubMed]
- Dréno B. The microbiome, a new target for ecobiology in dermatology. Eur J Dermatol. 2019;29:15-18.
[Crossref] [Google Scholar] [PubMed]
- Di Marzio L, Centi C, Cinque B, Masci S, Giuliani M, Arcieri A, et al. Effect of the lactic acid bacterium Streptococcusthermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp Dermatol. 2003;12(5):615-620.
[Crossref] [Google Scholar] [PubMed]
- Lise M, Mayer I, Silveira M. Use of probiotics in atopic dermatitis. Revista da Associação Médica Brasileira. 2018;64:997-1001.
[Crossref] [Google Scholar] [PubMed]
- Guillen JSQ, Fileto MB, Pinto CASDO, Rosado C, Baby AR, Velasco MVR. Approaches in the treatment of atopic dermatitis. BWS. 2021; 4, 1-18.
- Musthaq S, Mazuy A, Jakus J. The microbiome in dermatology. Clin Dermatol. 2018;36(3):390-398.
[Crossref] [Google Scholar] [PubMed]
- Wollina U. Microbiome in atopic dermatitis. Clinical, cosmetic and investigational dermatology. 2017:51-56.
[Crossref] [Google Scholar] [PubMed]
- Slattery MJ, Essex MJ, Paletz EM, Vanness ER, Infante M, Rogers GM et al. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol. 2011;128(3):668-671.
[Crossref] [Google Scholar] [PubMed]
- Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes and nutrition. 2011;6(3):261-274.
[Crossref] [Google Scholar] [PubMed]
- Yang HJ, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: A randomized, double-blind, placebo-controlled trial. Allergy Asthma Immunol Res. 2014;6(3):208-215.
[Crossref] [Google Scholar] [PubMed]
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EM et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649-667.
[Crossref] [Google Scholar] [PubMed]
- Vinderola G, Sanders ME, Salminen S. The concept of postbiotics. Foods. 11 (8): 1077.
[Crossref] [Google Scholar] [PubMed]
- Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb Cell Factories. 2020;19(1):1-22.
[Crossref] [Google Scholar] [PubMed]
- Holowacz S, Guinobert I, Guilbot A, Hidalgo S, Bisson JF. A mixture of five bacterial strains attenuates skin inflammation in mice. Antiinflamm Antiallergy Agents Med Chem (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents). 2018;17(2):125-137.
[Crossref] [Google Scholar] [PubMed]
- Lau S. Bacterial lysates in food allergy prevention. Curr Opin Allergy Clin Immunol. 2013;13(3):293-295.
[Crossref] [Google Scholar] [PubMed]
- Michels M, Córneo E, Rocha LB, Dias R, Voytena AP, Rossetto M, et al. Paraprobiotics strains accelerate wound repair by stimulating re-epithelialization of NIH-3T3 cells, decreasing inflammatory response and oxidative stress. Arch Microbiol. 2023;205(4):134.
[Crossref] [Google Scholar] [PubMed]
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96(4):523-526.
[Crossref] [Google Scholar] [PubMed]
- Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219-223.
[Crossref] [Google Scholar] [PubMed]
- Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clin diagn lab immunol. 1999;6(1):101-104.
[Crossref] [Google Scholar] [PubMed]
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29(1):15-21.
[Crossref] [Google Scholar] [PubMed]
- Rusu E, Enache G, Cursaru R, Alexescu A, Radu R, Onila O, et al. Prebiotics and probiotics in atopic dermatitis. Exp Ther Med. 2019;18(2):926-931.
[Crossref] [Google Scholar] [PubMed]
- Toh ZQ, Anzela A, Tang ML, Licciardi PV. Probiotic therapy as a novel approach for allergic disease. Front Pharmacol. 2012;3:171.
[Crossref] [Google Scholar] [PubMed]
- Leung B. Future perspectives in atopic dermatitis. In atopic dermatitis. CRC Press. 2002; 545-551.
[Crossref]
Citation: Rosseto MP, Jesus GFA, Voytena AP, Dal-Bó S, Feuser Z, Borges HDM, Michels M (2023) Safety and Effectiveness of Inactivated Streptococcus thermophilus ATCC 19258 (Neoimmuno Hilus Gb®) Lotion on Atopic Dermatitis: A Randomized Placebo-Controlled Clinical Trial. J Dermatitis. 8.202.
Copyright: © 2023 Rosseto MP ,e t al .This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.