Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2025) Volume 16, Issue 2
Role of Endophytes in the Management of Fungal and Bacterial Diseases and Mechanisms Involved
Aarthi R1*, Devanathan M1, Harish S1, Ganesan KN2 and Manivannan V32Department of Genetics and Plant Breeding, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
3Department of Agronomy, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
Received: 18-Jun-2024, Manuscript No. JPPM-24-26063; Editor assigned: 22-Jul-2024, Pre QC No. JPPM-24-26063 (PQ); Reviewed: 06-Aug-2024, QC No. JPPM-24-26063; Revised: 26-Jun-2025, Manuscript No. JPPM-24-26063 (R); Published: 03-Jul-2025, DOI: 10.35248/2157-7471.25.16.754
Abstract
Endophytes, which are typically bacteria or fungi, are vital for sustainable agriculture by inhabiting both inter and intracellular spaces within plants. Pathogens exert a significant impact on plants, leading to global annual crop losses. Due to their distinctive abilities, fungal and bacterial endophytes are essential in biocontrol, and the synthesis of bioactive compounds that effectively combat plant diseases. While pesticides and fungicides have the potential to control pathogen growth in plants and improve crop yield, their widespread use contributes to environmental pollution and poses various health risks to animals and humans. Consequently, there arises a necessity for alternative biocontrol agents, providing environmentally friendly methods to efficiently combat plant diseases. This review investigates the involvement of fungal and bacterial endophytes in addressing fungal and bacterial pathogens, delving into their biocontrol mechanisms.
Keywords
Fungal endophytes; Bacterial endophytes; Siderophore production
Introduction
The term "endophyte" originates from the Greek words "endon" and "phyton", which translate to “within” and “plant” respectively. Endophytes including bacteria and fungi are the microorganisms that reside within the plant's inner tissues without causing noticeable damage to the host plant. Endophytes can grow either inside or outside the cells of their host organism, exhibiting systemic or local growth without causing visible signs of infection or disease. Fungi and bacteria serve as the primary culprits, responsible for approximately 70-80% of plant infections, resulting in extensive damage to crops on a large scale. These pathogens infiltrate plants via various routes such as stomata, roots, or open wounds caused by human activities (such as machine handling and tools), adverse weather, vectors, and other insects. Subsequently, they induce plant diseases through the secretion of secondary metabolites, enzymes, or toxins. While chemical pesticides effectively control biotic stress and boost crop yields, they also cause environmental effects like soil acidification and groundwater pollution. These consequences hinder root growth in plants and disrupt the beneficial microorganisms in the rhizosphere [1].
He developed an effective alternative mechanism to alleviate various stress conditions. Endophytes produce and release secondary metabolites such as tannin, steroids, Phenols, Saponins, volatile oils, resins, steroids, alkaloids, terpenoids, and bioactive molecules. These compounds can prevent pathogen growth and reduce the harmful effects of plant infections. Some endophytes protect their host plants by triggering defense mechanisms in the plant. This is accomplished by Induced Systemic Resistance (ISR) or Systemic Acquired Resistance (SAR). Endophytic fungi, such as Trichoderma viride, offer distinct advantages due to their ability to target a diverse range of hosts compared to their biocontrol agents. Particularly from Spilanthes paniculata the broad-spectrum effectiveness of Trichoderma viride isolated, which demonstrates significant activity against Pythium aphanidermatum, Fusarium solani, and Colletotrichum capsici. As stated by, the absence of endophytes in a plant represents an atypical condition compared to the prevailing natural state. This review examines the crucial role played by stress-tolerant bacterial and fungal endophytes, as well as their defense mechanisms in biocontrol. These mechanisms include the synthesis of secondary metabolites, hydrolytic enzymes, and siderophores as well as the activation of Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR) [2].
Materials and Methods
Role of endophytes in alleviating bacterial and fungal pathogens
Bacterial endophytes: Bacterial endophytes are non-harmful bacteria that inhabit the inner tissues of plants. The most important entry points for bacterial colonization are wounds caused by nematode or microbial activity, root hairs, and root cracks, Intercellular gaps in the cortex and epidermis. Endophytic bacteria hold a superior position compared to rhizospheric bacteria due to their ability to directly interact with plant tissues. Moreover, they provide greater beneficial impacts on plants in contrast to bacteria located outside plant structures [3].
Various bacterial endophytes namely H. rubrisubalbicans, Acetobacter diazotrophicus, and Herbaspirillum seropedicae, have been reported from different crops. These bacterial endophytes demonstrate the ability to serve as effective bio-control agents against soil-borne pathogens, providing targeted protection to the host plant. These beneficial bacteria inhabit the host plant to combat various phytopathogens [4].
During host-pathogen interaction, microbial inoculants trigger various defense mechanisms that involve the activation of specific enzymes such as PAL, GLU, POX CHI, and PPO along with pathways like phenylpropanoids, MAPKs, and jasmonate, which contribute to the development of systemic resistance in plants. The simultaneous introduction of Bacillus atrophaeus B. subtilis, and Burkholderia cepacia has been shown to significantly diminish the severity of diseases in tomato crops. This combined inoculation also stimulates the increased expression and accumulation of defensive enzymes resulting in both direct control and Induced Systemic Resistance (ISR) against vascular diseases. Table 1 illustrate the defense mechanism employed by bacterial endophytes against pathogens [5].
| S. no | Name of the crop | Endophytes | Mode of defense against pathogens |
| 1 | Sugarcane (Saccharum officinarum) | Acidomonas methanolica, Asaia bogorensis, Bacillus altitudinis, Burkholderia gladioli and Nguyenibacter vanlangensis, Tanticharoeniaaidae |
Endophytes shows an effective antagonistic effect by producing volatile compounds, siderophores against Fusarium moniliforme |
| 2 | Rice (Oryza sativa L.) | Bacilllus altitudinis ssp. and Bacilllus aryabhattai ssp. |
Endophytes defend Fusarium moniliforme, and Rhizoctonia solani by producing bioactive compounds |
| 3 | Maize (Zea mays L.) | Chaetomium globosum chg-1 | Endophyte shows antagonist activity by the induction of antioxidant enzymes such as polyphenol oxidase and peroxidase against Cephalosporium maydis |
| 4 | Cotton (Gossypium hirsutum L.) | Bacillus altitudinis HNH7 and Bacillus velezensis HNH9 | Endophytes defend Verticillium dahlia by producing antimicrobial compounds suh as bacillomycin, surfacting fengycin, and bacillibactin |
| 5 | Wild and ancient maize (Zea spp.) | Burkholderia gladioli 3A12 | Endophytes show antagonistic activity by producing antifungal compounds such as phenazine and chitinase Sclerotinia homoeocarpa |
| 6 | Turmeric (Curcuma longa L.) | Bacillus sp. (ECL3), Bacillus cereus (ECL1), Bacillus pumilis (ECL4), Bacillus thuringiensis (ECL2), Clavibacter michiganensis (ECL) and Pseudomonas putida (ECL5) |
All the endophytes produce IAA and the strains ECL3, and ECL5 produce siderophores that defend against Alternaria alternata and Fusarium solani |
| 7 | Black pepper (Piper nigrum L.) | Pseudomonas putida BP25 | Endophytes produce antimicrobial volatile organic compounds such as 2-methyl pyrazine, 2-ethyl 5-methyl pyrazine, 2-ethyl 3, 6-dimethyl, 2, 5-dimethyl pyrazine, and Pythium myriotylumdimethyl trisulphide pyrazine against Phytophthora capsici and Rhizoctonia solani |
| 8 | Banana (Musa spp.) | Bacillus subtilis subsp. subtilis, Baccillus amyloliquefaciens, Bacillus thuringiensis | Endophytes defend Fusarium oxysporum f. sp cubense and Colletotrichum guaranicola by producing antimicrobial compounds namely fengycin |
| 9 | Sunflower (Helianthus annuus L.) | Pseudomonas spp. (EFP) | Endophytes produce antimicrobial volatile compounds, IAA, and hydrogen cyanide against F. oxysporum, Macrophomina phaseolina, Fusarium solani, and Rhizoctonia solani |
| 10 | Â Peanut (Arachis hypogaea L.) | Bacillus velezensis LHSB1 | Endophytes defend Sclerotium rolfsii by producing antifungal lipopeptides such as surfactin A, fengycin A, and bacillomycin A |
| Bacillus sp. F-1 and Burkholderia sp. R-11 | Endophytes defend Sclerotium rolfsii by increasing the activity of plant defense enzymes such as peroxidase, polyphenol oxidase, and phenylanalinase | ||
| 11 | Chilies (Capsicum annuum L.) | Bacillus tequilensis (CNU082075), Burkholderia cepacian (CNU082111), and Pseudomonas aeruginosa (CNU082137 and CNU082142) |
Endophytes produce volatile organic compounds against Fusarium oxysporum |
| 12 | Tomato (Solanum lycopersicum) | Bacillus sp. 2P2 | Endophytes elicit induced systemic resistance and increase the activity of ammonia-lyase, ascorbate oxidase, phenylalanine, peroxidase, and polyphenol oxidase against Sclerotium rolfsii, Fusarium oxysporum f.sp lycopersici, and Rhizoctonia solani |
| 13 | Black gram (Vigna mungo L.) | Klebsiella pneumoniae HR1 | Endophytes produce siderophore, HCN, and hydrolytic enzymes such as phenylalanine ammonia-lyase, peroxidase, and chitinase inhibit the growth of Macrophomina phaseolina |
| 14 | Green gram (Vigna radiata L.) | Streptomyces parvulus | Endophytes produce secondary metabolites with antifungal activity and lytic enzymes such as lipase, amylase, protease, and cellulase against Fusarium solani |
| 15 | Cowpea (Vigna unguiculata L.) | Bacillus amyloliquefaciens, Bacillus subtilis and Bacillus velezensis |
Endophytes exhibits antifungal antagonistic effects in both In vitro and in vivo studies against Rhizoctonia solani |
| 16 | Wheat | Bacillus subtilis XZ18-3 | Endophytes control Rhizoctonia cerealis by accumulating Reactive Oxygen Species (ROS) |
| 17 | Eggplant (Solanum melongena L.) | Bacillus sp. Enterobacter sp. Pseudomonas fluorescens |
Endophytes produce siderophore and IAA against Ralstonia solanacearum |
Table 1: Examples of bacterial endophytes inhibiting phytopathogens.
Some bacterial endophytes not only enhance plant growth by closely interacting with their hosts but also contribute to reducing the severity of pathogenic infections, either through indirect or direct means. The production of antibiotics by these bacteria contributes to reducing pathogenic effects. While some strains with these capabilities have been extensively researched and utilized, others, despite yielding positive effects, have not received much attention. The primary role of endophytic bacteria is to aid in plant disease management through three main mechanisms: (i) Enhancing nutrient availability and uptake (ii) Boosting stress tolerance and (iii) Conferring disease resistance [6].
Results and Discussion
Fungal endophytes
Fungal endophytes inhabit plant inner tissues without inducing any symptoms and have been documented in numerous plant components. The first documentation of an endophytic fungus was in 1772 when Sphaeriatyphena was described by Person who discovered Sphaeriatyphena. This fungus is currently recognized as Epichloetyphina (Pers.) Tul. Paleontological investigations have revealed the presence of endophytic fungi within plant fossils dating back 400 million years. Fungal endophytes are aptly termed "chemical synthesizers inside plants" owing to their capacity for synthesizing a broad spectrum of valuable bioactive compounds. Also, the fungal endophytes have the ability to generate secondary metabolites that resemble those produced by the plants themselves. Endophytic fungi play an important role in safeguarding plants through both direct and indirect strategies. They are widely distributed across various plant species and can be found in nearly every part of plants. These endophytic fungi establish mutually beneficial relationships with their host plants. Also, they establish interactions between different species through direct means like competition, parasitism, and antimicrobial effects, achieved by producing secondary metabolites, volatile compounds, or enzymes. Additionally, through indirect mechanisms like induced resistance, they protect the plants from pathogen invasion [7].
He investigated biological control as a means of managing root and collar rot in beans caused by Rhizoctonia solani. It was found that different Trichoderma species had inhibitory effects on the mycelial growth of the pathogenic fungus. Similarly, investigations conducted emphasized Trichoderma gamsii, as identified by Samuels and Druzhinina, as a potent biological control agent against R. solani. Another study is the mitigation of R. solani-induced root rot in beans. T. harzianum, G.S. Sm., Azotobacter chroococcum Beijerinck and Glomus intraradices N.C. Schenckwasan agent that notably decreased disease severity. The combination and conductedgents were found to be more effective than their individual effects conducted an assessment of Trichoderma isolates in Iran for their efficiency in controlling Rhizoctonia root rot in beans. This study concluded that Trichoderma primarily employs mycoparasitism to exert antagonistic activity against the pathogen. Additionally, endophytes can mimic plant cell responses [8].
Endophytes serve as promising sources of biotic elicitors due to their capability to imitate plant cell responses to diseases. The ability of endophytes to produce and store secondary metabolites within their hosts' tissues has made them stand out. These metabolites can affect how antioxidant enzymes function, thereby triggering a cascade of defense signals and promoting the positive regulation of key enzyme gene expression during the synthesis of secondary metabolites. Endophytic fungi found worldwide have been a prolific source of diverse secondary metabolites, encompassing steroids, polyketides, phenols, flavonoids, terpenoids, peptides, alkaloids quinols, and various halogenated compounds (Figure 1). This rich array of bioactive compounds, as documented by exhibits notable cytotoxic, antimicrobial, insecticidal, and anticancer properties. Table 2 illustrates the defense mechanism employed by fungal endophytes against pathogens [9].
| S. no | Name of the crop | Endophytes | Inhibited phytopathogen |
| 1 | Sugarcane (Saccharum officinarum L.) | Epicoccum nigrum | Fusarium verticillioides |
| Trichoderma asperellum and Trichoderma longibrachiatum | Colletotrichum falcatum | ||
| 2 | Rice (Oryza sativa L.) | Trichoderma longibrachiatum EF5 | Endophytes controls Rhizoctonia solani, Macrophomina phaseolina, and Magnoporthe grisea by producing secondary metabolites such as aliphatic organic acids, volatile metabolites such as tetramethyl hexahydro napthalenone, 2,3- butanediol, and pentadecane |
| Trichoderma virens Aspergillus fumigatus Trichoderma harzianum | Endophytes produce secondary metabolites, especially T. virens show mycoparasitic activity against Rhizoctonia solani | ||
| 3 | Maize (Zea mays L.) | Chaetomium spp. | Endophyte control by mycoparasitic activity and increase in the defense-related enzymes such as polyphenol oxidase, chitinase, and peroxidase against Cephalosporium maydis |
| 4 | Cotton (Gossypium hirsutum L.) | Fusarium solani CEF559 | Endophyte indue increase of pathogenesis-related genes such as acidic chitinase, chitinase, β-1,3-glucanase and 4CL against Verticillium dahlia |
| Cladorrhinum foecundissimum | Endophytes inhibit Rhizoctonia solani by producing phenolic compounds | ||
| 5 | Tomato (Solanum lycopersicum L.) | Chaetomium globosum Cg 40 | Endophyte produces volatile compounds such as 1,2-epoxy-5,9-cyclododecadiene, and hexadecenoic acid, against Fusarium sp. |
| 6 | Banana (Musa spp.) | Sarocladium brachiariae HND5 | Endophytes show antagonistic activity by producing volatile organic compounds such as 3,4-dimethoxystyrol, caryophyllene, and 2-methoxy-4-vinylphenol against Fusarium oxysporum f. sp. cubense |
| 7 | Sunflower (Helianthus annuus L.) | Talaromyces assiutensis and T. trachyspermus |
Endophytes stimulate the production of plant defense by salicylic acid and polyphenolic content salicylic acid against F. oxysporum, Macrophomina phaseolina, Rhizoctonia solani, and Fusarium solani |
| 8 | Peanut (Arachis hypogaea L.) | Phomopsis liquidambaris B3 | Endophyte control Fusarium oxysporum by increasing the activities of pathogenesis-related proteins |
| Trichoderma virens, Penicillium decaturense, Penicillium rubens, Trichoderma virens, Trichoderma viride, and Aspergillus flavus | Endophytes show antagonistic effect by mycoparasitism mechanism against Sclerotium rolfsii | ||
| 9 | Lettuce | Trichoderma spirale T76-1 and Trichoderma asperellum T1 |  Endophytes shows antagonistic activity against Curvularia aeria and Corynespora cassiicola by producing extracellular enzymes such as POD, polyphenyloxidase, chitinase and β-1,3-glucanase |
| 10 | Chickpea (Cicer arietinum L.) | Fusarium sulawesiense, Fusariumincarnatum, Fusarium nygamai, Fusarium equeseti, Fusarium proliferatum, Aspergillus nidulans and Trichoderma asperellum | Endophytes shows antifungal activity by producing IAA against Rhizoctonia bataticola |
| 11 | Finger millet (Eleusine coracana L.) | Aspergillus spp. Penicillium spp.and Phoma spp. | Endophytes show antagonistic effect by producing antifungal compounds such as alternariol-monomethyl ether, tenuazonic acid, viridicatol, and alternariol against Fusarium graminearum |
| 12 | Cacao (Theobroma cacao) | DIS 219f (T. harzianum), DIS 110a(Trichoderma harzianum), DIS 219b (T. hamatum), and TA (T. asperellum) | Endophytes inhibit Moniliophthora roreri by producing secondary metabolites and mycoparasitic the pathogen |
| 13 | Black pepper (Piper nigrum L.) | Trichoderma viride and Trichoderma asperellum | Endophytes induced the systemic resistane by increasing the activity of phenylalanine ammonia-lyase, peroxidase, and chitinase activity against Phytophthora capsica |
Table 2: Examples of fungal endophytes against phytopathogens.
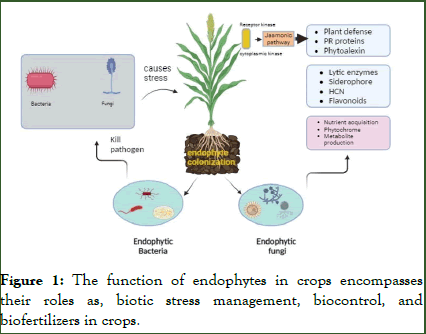
Figure 1: The function of endophytes in crops encompasses their roles as, biotic stress management, biocontrol, and biofertilizers in crops.
Mechanisms of action of endophytes in managing plant diseases
Hydrolytic enzymes: Hydrolytic enzymes possess an antagonist characteristic that can impede or withstand pathogens through the hyper-parasite mechanism, thereby playing a crucial role in enhancing crop fertility through effective biocontrol. In 1877, Wilhelm Friedrich Kuhne was the pioneer in introducing the term “enzyme”, which is also referred to as biocatalysts. One of the functions performed by endophytes that inhabit plants is the synthesis of enzymes that break down the cell walls of plants.
These enzymes encompass 1,3-glucanase chitinase, cellulase and protease. Some of the lytic enzymes synthesized by endophytes can break down diverse polymeric substances such as cellulose, lipids, chitin, and proteins. Endophytes, which colonize the bacterial cell wall, utilize hydrolytic enzymes like amylase, xylanase, carboxylase, and peptidase to degrade the protective peptidoglycan or murein layer. Endophytic bacteria play a direct role in alleviating Ethylene (ET) levels within plants. When plants face pathogens or stress ethylene levels often increase. Multiple reports highlight enhanced protection in plants that have been inoculated with bacterial endophytes. This protective effect stems from the bacterial synthesis of the enzyme 1- aminocyclopropane-1-carboxylate (ACC). ACC acts by cleaving ethylene into ketobutyrate and ammonia, thereby reducing the presence of this stress-related compound in plants and minimizing physiological damage [10].
Endophytic hydrolytic enzymes have the ability to break down the cell walls of pathogenic fungi, thereby protecting plants during fungal pathogens. Chitinase facilitates the breakdown of chitin, a primary component of fungal cell walls. Consequently, the release of these enzymes may exhibit a defensive mechanism by disrupting the integrity of the fungal cell wall, thereby compromising the pathogen’s survival. For example, chitinase produced by endophytic Streptomyces hygroscopicus has been observed to impede the growth of various fungal strains or fungus-like organisms, including Ralstonia solani, Fusarium oxysporum, Aspergillus niger, Sclerotium sclerotiorum, Aspergillus flavus, Alternaria alternate and Botrytis cinerea. Lytic enzymes such as chitinases, proteases and 1-3 glucanases released by Trichoderma viride and Trichoderma harzianum effectively decreased the occurrence of collar rot disease caused by Aspergillus niger [11].
Competition: Nutrients are essential for promoting spore germination and controlling the growth of pathogens or endophytes within the host. Competition represents a potential strategy utilized by endophytes to hinder the colonization of host tissues by pathogens. Biotrophic pathogens extract nutrients from host tissues. When these pathogens invade plant tissue. Endophytes are widespread and can resist pathogen attacks by colonizing the tissue and competing for resources that would otherwise be available to the pathogens due to the niche overlap. The competition mechanisms primarily exert direct effects; therefore, for more effective antagonism against pathogens, an endophyte must systematically colonize the plant. investigated the competitive relationship between endophytes and the aggressive pathogen Ophiostoma novoulmi, responsible for Dutch elm disease. Through carbon utilization profiles, they demonstrated that endophytes shared a considerable niche overlap with the virulent pathogen. However, due to their superior efficiency in utilizing carbon substrates, the endophytes outcompeted the pathogens. Fungi located in the Phyllosphere utilize the thigmotrophism mechanism to suppress rust-induced Phytophthora infestans in potatoes, limiting access to stomata and thereby impeding the germination of rust spores [12].
Mycoparasitis: A Fungi which exhibit parasitic effects on other fungi are referred to as mycoparasites. In mycoparasitism, endophytic fungi protect the host’s ecological balance by directly combating phytopathogenic fungi. Typically, mycoparasitic interactions entail stages such as host recognition, directional growth towards the host attachment, coiling around the host, penetration, and acquiring nutrients. Endophytic fungal spores may encounter the host fungi, imitating germination and the extension of a germ tube, eventually leading to further growth towards the host. Mycoparasite interacts with pathogens in either necrotrophic or biotrophic manners, employing hydrolytic enzymes, secondary metabolites, or antibiotics to exert antagonistic effects. They obtain nutrients from virulent fungi through a biotrophic interface. Likewise, endophytic fungi obtained from common reed suppressed the proliferation of soil-borne fungal pathogens by encircling their hyphae, leading to the breakdown of hyphal cytoplasm upon cell penetration. This breakdown of fungal hyphae entails the release of different cell wall-degrading enzymes by endophytic fungi [13].
For example, Certain Trichoderma strains parasitize Fusarium oxysporum through the formation of haustoria and the production of enzymes or secondary metabolites, facilitating the absorption of nutrients from the pathogenic fungus. Ampelomyces species that are endophytic parasitize powdery mildew, which are biotrophs, primarily employing antibiosis and mycoparasitism as their main modes of antagonistic action. Trichoderma harzianum and T. hamatum exhibit increased antagonistic effects against Phytophthora capsica, a pathogen contributing to root rot disease in Capsicum pubescens. It was noted that, in comparison to T. harzianum, T. hamatum, demonstrates a robust mycoparasitic effect. Trichoderma harzianum typically combats Sclerotinia sclerotiorum through direct parasitism, whereby Trichoderma coils around and breaks down the hyphae of the target organism. Mycoparasitism entails intermicrobial signaling, involving the activation of signal transduction pathways that lead to the repression of multiple genes or transcriptional activation. Trichoderma is known for its well-established signal transduction mechanism mediated by MAP kinase pathways and G-protein. In experiments, the silencing of a G-Protein-Coupled Receptor (GPCR), GPR1, in T. atroviride P1 resulted in the incapacity of the fungus to attach to its host fungus, Rhizoctonia solani [14].
Production of siderophores: Iron (Fe) is a trace element with redox properties that act as a cofactor for numerous enzymes. The primary function of siderophores is to sequester iron (Fe) within cells and inhibit the proliferation of pathogenic organisms. These siderophores, produced by endophytic bacteria, support plant growth by supplying iron to the plants. Many bacterial endophytes possess the ability to chelate iron, including Bacillus, Azotobacter, Arthrobacter, Nocardia, Streptomyces, and Enterobacter (Figure 2) [15].
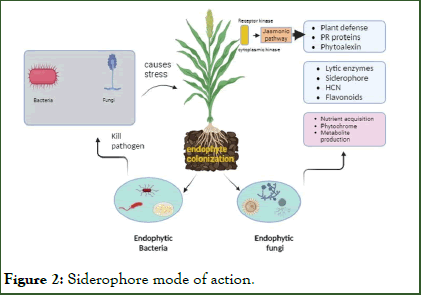
Figure 2: Siderophore mode of action.
In their study, examined the extracellular production of siderophores, as well as the synthesis of antioxidant and antibacterial compounds, in foliar endophytic fungi found in Labrador tea (Rhododendron tomentosum Harmaja) and Scots pine (Pinus sylvestris L.). Their findings indicated that the siderophore is produced in vivo interaction between the plant host and endophyte. Another instance involves the endophyte Talaromyces pinophilus, which inhabits strawberry trees and produces plant growth-promoting siderophore ferrirubin. In a study by they utilized bacterial endophytes called Streptomyces sp., previously isolated from jasmine rice, along with its siderophore mutant (desD). Their findings indicated a significant improvement in plant growth, accompanied by a notable increase in plant biomass, observed in both mungbean and rice treated with the siderophore-producing endophytes. The increase in plant growth or biomass was significantly greater in plants treated with Streptomyces-producing siderophore compared to those treated with siderophore-deficient desD mutant and untreated control plants. Siderophores of bacterial origin, including both hydroxamate and catechol types, obtained from F. rubra, Agrostis capillaris grown in heavy metal-contaminated areas, markedly enhanced the growth rate of Brassica napus and Festuca rubra [16].
Production of secondary metabolites
Secondary metabolites are active biological compounds that play a crucial role in signaling for defense, competitive processes, and ecological interactions. Many endophytes are renowned for their capacity to synthesize secondary metabolites with potent antifungal and antibacterial properties, which impede the growth of harmful microorganisms. Various metabolites, such as terpenoids, peptides, steroids, alkaloids, phenols, and flavonoids, have been isolated from fungal and bacterial endophytic strains. Therefore, secondary metabolites produced by endophytes are employed as biocontrol agents to safeguard plants and enhance crop characteristics. While plants generate bioactive compounds with variable and often inadequate quality, microbial metabolites are consistent, high-quality, and exhibit maximum efficacy in terms of their biocontrol capabilities [17,18].
Certain endophytes, like Piriformospora indica, and Colletotrichum gloeosporioides have been utilized to enhance the production of important plant secondary metabolites, such as asiaticoside. The endophytic bacterium Sphingomonasmelonis, discovered in paddy, demonstrates resistance to disease-prone traits through the synthesis of anthranilic acid. When paddy is infected by Burkholderia plantarii, S. melonis releases ammonia acetic acid which is the extracellular signaling molecule to coordinate the host response. Subsequently, it interferes with the control of the RpoS transcriptional cascade, which relies on the biosynthesis pathway of virulence factors in B. plantarii. This interference promotes the accumulation of plant secondary metabolites and prevents B. plantarii infection. Various researchers have investigated the antipathogenic effects of fungal endophyte secondary metabolites and explored their potential use in controlling diseases in agriculture. Volatile Organic Compounds (VOCs) like 2-methoxy 4-vinylphenol, caryophyllene, and 3, 4- dimethoxystyrol, which exhibit antifungal properties and are emitted by Sarocladium brachiariae endophytic fungi, have been demonstrated to be efficacious against Fusarium oxysporum. Acremonium sp. Ld-03 demonstrates antifungal effects against pathogens such as Botrytis cinerea, Botryosphaeria dothidea, Fusarium oxysporum and Fusarium fujikuroi which are to infect Allium tuberosum. This preventive action against infection is achieved through the production of secondary metabolites including peptides, cyclic dipeptides, valyl aspartic acid, and xanthurenic acid [19].
Induced Systemic Resistance (ISR) and Systemic Acquired Resistance (SAR)
Plants defend themselves against microbial pathogens through induced resistance mechanisms. Systemic Acquired Resistance (SAR) is one such mechanism activated by pathogens, prompting the plant to display a hypersensitive reaction in noninfected areas to show resistance against pathogens. Endophytes employ an indirect mechanism to hinder pathogens, possessing the ability to reduce disease susceptibility in their host plants upon pathogen invasion by eliciting induced resistance. Induced Systemic Resistance (ISR) typically relies on the regulation of ethylene or jasmonic acid and does not involve the upregulation of Pathogenicity-Related (PR) proteins. In contrast, Systemic Acquired Resistance (SAR) is commonly activated in response to pathogen infection and is mediated by salicylic acid, ultimately resulting in the accumulation of PR proteins. Microbe-or Pathogen-Associated Molecular Patterns (MAMPs/PAMPs) are fundamental structures essential for microbial survival. Plants, in turn, have evolved diverse families of receptor proteins to detect these patterns and initiate the activation of their immune system. Pattern-Recognition Receptors (PRRs) have developed to identify typical microbial molecules, like bacterial flagellin or fungal chitin know as Pathogen-or Microbe-Associated Molecular Patterns (PAMPs or MAMPs). This recognition process leads to the initiation of a primary defense mechanism known as PAMPTriggered Immunity (PTI), which effectively manages the majority of potential invaders. Induced defense mechanisms involve various responses, including the production of phytoalexins, nitrogen oxide, Reactive Oxygen Species (ROS), and phenolic compounds. Additionally, they may involve the redistribution of nutrients, the synthesis of Pathogenesis-Related (PR) proteins and antimicrobial metabolites, as well as the reinforcement of physical barriers such as modifications of cuticles, cell walls, and closure of stomata. For example, Trichoderma arundinaceum emits Volatile Organic Compounds (VOCs), such as trichodiene, which influence Botrytis cinerea by stimulating the expression of defense-related genes in tomato plants. These genes are responsible for encoding Jasmonate (JA) and Salicylic Acid (SA), both crucial components of the plant’s defense mechanisms. He observed that there is an increase in phenylalanine activity and peroxidase activity in chickpea plants treated with Bacillus strain CL05 [20].
Conclusion
In conclusion, the multifaceted impacts of endophytes on plant growth, stress tolerance, and disease resistance make them indispensable allies in sustainable agriculture. The intricate interplay between endophytes and their host plants, involving the synthesis of beneficial compounds, modulation of hormonal balance, and activation of defense mechanisms, highlights the potential of harnessing endophytes for the development of ecofriendly agricultural practices. As research in this field advances, further insights into the diverse roles of endophytes and their applications in different crops and environmental conditions will likely emerge, paving the way for more targeted and effective utilization of these microorganisms in agriculture and environmental management.
Acknowledgement
The authors fully acknowledge the guidance and inputs received from prof. (Dr.) M. Devanathan.
Conflict of Interests
The authors declare that there is no conflict of interests.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Aamir M, Rai KK, Zehra A, Kumar S, Yadav M, Shukla V, et al. Fungal endophytes: Classification, diversity, ecological role, and their relevance in sustainable agriculture. Microbial Endophytes 2020;12:291-323.
- Agisha VN, Kumar A, Eapen SJ, Sheoran N, Suseelabhai R. Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci Technol. 2002;29:1069-1089.
- Agri U, Chaudhary P, Sharma A, Kukreti B. Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil. 2002;474:451-468.
- Albelda-Berenguer M, Monachon M, Joseph E. Siderophores: From natural roles to potential applications. Adv App Microbiol. 2001;106:193-225.
[Crossref] [Google Scholar] [PubMed]
- Araújo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JWL, Azevedo JL. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. App Env Microbiol. 2002;68:4906-4914.
- Aravind R, Kumar A, Eapen SJ, Ramana KV. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Letter App Microbiol. 2019;48:58-64.
[Crossref] [Google Scholar] [PubMed]
- Awodun M, Oladele S, Adeyemo A. Efficient Nutrient Use and Plant Probiotic Microbes Interaction. Probiot Agro. 2018;217-232.
- Bailey BA, Bae H, Strem MD, Crozier J, Thomas SE, Samuels GJ, et al. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol control. 2013;46:24-35.
- Baiyee B, Pornsuriya C, Ito Si, Sunpapao A. Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol cont. 2019;129:195-200.
- Blumenstein K, Albrectsen BR, Martín JA, Hultberg M, Sieber TN, Helander M, et al. Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. Bio Cont. 2015;60:655-667.
- Bokhari A, Essack M, Lafi FF, Andres-Barrao C, Jalal R, Alamoudi S, et al. Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci Rep. 2019;9:18154.
[Crossref] [Google Scholar] [PubMed]
- Bolívar-Anillo HJ, Garrido C, Collado IG. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phyto Chem Rev. 2019;19:721-740.
- Brader G, Compant S, Vescio K, Mitter B, Trognitz F, Ma LJ, et al. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Ann Rev Phytopathol. 2016;55:61-83.
[Crossref] [Google Scholar] [PubMed]
- Busby PE, Ridout M, Newcombe G. Fungal endophytes: Modifiers of plant disease. Plant Mol Biol. 2011;90:645-655.
[Crossref] [Google Scholar] [PubMed]
- Cao R, Liu X, Gao K, Mendgen K, Kang Z, Gao J, et al. Mycoparasitism of endophytic fungi isolated from reed on soilborne phytopathogenic fungi and production of cell wall-degrading enzymes in vitro. Curr Microbial. 2011;59:584-592.
[Crossref] [Google Scholar] [PubMed]
- Card S, Johnson L, Teasdale S, Caradus J. Deciphering endophyte behaviour: the link between endophyte biology and efficacious biological control agents. FEMS Microbiol Ecol. 2016;92:114.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Wu YD, Chong XY, Xin QH, Wang DX, Bian K. Seedâ?borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotium rolfsii. J App Microbiol. 2016;128:803-813.
[Crossref] [Google Scholar] [PubMed]
- Cheng YT, Zhang L, He SY. Plant-microbe interactions facing environmental challenge. Cell Host Microbe. 2012;26:183-192.
[Crossref] [Google Scholar] [PubMed]
- de Ita MÁV, Fátima JH, Lezama CP, Simón AB, Cortés GL, Romero-Arenas O, et al. Bio-controller effect of four native strains of Trichoderma spp., on Phytophthora capsici in Manzano chili (Capsicum pubescens) in Puebla-Mexico. J Pure Appl Microbiol. 2015;15:998-1005.
- Dey S, Dutta P, Majumdar S. Biological Control of Macrophomina phaseolina in Vigna mungo L. by Endophytic Klebsiella pneumoniae HR1. J Bio Sci. 2016;12:21-29.
Citation: Aarthi R, Devanathan M, Harish S, Ganesan KN, Manivannan V (2025) Role of Endophytes in the Management of Fungal and Bacterial Diseases and Mechanisms Involved. J Plant Pathol Microbiol. 16:754.
Copyright: © 2025 Aarthi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.