Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2023) Volume 11, Issue 2
Review on IVC injuries and current management strategies
Aravinda Nanjundappa*, Amar Krishnaswamy, Samir Kapadia, Scott Cameron, Deborah Hornace and Calvin ShengReceived: 03-Feb-2023, Manuscript No. JVMS-23-19779; Editor assigned: 07-Feb-2023, Pre QC No. JVMS-23-19779 (PQ); Reviewed: 27-Feb-2023, QC No. JVMS-23-19779; Revised: 06-Mar-2023, Manuscript No. JVMS-23-19779 (R); Published: 13-Mar-2023
Abstract
IVC injuries are rare but are associated with high morbidity and mortality. Most are traumatic (blunt versus penetrating), which often require surgical management, either repair or ligation, as first-line depending on clinical stability. Unlike advancements in endovascular approach for arterial disease, the role for endovascular therapy such as balloon occlusion or stent graft remains unclear. In this review, we discuss management of IVC injuries based on anatomic and clinical considerations and various treatment options including traditional, standard-of-care surgical approach versus novel, off-label endovascular repair. In the trauma setting, up to half of patients will die before even reaching the hospital, and of those who arrive to the hospital, roughly half will not survive to discharge.
Keywords
IVC injury; Endovascular repair; Stent graft
INTRODUCTION
Injuries to the Inferior Vena Cava (IVC) are rare but are associated with significant morbidity and mortality. Majority of cases are due to traumatic insult with overall incidence of 0.5-5% in penetrating trauma and 0.6-1% in blunt trauma whereas reported incidence of iatrogenic injury during intraabdominal surgery is 0.01-1.9% [1,2]. In the trauma setting, up to half of patients will die before even reaching the hospital, and of those who arrive to the hospital, roughly half will not survive to discharge [3,4]. Despite improvements in pre-hospital care and advances in management of vascular trauma, mortality remains high and unchanged over the past several decades [1].
In this review, we first delve into anatomic and clinical considerations when managing IVC injuries and then discuss various treatment options including traditional, standard-of-care surgical approach versus novel, off-label endovascular repair. We also propose a workflow scheme for how to approach these injuries depending on variables like anatomic and clinical factors discussed in great detail below (Figure 1).
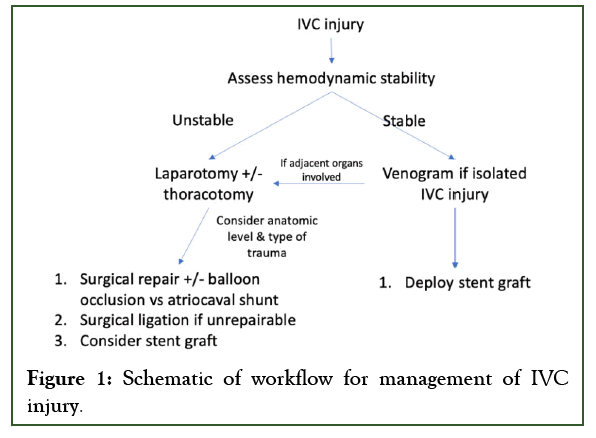
Figure 1: Schematic of workflow for management of IVC injury.
Literature Review
Anatomic and clinical factors
The IVC is a large retroperitoneal vein that drains venous return from the lower extremities, pelvis, and abdominal viscera to the right atrium of the heart. It is formed by the confluence of the iliac veins at L5 and receives numerous tributaries such as lumbar, gonadal, renal, and hepatic veins as it ascends into the thoracic cavity through the diaphragmatic hiatus at T8. Anatomically, the IVC can be divided into four segments in terms of location of injury for management considerations including infrarenal, juxta-renal, retrohepatic, and suprahepatic.
Typically, infrarenal IVC injuries have the best survival due to the relative ease of access and ability to tamponade bleeding whether surgically or endovascularly [5]. Suprarenal IVC, while still accessible, tends to have more adjacent structures to dissect apart and tend to have associated renal and liver injuries. For instance, retrohepatic IVC injury commonly also involves liver parenchyma damage, which requires mobilization of the liver and exposure of hepatic vasculature, leading to increased complexity. Morality for such cases may range from 50% to 80% [6]. Suprahepatic IVC presents even more difficulty controlling bleeding and is near universally fatal with rare case reports of survival [1,7,8]. Patients can present with cardiac tamponade, and clamping and draining of IVC for emergent cardiopulmonary bypass can be technically challenging, especially if the IVC is torn. In the largest available prospective registry (PROOVIT), overall mortality was 42% (infrarenal 33% versus suprarenal 66%, p<0.001) [9].
Not surprisingly, commonly reported clinical predictors of mortality include factors such as hypotension, higher injury severity score, lower Glasgow coma score, and need for thoracotomies [5,10,11].
Discussion
Surgical approach
Primary ligation vs repair: There is currently a lack of expert consensus in terms of best approach for managing IVC injuries, in part due to rare incidence limiting outcomes data to small single center studies without standardized details for reporting across institutions.
Factors such as patient’s clinical status, type of trauma, and IVC level of injuries, surgeon experience, and patient anatomy all contributed to the decision for ligation versus repair. Time is of the essence when it comes to the management of IVC injuries. In a stable patient with a simple injury, repair, if feasible, is generally favored. However, in an acute unstable patient who is in hemorrhagic shock, damage control with IVC ligation may be a reasonable option, particularly for complex injuries [12]. Previously in a propensity score matching analysis using the National Trauma Data Bank (NTDB) from 2007-2014, there was no difference in mortality between IVC ligation and IVC repair (41.3% vs 39.0%; OR 1.10; 95% CI 0.80 to 1.52), but ligation was associated with higher complication rates such as extremity compartment syndrome (OR 5.23; 95% CI 1.50 to 18.24) and hospital length of stay (17.0 days (interquartile range 1.0 to 35.0 days) vs 9.0 days (interquartile range 1.0 to 22.0 days); p=0.002) [13,14]. One major limitation is the lack of data granularity such as reporting of IVC anatomic level of injury and type of trauma. For instance, suprarenal IVC injury has been reported as an independent predictor of mortality [12,15]. Similarly, blunt trauma is associated with higher mortality than penetrating trauma since the nature of the injury requires significant energy which leads to numerous other injuries.
In a more recent comprehensive meta-analysis of 14 studies, IVC ligation was associated with higher mortality than IVC repair (OR 3.12; 95% CI 1.58 to 6.15; p<0.01, I2=49%) in all-comers [12]. However, in a subset analysis on the impact of ligation versus repair specific to infrarenal IVC injury, there was again no statistical difference on mortality (OR 3.13; 95% CI 0.83 to 11.75, p=0.0917, I2=61%), which would be consistent with prior studies using NTDB [13,14]. Alternatively, this also raises concern for a type II error due to smaller sample size of 193 patients. In a large single center series of IVC injury outcomes involving 100 patients, 25 underwent ligation of the IVC, including 22 infrarenal IVC and 3 suprarenal, versus 29 who underwent IVC repair [16]. Infrarenal IVC ligation resulted in early mortality of 41% compared to 21% for repair (p=0.11), albeit statistically insignificant, and was associated with increased length of stay. For juxtarenal injuries, 3 were ligated and 18 repaired. Notably, all 3 ligations were performed as salvage after repair failed (2 died on the table). Overall, 10 patients with IVC ligation (9 infrarenal, 1 suprarenal) survived to hospital discharge, and at an average of 42 months (11-117 months), no patients have significant complications such as lower extremity edema.
Atriocaval shunt: Also known as the Schrock shunt, the Atriocaval Shunt (ACS) was first described in 1968 as a last-ditch effort in trauma patients with suprarenal IVC injury to control hemorrhage by placing a tube inserted through the right atrium into the suprarenal IVC to create a shunt bypassing the defect. Survival in patients with ACS is abysmal, roughly 19%-22% [17,18]. In the era of endovascular therapies, ACS is becoming more obsolete.
Endovascular approach
Despite advances in endovascular technique for arterial disease, there has been limited adoption of endovascular approach for IVC injury, used in 2% of cases in the largest prospective registry (PROOVIT) [9]. The main advantage of endovascular approach (balloon occlusion and stent graft) is potentially the relative ease and speed for controlling active hemorrhage, leading to decreased subsequent coagulopathy and reduced procedure time. In general, it can be considered an adjunct to open surgery as there are often concomitant traumas with the IVC injury.
Several unanswered questions remain in terms of long-term durability of stent graft and management of postoperative anticoagulation. In the early months, the stent is highly thrombogenic until endothelization of the endoluminal surface. However, there is limited data on anticoagulation regimen after IVC endografting. An experimental canine model suggested use of coumadin led to greater patency of PTFE-covered stents compared with controls [19]. Routine use of antiplatelet therapy has also not been well established, though mechanistically, platelet inhibition, especially immediately after stenting, would seem beneficial [20].
Balloon occlusion: Resuscitative Endovascular Occlusion of The Aorta (REBOA) is a well described technique in the aorta for traumatic life-threatening hemorrhage below the diaphragm [21]. Case reports have described similar endovascular approach for balloon occlusion of the IVC (REBOVC) as an adjunctive to temporize bleeding and allow for surgical repair, which is particularly helpful in difficult retrohepatic and suprahepatic cases [22-24]. In a proof-of-concept swine model of trauma (n=13), REBOVC demonstrated superior control of bleeding with significantly less blood loss [25]. In one case series of 5 patients, REBOVC served as a useful adjunct in the management of IVC injury and demonstrated the feasibility of hybrid open-endovascular approach with 80% survival to discharge [26]. Four of the 5 underwent IVC repair whereas one underwent ligation.
Stent graft: To date, there have been only 15+ single case reports of using arterial devices for treating IVC injuries, ranging from stent grafts to aortic cuff to self-expanding covered stents summarized in Table 1 [27-41]. Overall, while biased reporting, all 15 endovascular procedures were successful with 14/15 (93.3%) surviving to discharge. One patient died two days postop due to traumatic brain injury. Seven cases were due to blunt trauma, 3 due to penetrating trauma, and remaining 5 due to iatrogenic causes during other invasive procedures. Four cases were infrarenal while the remaining 11 cases were suprarenal. The latter of which, as previously discussed, portends worse outcomes, and implantation of an endovascular stent as adjuvant to control bleeding may be life-saving in these instances. In the 5 case studies that reported duration of procedure, all were completed within an hour (ranging from 8 to 53 minutes), which again highlights the advantages of endovascular approach. Only 9 of the 15 cases reported some form or combination of antiplatelet/ anticoagulation therapy on discharge, though it remains unclear whether there is clinical benefit for endograft patency given paucity of data. Limited follow-up ranged from 1 to 18 months with patent stent graft.
| Case report | Year | Demographic | Anatomic location | Type of injury | Endovascular repair | Duration of procedure (mins) | Outcome | Anticoagulant/ antiplatelet |
|---|---|---|---|---|---|---|---|---|
| Watarida, et al. | 2002 | 62 yoM | juxtahepatic | blunt (MVA) | 2 self-expanding Gianturco Z stents (30 mm × 50 mm and 30 mm ×50 mm) | 52 | patent endograft at 16 months | warfarin |
| Erzurum, et al. | 2003 | 37 yoF | retrohepatic | iatrogenic during resection of RP leiomyosarcoma | Endoluminal stent graft (2 AnuRx aortic extension cuffs 28 mm × 37.5 mm) | patent endograft at 6 months | ||
| de Naeyer, et al. | 2005 | 51 yoF | infrahepatic | iatrogenic during lumbar vertebral fusion | Talent endoluminal stent graft 44 mm | patent endograft at 18 months | warfarin | |
| Castelli, et al. | 2005 | 65 yoF | infrarenal | blunt (MVA) | Stent graft 31 mm × 14 mm × 150 mm Gore | 9 | died 2 days post-op due to brain injury | |
| Sam, et al. | 2006 | 62 yoM | infrarenal | Blunt (construction) | Gore TAG × 2 (28.5 mm endograft cuffs) | patent endograft at 14 months | warfarin (popliteal DVT) | |
| Hommes, et al. | 2010 | 29 yoF | juxtahepatic | Penetrating (stabbing) | Gore aortic extenders × 2 (32 mm × 45 mm and 32 mm × 45 mm) | discharged | ||
| Filippini, et al. | 2013 | 25 yoM | juxtahepatic | blunt (MVA) | NuMED Covered CP stent × 2 | patent stents at 15 months | aspirin | |
| Pittaretti, et al. | 2013 | 23 yoM | suprarenal | blunt (MVA) | Zenith 32 mm × 58 mm endograft | 8 | patent endograft at 12 months | |
| Briggs, et al. | 2014 | 46 yoF | retrohepatic | iatrogenic (adrenalectomy) | Talent covered stent graft × 2 (32 × 115 mm and 34 × 158 mm) | patent endograft at 13 months | warfarin | |
| Marsala, et al. | 2018 | 72 yoF | infrarenal | Iatrogenic (IVC filter removal) | Gore aortic extender stent graft × 2 (23 mm × 33 mm) and Gore tag × 1 (21 × 100 mm) | patent endograft at 3 months but occluded at 12 months | ||
| Frenk, et al. | 2019 | 75 yoM | infrarenal | Iatrogenic (vertebroplasty) | Endurant II aortic extension stent graft 32 mm × 57 mm | patent endograft at 1 month | aspirin + plavix | |
| Tariq, et al. | 2019 | 27 yoF | suprahepatic | blunt (MVA) | Endurant endografts × 2 (13 mm × 82 mm and 16-13 mm taper × 124 mm) | 50% endograft clot burden after stopping warfarin | warfarin × 6 weeks; aspirin + plavix indefinitely | |
| El Khoury, et al. | 2019 | 22 yoM | suprarenal | penetrating (GSW) | Endurant endografts × 2 (28 mm × 82 mm and 28 mm × 70 mm) | patent endograft at 2 months | ||
| AllMulhim, et al. | 2021 | 52 yoM | retrohepatic | blunt (MVA) | BENTYL Be graft 24 × 48 mm; balloon mounted covered stent | 12 | discharged | aspirin + 5 months warfarin |
| Santini, et al. | 2022 | 40 yoM | suprarenal | penetrating (GSW) | Gore × 2 (28.5 mm × 33 m and 28 mm × 45 mm endograft cuffs) | 53 | patent endograft at 1 month | aspirin + plavix × 1 month, then plavix for 6 months |
Table 1: Summary of case reports for use of endografts for various IVC injuries.
A variety of stent grafts, aortic extender cuffs, and covered stents were utilized, in part likely limited by equipment availability at the respective facilities. Notably, all commercially available devices are not intended for veins, which have theoretical risks for erosion and rupture of the IVC. Depending on the anatomy and anatomic level of injury, there may also be concern for branch occlusion, necessitating need for fenestrated endografts but would not be readily available. In a proof-of-concept canine model, the use of stent graft showed drastic improvement in outcome from 100% mortality to 100% survival rate in the setting of juxtahepatic IVC injury (n=20) [42].
Furthermore, once the decision to place stent graft is made, identifying level of IVC injury via venogram and selecting appropriate size of stent graft are crucial. Angiographic sizing can be challenging due to dynamic respirophasic nature of the IVC, depending on the hemodynamic and volume status of patient. If time allow, intravascular ultrasound can be helpful to obtain more objective diameter measurements. Given lower pulse pressure and thinner layers of the IVC as opposed to aorta, less aggressive oversizing of the endograft such as oversizing by 10%-15% based on transverse diameter may be reasonable.
Conclusion
IVC injuries, particularly due to blunt trauma and suprarenal, can be devastating and often fatal. Rapid control of bleeding is essential. There is lack of expert consensus in terms of best approach to IVC injuries given infrequent incidence and scarce prospective data. Open surgery is typically first-line, especially in unstable patients with multiple visceral and organ injuries. Repair is preferred over ligation, latter of which is considered in damage-control situations and leads to higher complication rates such as lower extremity swelling and prolonged hospitalization. Newer endovascular therapies including balloon occlusion and stent graft have shown promise in case reports and can be considered as adjuncts for controlling bleeding and primary therapy options.
References
- Giannakopoulos TG, Avgerinos ED. Management of peripheral and truncal venous injuries. Front Surg. 2017;4:46.
[Crossref] [Google Scholar] [PubMed]
- Washita Y, Uchida H, Takayama H, Ichimanda M, Taniguchi K, Kiguchi H, et al. Control of inferior vena cava injury during laparoscopic surgery using a double balloon-equipped central venous catheter: Proof of concept in a live porcine model. Surg Endosc. 2018;32:2397-2401.
[Crossref] [Google Scholar] [PubMed]
- Buckman RF, Pathak AS, Badellino MM, Bradley KM. Injuries of the inferior vena cava. Surg Clin North Am. 2001;81:1431-1447.
[Crossref] [Google Scholar] [PubMed]
- Maciel JD, Plurad D, Gifford E, DeVirgilio C, Koopmann M, Neville A, et al. Predictors of mortality in patients with penetrating inferior vena cava injuries surviving to the operating room. Am Surg. 2015;81(10):1000-1004.
[Crossref] [Google Scholar] [PubMed]
- Ombrellaro MP, Freeman MB, Stevens SL, Diamond DL, Goldman MH. Predictors of survival after inferior vena cava injuries. Am Surg. 1997;63:178-183.
[Crossref] [Google Scholar] [PubMed]
- Buckman Jr RF, Miraliakbari R, Badellino MM. Juxtahepatic venous injuries: A critical review of reported management strategies. Journal of Trauma and Acute Care Surgery. 2000;48:978-984.
[Crossref] [Google Scholar] [PubMed]
- Prabhu AD, Karim RA, Thazhkuni IE, Rajendran S, Thamaran RA, Vellachamy KA, et al. Traumatic suprahepatic inferior vena cava injury: surgical management. Innovations. 2007;2(4):213-214.
[Crossref] [Google Scholar] [PubMed]
- Rooke DA, Burke CR, Bulger EM, van Eaton E, Nandate K. Traumatic Suprahepatic inferior vena cava injury survival of a rare case. Trauma Case Rep. 2021;36:100535.
[Crossref] [Google Scholar] [PubMed]
- Stonko DP, Azar FK, Betzold RD, Morrison JJ, Fransman RB, Holcomb J, et al. Contemporary management and outcomes of injuries to the inferior vena cava: a prospective multicenter trial from prospective observational vascular injury treatment. Am Surg. 2021; 13:00031348211038556.
[Crossref] [Google Scholar] [PubMed]
- Huerta S, Bui TD, Nguyen TH, Banimahd FN, Porral D, Dolich MO. Predictors of mortality and management of patients with traumatic inferior vena cava injuries. Am Surg. 2006;72:290-296.
[Crossref] [Google Scholar] [PubMed]
- Cudworth M, Fulle A, Ramos JP, Arriagada I. GCS as a predictor of mortality in patients with traumatic inferior vena cava injuries: A retrospective review of 16 cases. World J Emerg Surg. 2013;8:1-5.
[Crossref] [Google Scholar] [PubMed]
- Byerly S, Tamariz L, Lee EE, Parreco J, Nemeth Z, Palacio A, et al. A Systematic Review and Meta-Analysis of Ligation Versus Repair of Inferior Vena Cava Injuries. Ann Vasc Surg. 2021;75:489-496.
[Crossref] [Google Scholar] [PubMed]
- Byerly S, Cheng V, Plotkin A, Matsushima K, Inaba K, Magee GA. Impact of inferior vena cava ligation on mortality in trauma patients. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2019;7:793-800.
[Crossref] [Google Scholar] [PubMed]
- Matsumoto S, Jung K, Smith A, Coimbra R. Management of IVC injury: repair or ligation? A propensity score matching analysis using the National Trauma Data Bank. J Am Coll Surg. 2018;226:752-759. e2.
[Crossref] [Google Scholar] [PubMed]
- Coimbra R, Hoyt D, Winchell R, Simons R, Fortlage D, Garcia J. The ongoing challenge of retroperitoneal vascular injuries. Am J Surg. 1996;172:541-545.
[Crossref] [Google Scholar] [PubMed]
- Sullivan PS, Dente CJ, Patel S, Carmichael M, Srinivasan JK, Wyrzykowski AD, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg. 2010;199(4):500-506.
[Crossref] [Google Scholar] [PubMed]
- Burch J, Feliciano DV, Mattox KL. The atriocaval shunt. Facts and fiction. Ann Surg. 1988;207:555.
[Crossref] [Google Scholar] [PubMed]
- Kudsk KA, Sheldon GF, Lim Jr RC. Atrial-caval shunting (ACS) after trauma. J Trauma. 1982;22:81-85.
[Crossref] [Google Scholar] [PubMed]
- Makutani S, Kichikawa K, Uchida H, Maeda M, Konishi N, Hiasa Y, et al. Effect of antithrombotic agents on the patency of PTFE-covered stents in the inferior vena cava: an experimental study. Cardiovasc Intervent Radiol. 1999;22:232-238.
[Crossref] [Google Scholar] [PubMed]
- Robbins MR, Assi Z, Comerota AJ. Endovascular stenting to treat chronic long-segment inferior vena cava occlusion. J Vasc Surg. 2005;41:136-140.
[Crossref] [Google Scholar] [PubMed]
- Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):e000154.
[Crossref] [Google Scholar] [PubMed]
- Bisulli M, Gamberini E, Coccolini F, Scognamiglio G, Agnoletti V. Resuscitative endovascular balloon occlusion of vena cava: An option in managing traumatic vena cava injuries. J Trauma Acute Care Surg. 2018;84:211-213.
[Crossref] [Google Scholar] [PubMed]
- Bui TD, Mills JL. Control of inferior vena cava injury using percutaneous balloon catheter occlusion. Vasc Endovascular Surg. 2009;43:490-493.
[Crossref] [Google Scholar] [PubMed]
- Angeles AP, Agarwal N, Lynd Jr C. Repair of a juxtahepatic inferior vena cava injury using a simple endovascular technique. J Trauma. 2004;56:918-921.
[Crossref] [Google Scholar] [PubMed]
- Reynolds CL, Celio AC, Bridges LC. REBOA for the IVC? Resuscitative balloon occlusion of the inferior vena cava (REBOVC) to abate massive hemorrhage in retrohepatic vena cava injuries. J Trauma Acute Care Surg. 2017;83:1041-1046.
[Crossref] [Google Scholar] [PubMed]
- Howell EC, Kulkarni SS, Walker PF, Morrison JJ, Kundi R, Scalea TM. Endovascular balloon occlusion of the inferior vena cava in trauma: A single-center case series. J Am Coll Surg. 2022.
[Crossref] [Google Scholar] [PubMed]
- Watarida S, Nishi T, Furukawa A. Fenestrated stent-graft for traumatic juxtahepatic inferior vena cava injury. J Endovasc Ther. 2002;9:134-137.
[Crossref] [Google Scholar] [PubMed]
- Erzurum VZ, Shoup M, Borge M, Kalman PG, Rodriguez H, Silver GM. Inferior vena cava endograft to control surgically inaccessible hemorrhage. J Vasc Surg. 2003;38:1437-1439.
[Crossref] [Google Scholar] [PubMed]
- Castelli P, Caronno R, Piffaretti G, Tozzi M. Emergency endovascular repair for traumatic injury of the inferior vena cava. Eur J Cardiothorac Surg. 2005;28:906-908.
[Crossref] [Google Scholar] [PubMed]
- de Naeyer G, Degrieck I. Emergent infrahepatic vena cava stenting for life-threatening perforation. J Vasc Surg. 2005;41(3):552-554.
[Crossref] [Google Scholar] [PubMed]
- Sam II AD, Frusha JD, McNeil JW, Olinde AJ. Repair of a blunt traumatic inferior vena cava laceration with commercially available endografts. J Vasc Surg. 2006;43:841-843.
[Crossref] [Google Scholar] [PubMed]
- Hommes M, Kazemier G, van Dijk L. Complex liver trauma with bilhemia treated with perihepatic packing and endovascular stent in the vena cava. J Trauma. 2009;67:E51-E53.
[Crossref] [Google Scholar] [PubMed]
- Piffaretti G, Carrafiello G, Piacentino F, Castelli P. Traumatic IVC injury repair: the endovascular alternative. Endovascular today. 2013:39-43.
- Filippini S, Desebbe O, Gamondes D, Henaine R. Synergy between stents and extracorporeal membrane oxygenation in multitrauma patients with inferior vena cava injury. Eur J Cardiothorac Surg. 2013;44:1140-1142.
[Crossref] [Google Scholar] [PubMed]
- Briggs CS, Morcos OC, Moriera CC, Gupta N. Endovascular treatment of iatrogenic injury to the retrohepatic inferior vena cava. Ann Vasc Surg. 2014;28:1794. e13-1794. e15.
[Crossref] [Google Scholar] [PubMed]
- Marsala A, Hadduck T, Baril D, Kee S. Rupture of the inferior vena cava during filter removal. J Vasc Interv Radiol. 2018;29:1618-1619.
[Crossref] [Google Scholar] [PubMed]
- Tariq U, Petit J, Thomas A, Abt P, Toy F, Lopez R, et al. Traumatic inferior vena cava laceration acutely repaired with endovascular stent graft and associated complications salvaged by surgery. J Vasc Interv Radiol. 2019;30(2):273-276.
[Crossref] [Google Scholar] [PubMed]
- El Khoury R, Kunda NM, Keldahl ML. Endovascular treatment of a penetrating injury of the suprarenal inferior vena cava. J Vasc Surg Venous Lymphat Disord. 2019;7:247-250.
[Crossref] [Google Scholar] [PubMed]
- Frenk NE, Salazar GM, Vazquez R, Irani Z. Intravascular cement leak after vertebroplasty treated with stent graft placement in the inferior vena cava. J Vasc Interv Radiol. 2019;30:74-75.
[Crossref] [Google Scholar] [PubMed]
- AlMulhim J, AlMutairi B, Qazi S, Mohammed MF. Retrohepatic IVC injury: A new treatment approach with arterial stent graft. Radiol Case Rep. 2021;16:560-563.
[Crossref] [Google Scholar] [PubMed]
- Santini A, Dargy N, Umstot R, Adkins N, Nanjundappa A. Endovascular repair of a traumatic inferior vena cava injury after exploratory laparotomy. J Vasc Surg Cases Innov Tech. 2022;8:694-697.
[Crossref] [Google Scholar] [PubMed]
- Porta RM, Poggetti RS, Pereira O, Chammas C, Fontes B, Fratezi A, et al. An experimental model for the treatment of lethal bleeding injury to the juxtahepatic vena cava with stent graft. J Trauma. 2006;60(6):1211-1220.
[Crossref] [Google Scholar] [PubMed]
Citation: Nanjundappa A, Sheng CC (2023) Review on Inferior Vena Cava (IVC) Injuries and Current Management Strategies. J Vasc Surg. 11:507.
Copyright: © 2023 Nanjundappa A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

