Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 1
Relative Risk of Haemolytic Disease of the Foetus and New-born Based on Maternal Alloantibody Specificity: Systematic Review and MetaAnalysis
Carmine H and Denise E. JacksonReceived: 15-Dec-2022, Manuscript No. JBDT-22-19576; Editor assigned: 17-Dec-2022, Pre QC No. JBDT-22-19576 (PQ); Reviewed: 06-Jan-2023, QC No. JBDT-22-19576; Revised: 13-Jan-2023, Manuscript No. JBDT-22-19576 (R); Published: 20-Jan-2023, DOI: 10.4172/2155-9864.22.14.543
Abstract
Haemolytic Disease of the Foetus and Newborn (HDFN) is a common cause of foetal morbidity involving the incompatibility of alloimmunised maternal erythrocyte IgG antibodies and foetal erythrocytes causing haemolysis in the neonate. HDFN severity will depend upon the maternal erythrocyte antibody specificity and the antibody titre strength of the antibody. The primary aim of this review is to determine what is the relative risk of neonatal hyperbilirubinaemia and anaemia when comparing different erythrocyte antibody specificities present in pregnant mothers known to cause haemolytic disease of the foetus and newborn? To obtain appropriate papers to be used in this study, Scopus, Pubmed and Embase databases employed using the search dates from January 1st 2012 until August 31st 2022 using a range of keywords. Meta-analysis was conducted on these papers using Openmeta analyst software using binomial random effects proportion-based analysis employing the Arcsine transformed proportion metric with a maximum likelihood random effects method. Upon analysis of included studies maternal anti-D had the greatest risk of causing neonatal anaemia of 34.9% (95% CI (0.195-0.522), p<0.001), followed by anti-c with a relative risk of 26.2% (95% CI (0.120-0.435), p<0.001) and with anti-Kell with a relative risk of 15.4% (95% CI (0.041–0.321), p<0.001). Maternal anti-c appears to have the highest relative risk of hyperbilirubinaemia with 65.2% (95% CI (0.412-0.857), p<0.001), anti-D with a relative risk of 55.5% (95% CI (0.291-0.804), p<0.001) and then anti-Kell with a relative risk of 30.0% (95% CI (0.049-0.648), p=0.001).
Keywords
Haemolytic Disease of the Foetus and Newborn (HDFN); Erythrocyte antibody; Haemolysis; Alloimmunisation; Hyperbilirubinaemia
INTRODUCTION
Haemolytic disease of the foetus and new-born
Haemolytic Disease of the Foetus and Newborn (HDFN) is an immunological condition that occurs when IgG alloantibodies to specific blood group antigens in a pregnant mother’s serum pass through the placenta and react with foetal erythrocytes possessing the corresponding antigen causing haemolysis [1]. HDFN range from mild to severe depending on the specificity and the titer of the maternal alloantibody that manages to cross the placenta and interact with foetal erythrocytes. Severe cases of HDFN can cause foetal anaemia and hypo-regenerative anaemia which can lead to Foetal Death In Utero (FDIU) or other sequelae if not treated correctly [1].
Maternal alloimmunisation
Alloimmunisation of erythrocyte antigens can happen during blood transfusion or during traumatic events during pregnancy such as falls or car accidents. The most common sensitising event is giving birth itself as it is highly traumatic with a high chance of foetal erythrocytes being transferred into the maternal circulatory system. Therefore, there is an increased risk of HDFN in multigravida women for each subsequent pregnancy. This is especially true in cases of mothers with children from multiple fathers as each father’s erythrocytes have their own red cell antigen phenotype leading to the potential for different antigenic phenotypes being present on foetal erythrocytes [2].
Alloantibody prevalence in HDFN
Historically the most prevalent maternal alloantibody that caused HDFN is Anti Rhesus D (Rh(D)). This is due to the Rhesus D antigen being highly immunogenic with approximately 42.7% alloimmunisation rate in Rh(D) negative patients when exposed to Rh(D) positive blood in one study [3]. Due to routine prophylaxis of Rh(D) negative females with commercially produced Anti-D in developed countries, the alloimmunisation of Rh(D) negative mothers to the Rh(D) antigen has declined to approximately 10% in one study [4]. Depending on maternal and paternal ethnicity Anti-E has become the most encountered clinically significant maternal alloantibody that causes HDFN followed by Anti-K [5].
The use of prophylactic anti Rh(D) to reduce the risk of HDFN
In most developed countries commercially produced Anti Rh(D) IgG is administered to pregnant mothers who have an Rh(D) negative phenotype. In Australia, according to the National Blood Authority’s “Guidelines on the prophylactic use of Rh D immunoglobulin in obstetrics” (NBA) anti-Rh(D) is routinely administered at 28 and 34 weeks of pregnancy (625 IU) and postpartum (625 IU). Anti Rh(D) is also administered during sensitising events taking place throughout the entire pregnancy with a dose of 250 IU in the first trimester and 625 IU beyond the first trimester [6]. The use of administered anti Rh(D) is to bind to any Rh(D) positive foetal erythrocytes present in the maternal circulation. Once the anti Rh(D) binds to the Rh(D) antigen on the foetal erythrocytes the macrophages in the spleen of the mother will phagocytose the foetal erythrocytes reducing the risk of alloimmunisation of anti Rh(D) in the mother [7].
Laboratory monitoring of HDFN and sensitising events
If a pregnant mother is believed to be at risk of a sensitising event, a Kleihauer-Betke test may be ordered. This test involves the collection of maternal peripheral blood and after smearing and staining on a microscope slide, the peripheral blood film is assessed to see how much foetal blood is present in the maternal circulatory system. [8] Once the foetal bleed has been calculated, a corresponding amount of anti Rh(D) is administered to Rh(D) negative mothers to prevent alloimmunisation of anti Rh(D). If the Kleihauer-Betke test is positive for foetal blood, a Haemoglobin-F flow cytometry test may be ordered as a more accurate quantification of the foetal bleed that has taken place [8].
If a pregnant mother has already been alloimmunised with an erythrocyte antibody, antibody titres are conducted throughout the pregnancy to monitor the concentration of the alloantibody present in the maternal circulation [9].
Rationale of the review
Preventing alloimmunisation in women of childbearing age is paramount in reducing the risk of HDFN. Many countries have routine prophylaxis programs to prevent Rh(D) alloimmunisation while also preventing women of childbearing age of receiving Kell antigen positive blood products to reduce the risk of forming anti-Kell [10]. This is because the Kell antigen is highly immunogenic and can cause severe HDFN if a pregnant mother is alloimmunised.
HDFN is still a cause of neonatal mortality even after the introduction of routine prophylaxis in developed countries [11]. It is important that the most clinically significant erythrocyte antibodies that cause HDFN are identified so that further precautions can be taken when transfusing females of childbearing age, such as selecting blood products that are negative for these antigens.
Aim of the study
The primary aim and research question of this study is: What is the relative risk of neonatal hyperbilirubinaemia and anaemia when comparing different erythrocyte antibody specificities present in pregnant mothers known to cause haemolytic disease of the foetus and newborn?
This study examines mothers who have given birth while having an erythrocyte antibody known to cause HDFN and examining haemoglobin and bilirubin levels present in the neonatal after birth. Each antibody specificity will then be assessed on its relative risk to cause foetal anaemia and hyperbilirubinaemia. This study will provide insight into which antibody sensitivities have the highest statistical risk to cause foetal anaemia and hyperbilirubinaemia.
Study hypothesis
Pregnant mothers with alloimmunised anti-Rh(D) will present with the highest relative risk of neonatal hyperbilirubinaemia and anaemia, compared with mothers who have other antibody specificities.
Methodology
Study design
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols were employed within this systematic review to aide in the identification and collation of peer reviewed studies [12]. Papers selected via these protocols were also subject to quality assessment through the application of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [13].
Search strategy
To obtain appropriate papers to be used in this study, Scopus, Pubmed and Embase databases employed using the search dates from January 1st 2012 until August 31st 2022. Search terms included the phrases “Anti D alloimmunisation”, “Rh(D) alloimmunisation”, “Haemolytic disease of the foetus and Newborn Rh(D)”, “Foetal Maternal Haemorrhage Rh(D)”. Alternate combinations of these phases were also used in conjunction with American English spelling of the words “Alloimmunization” and “Fetus”. Further papers were identified through manual searches that were missed with database searches. These papers were identified using Google Scholar. The number of pages obtained in these searches and papers included in this study and highlighted within the PRISMA Flowchart in Figure 1.
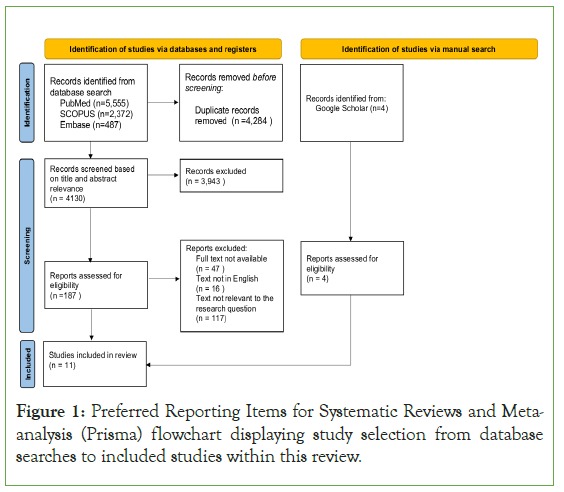
Figure 1: Preferred Reporting Items for Systematic Reviews and Metaanalysis (Prisma) flowchart displaying study selection from database searches to included studies within this review.
Selection criteria
Papers identified using the search terms discussed above were assessed based on their titles and abstracts. Eligible articles included studies of pregnant women who were alloimmunised with an erythrocyte antibody and the effect on their newborn baby and any treatment the neonate received. Articles were excluded from this analysis based on the following criteria: Language not in English, Full text not available, results not containing antibody specificities or neonatal treatment, single case study or meta-analysis. Each article that was eligible for inclusion in this analysis was subject to examination through the STROBE checklist [13].
Data extraction
Data from included studies to be used in this analysis have been organized in Table 1 with the primary authors name, year published, country of origin, study period and information regarding sample size and relevant data for the review. Raw data used in the statistical analysis of the review have been placed in Table 2 organized by article, antibody specificity and neonatal treatment in relation to HDFN.
| Study | Study design | Study period | Country of study | Number of pregnancies | Incidence of allo-immunisation | Patients with Anti-D | Patients with non-Anti-D antibodies |
|---|---|---|---|---|---|---|---|
| Gottvall, et al. 2008 [14] | Retrospective | 1992-2005 | Sweden | 78,145 | 376 | 120 | 196 |
| Hassan, et al. 2014 [15] | Cross-Sectional | 2009 | Malaysia | 5,163 | 30 | 3 | 44 |
| Rath, et al. 2013[16] | Retrospective | 2000-2011 | Netherlands | (Not Displayed) | 393 | 296 | 17 |
| Mandal, et al. 2021 [17] | Observational | (Not Displayed) | India | 652 | 18 | 13 | 4 |
| Koelewijn, et al. 2008 [18] | Prospective | 2003-2004 | Netherlands | 3,05,000 | 2,359 | (Not assessed) | 2359 |
| Karim, et al. 2014 [19] | Descriptive | (Not Displayed) | Pakistan | 1,000 | 18 | 2 | 16 |
| Yang, et al. 2019[20] | Retrospective | 2012-2017 | South Korea | 1,508 | 37 | 7 | 30 |
| Chatziantoniou, et al 2017 [21] | Retrospective descriptive | 2006-2013 | United Kingdom | 130 | 93 | 48 | 45 |
| Bollason, et al 2017 [22] | Retrospective | 1996-2015 | Iceland | 87,437 | 648 | 150 | 498 |
| Dajak, et al 2011 [23] | Retrospective | 1993-2008 | Croatia | 85,600 | 1,105 | 196 | 909 |
| Chandrasekar, et al 2001[24] | Retrospective | 1999-2000 | Ireland | 34,913 | 85 | (Not assessed) | 85 |
Note: *Transfusion required includes
Abbreviations: IUT : Intrauterine Transfusion; ET: Exchange Transfusion; BT=Blood Transfusion
Table 1: Characteristics of eligible studies including the patients antibodies.
Statistical analysis
Open Meta Analyst Version 12.11.4 was utilized to combine data from included studies to form the basis of the meta-analysis [14]. Using the Open Meta Analyst software, the extracted data from included studies in Table 2 was subject to binomial random effects proportion-based analysis using the Arcsine transformed proportion metric with a maximum likelihood random effects method employed. Using this method forest plots were created examining the relative risk of neonatal anemia and hyperbilirubinaemia based on maternal erythrocyte antibody specificity. In this review, a p-value of <0.05 is considered statistically significant.
| Study | Anti-Rh(D) | Anti-K | Anti- Rh(c) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transfusion Required* |
Phototherapy Required |
Total | Transfusion required* |
Phototherapy Required |
Total | Transfusion Required* |
Phototherapy Required |
Total | |||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||||
| Gottvall, et al. 2008 [14] |
21 | 50 | 11 | 60 | 71 | 4 | 19 | 23 | |||||||
| Hassan et al. 2014[15] |
1 | 2 | 3 | 0 | 3 | 0 | 2 | 2 | 0 | 2 | |||||
| Rath, et al. 2013 [16] |
61 | 42 | 101 | 2 | 103 | 11 | 11 | 21 | 1 | 22 | |||||
| Mandal, et al. 2021 [17] |
7 | 2 | 6 | 3 | 9 | ||||||||||
| Koelewijn, et al. 2008 [18] |
5 | 14 | 8 | 11 | 19 | 12 | 106 | 39 | 79 | 118 | |||||
| Karim, et al. 2014 [19] |
1 | 3 | 4 | ||||||||||||
| Yang, et al. 2019 [20] |
3 | 4 | 7 | ||||||||||||
| Chatziantoniou, et al. 2017 [21] |
4 | 18 | 11 | 11 | 22 | 0 | 1 | 0 | 1 | 1 | 2 | 7 | 5 | 4 | 9 |
| Bollason, et al. 2017 [22] |
13 | 115 | 29 | 99 | 128 | ||||||||||
| Dajak, et al. 2011 [23] |
66 | 104 | 170 | 2 | 4 | 6 | 20 | 14 | 34 | ||||||
| Chandrasekar, et al. 2001 [24] |
1 | 35 | 1 | 35 | 36 | 2 | 18 | 10 | 10 | 20 | |||||
Table 2: Neonatal Treatment by antibody specificity in reported studies.
Results
Study selection
Using the search strategy described previously 8,414 articles were obtained from Pubmed, Scopus and Embase databases. With the use of Endnote citations manager software duplicate articles were removed from the database search yielding 4,130 articles. The following articles were then screened for relevance to the research question based on their titles and abstracts leaving 187 articles to be assessed for eligibility. These 187 articles were then examined and 180 were removed based on the selection criteria above. Manual searching on Google Scholar yielded a further 4 papers that were outside the date range for the database searches. In total 11 articles were accepted and are outlined in Table 1.
Search characteristics
11 relevant studies were included in this meta-analysis after screening through the literature. These 11 studies describe HDFN based on maternal erythrocyte antibody specificity and describe neonatal treatment that was required based on severity of neonatal hyperbilirubinaemia and anaemia. These 11 studies were a mix of retrospective, cross-sectional, observational, prospective and descriptive study designs [15-25]. The studies were conducted in 10 different countries and included a myriad of antibody specificities which were observed to cause HDFN, however, only anti Rh(D), anti-Kell and anti-Rh(c) are covered in this review [15-25]. Within this review, neonatal anaemia is classified as the neonatal requiring Intrauterine Transfusion (IUT), Exchange Transfusion (ET) or Blood Transfusion (BT) and neonatal hyperbilirubinaemia is classified as the neonatal requiring phototherapy. Each of these studies described how many pregnancies took place with different antibody specificities and the treatment that was required for the neonatal of each pregnancy pre-natal and postpartum [15-25].
Quality assessment of included studies
Each of the studies included in this review were analysed using the STROBE checklist for methodological quality. Table 3 displays all the included studies in this review alongside relevant criteria taken from the STROBE checklist. All the studies included in this review were of high quality and fulfilled most of not all the relevant STROBE criteria. However, it should be noted that two articles failed to describe a timeframe for their study period decreasing the value of their results [18,20].
| Gottvall, et al. 2008 [14] | Hassan, et al. 2014 [15] | Rath, et al. 2013 [16] | Mandal, et al. 2021 [17] | Koelewijn, et al. 2008 [18] | Karim, et al. 2014 [19] | Yang, et al. 2019 [20] | Chatziantoniou, et al 2017 [21] | Bollason, et al 2017 [22] | Dajak, et al 2011 [23] | Chandrasekar, et al 2001 [24] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Title and abstract | |||||||||||
| Includes study design | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Includes balanced summary of the study | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Introduction | |||||||||||
| Explains scientific rational for the study | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| States objectives of the study | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Methods | |||||||||||
| Describes study period dates and locations | Y | Y | Y | N* | Y | N* | Y | Y | Y | Y | Y |
| Gives eligibility criteria of participants | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Results | |||||||||||
| Report numbers of outcome events | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Discussion | |||||||||||
| Summarise key results and findings | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Note: *Study period not defined
Table 3: Evaluation of included studies using the STROBE Checklist.
Meta-analysis of incidence of alloimmunisation
A forest plot was created representing the incidence of maternal alloimmunisation. All the included studies which displayed a sample size were included, (Rath, et al. was excluded from this forest plot due to only displaying pregnancies with maternal alloimmunised antibodies.) The one arm proportion analysis for maternal alloimmunisation showed the incidence was 1.6% (95% CI (0.012–0.021); p<0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2= 99.136; p<0.001).
Meta-analysis of risk of anaemia due to maternal alloimmunisation
Forest plots were created representing the relative risk of neonatal anaemia requiring treatment through transfusion for included studies with relevant data. The one arm proportion analysis for mothers with an Anti-Rh(D) antibody specificity were shown to have a moderate risk of causing neonatal anaemia requiring treatment through HDFN, with a relative risk of 34.9% (95% CI (0.195–0.522); p<0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2= 91.527; p<0.001).
The one arm proportion analysis for mothers with an Anti-Rh(c) antibody specificity were shown to have a moderate risk of causing neonatal anaemia requiring treatment through HDFN, with a relative risk of 26.2% (95% CI (0.120–0.435); p<0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2= 83.459; p< 0.001).
The one arm proportion analysis for mothers with an Anti-Kell antibody specificity were shown to have a low risk of causing neonatal anaemia requiring treatment through HDFN, with a relative risk of 15.4% (95% CI (0.041-0.321); p<0.001). Included studies for this parameter showed a moderate level of heterogeneity (I2=46.224; p=0.051).
Meta-analysis of risk of hyperbilirubinaemia due to maternal alloimmunisation
Forest plots were created representing the relative risk of neonatal hyperbilirubinaemia requiring treatment through phototherapy for included studies with relevant data. The one arm proportion analysis for mothers with an Anti-Rh(D) antibody specificity were shown to have a high risk of causing neonatal hyperbilirubinaemia requiring treatment through HDFN, with a relative risk of 55.5% (95% CI (0.291-0.804); p<0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2=95.082; p<0.001).
The one arm proportion analysis for mothers with an Anti-Rh(c) antibody specificity were shown to have a high risk of causing neonatal hyperbilirubinaemia requiring treatment through HDFN, with a relative risk of 65.2% (95% CI (0.412-0.857); p<0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2=82.959; p<0.001).
The one arm proportion analysis for mothers with an Anti-Kell antibody specificity were shown to have a moderate risk of causing neonatal hyperbilirubinaemia requiring treatment through HDFN, with a relative risk of 30.0% (95% CI (0.049-0.648); p=0.001). Included studies for this parameter showed to have a high degree of heterogeneity (I2=79.468; p<0.001).
Discussion
HDFN has always been a major cause of foetal and neonatal fatality even after the introduction of routine prophylaxis to prevent maternal anti D alloimmunisation in developed countries. After conducting a meta-analysis on the three most prevalent erythrocyte antibody specificities known to cause HDFN several findings were observed.
Firstly, after assessing all the pregnancy data within the included studies, the incidence of alloimmunisation in pregnancy is approximately 1.6% (95% CI (0.012-0.021) p<0.001). The forest plot in Figure 2 included all the included studies in the review apart from Rath, et al. [16,17]. This is because Rath, et al. did not include a sample size and only included alloimmunised cases within their study. Including this study within the forest plot would have placed their alloimmunisation rate at 100% creating a false outlier on the plot negatively affecting the incidence of alloimmunisation result. When viewing the forest plot, it is observed that Chatziantoniou, et al. is a major outlier sitting above the mean at approximately 71.5% alloimmunisation rate [21,22]. This is because the data within their study only included high risk pregnancies which happened to include many pregnancies with maternal alloimmunisation. When excluding Chatziantoniou, et al. the incidence of alloimmunisation within included studies are approximately 1% (95% CI (0.06-0.016)) [21,22].
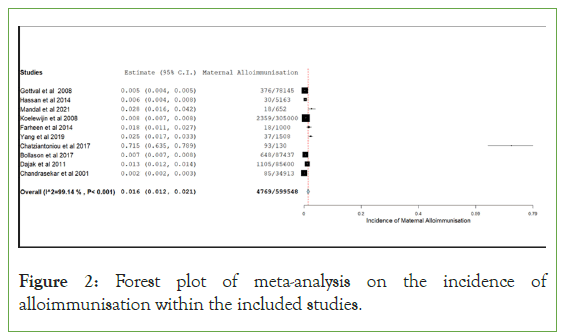
Figure 2: Forest plot of meta-analysis on the incidence of alloimmunisation within the included studies.
Secondly when viewing the results of the relative risk of anaemia requiring treatment forest plots, maternal anti-D appears to have the highest relative risk with 34.9% (95% CI (0.195-0.522), p<0.001), followed by anti-c with a relative risk of 26.2% (95% CI (0.120-0.435), p<0.001) and with anti-Kell having the lowest relative risk of 15.4% (95% CI (0.041-0.321), p<0.001). Anti-D having the highest risk of causing HDFN is quite well known and numerous measures are taken to prevent alloimmunisation of women of childbearing age with anti-D, such as, Anti-D prophylaxis throughout pregnancy and during sensitising events and avoiding giving Rh(D) positive blood products to women of childbearing age. However, when viewing the results of the relative risk of anaemia it should be noted that anti-Rh(c) also has quite a similar chance to cause neonatal anaemia requiring treatment as anti-D. These results show that maybe more should be done to prevent the alloimmunisation of women of childbearing age to Anti-c such as matching Rh-(c) when transfusing women of childbearing age or maybe developing an Rh(c) prophylaxis program potentially in the future.
Finally, when observing the results of the relative risk of hyperbilirubinaemia requiring treatment forest plots, maternal anti-c appears to have the highest relative risk with 65.2% (95% CI (0.412- 0.857), p<0.001), followed by anti-D with a relative risk of 55.5% (95% CI (0.291-0.804), p<0.001) and then finally anti-Kell with a relative risk of 30.0% (95% CI (0.049-0.648), p=0.001). When observing these results, it should be noted that all these risk percentages are quite high when compared to the risk percentages of neonatal anaemia. This is due to phototherapy being an early more conventional treatment of HDFN whereas transfusions are more invasive and are usually for more severe cases of HDFN. Anti-Kell HDFN is more known to cause hypo-regenerative anaemia due to suppression of foetal/neonatal erythropoiesis. However, from the results of the forest plots it is also important to know that the extravascular haemolysis component of maternal anti-Kell HDFN is still significant [26]. Hassan, et al. noted that all newborns from anti-Kell alloimmunised mothers developed mild jaundice Figure 3 [15,27].

Figure 3: Forest plots of meta-analysis on the risk of neonatal anaemia requiring treatment due to maternal alloimmunisation for maternal Anti-D, Anti-c and Anti-Kell. Included studies which contained data based on each antibody specificity and outlined treatment of neonatal anaemia were included in these forest plots.
Since the studies included in this analysis took place all over the world this would mean that the data gathered would be from mothers and newborns of different ethnic backgrounds. From what is known in the literature, different ethnicities tend to have different erythrocyte antigen frequencies [28]. For example approximately 15% of the Caucasian population is believed to be Rh(D) negative, whereas only 3-5% of the black African population are known to be Rh(D) negative. This variance in antigen frequency would help account for the high degree of heterogeneity shown within all the forest plots produced within this review as since the included studies are from different countries the difference in ethnic backgrounds of the participants would vary the alloimmunisation results of each antibody specificity significantly [28].
When examining limitations of this review it must be noted that using patient treatment statistics as an assessment of anaemia and hyperbilirubinaemia could affect the results significantly. This is because those different hospitals have their own guidelines for treatment and use different haemoglobin and bilirubin thresholds before administering treatment. This is especially true for the included studies of this review as they are from different nationalities and include different regimens. For example, Gottvall, et al. described postpartum ET taking place when Hb<120 g/L or bilirubin>110 mmol/L [14,15]. Hassan, et al. only had one baby who needed a blood transfusion in their study in Malaysia at Hb 116 g/L [15,16]. Rath, et al. had ET take place at Hb 82.6 g/L on average for maternal anti-c antibodies and for Hb 82.1 g/L on average for maternal anti-D antibodies in the Netherlands. These differences in treatment regimens would cause variance between neonatal treatment data between hospitals and nationalities and may cause inaccuracy of results when examining risk of neonatal anaemia and hyperbilirubinaemia when compared to examining direct neonatal bilirubin and haemoglobin results Figure 4 [17].
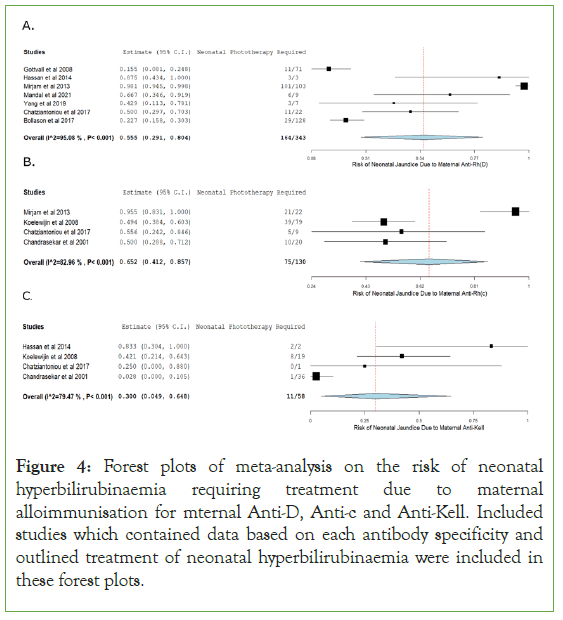
Figure 4: Forest plots of meta-analysis on the risk of neonatal hyperbilirubinaemia requiring treatment due to maternal alloimmunisation for mternal Anti-D, Anti-c and Anti-Kell. Included studies which contained data based on each antibody specificity and outlined treatment of neonatal hyperbilirubinaemia were included in these forest plots.
The final limitation of the review involves the lack of description of antibody titer strength that was included in each case within the included studies. Antibody titers are used by blood bank scientists as a means of monitoring maternal erythrocyte concentration throughout pregnancy. Antibody titration involves performing serial dilutions on maternal plasma which contains a known erythrocyte antibody known to cause HDFN. The plasma is reacted with erythrocytes expressing the corresponding antigen using the Indirect Antiglobulin Test (IAT) test method [9]. According to the literature, high titer maternal erythrocyte antibodies usually produce more severe HDFN then lower titer antibodies of the same specificity. Gottvall, et al. showed that increasing anti-D titer will cause the severity of haemolysis and anaemia to increase proportionally. Because of this, since the maternal antibody titer is not displayed in all the cases within the included studies it is assumed that all the data produces a range of antibody titers for each specificity [14,15].
Conclusion
Through conducting this meta-analysis, it can be deduced that in cases where a neonate has HDFN, maternal anti-D has the highest relative risk of causing anaemia requiring treatment in the newborn, followed by anti-c, then anti-Kell. Maternal anti-c has the highest relative risk of causing hyperbilirubinaemia requiring treatment in the newborn, followed by anti-D, then anti-Kell. Since HDFN is a major cause of neonatal and foetal mortality, it is important that healthcare professionals follow protocols to reduce the risk of maternal erythrocyte alloimmunisation through the use of prophylactic anti-D. Results of high relative risk of hyperbilirubinaemia and anaemia for maternal anti-c erythrocyte antibodies also show that further measures should be taken into preventing alloimmunisation of anti-c whether that is through expanding prophylaxis programs or providing Rh(c) phenotype matched blood for women of child baring age.
Declaration
Conflict of interest
Authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Azuonwu O, Nnenna I, Douglass AS, Ntaa NB. Consequences of haemolytic disease of the fetus and newborn (HDFN) and the clinical significance of antibody screening in prenatal diagnosis: a study of multigravidal and primigravidal women in Port Harcourt, Niger Delta. J Clin Lab Med. 2016;1(1).
- Sankaralingam P, Jain A, Bagga R, Kumar P, Marwaha N. Red cell alloimmunization in RhD positive pregnant women and neonatal outcome. Transfus Apher Sc. 2016 1;55(1):153-158.
[Crossref] [Google Scholar] [PubMed]
- Yazer M, Triulzi D, Sperry J, Corcos A, Seheult J. Rate of RhD-alloimmunization after the transfusion of RhD-positive red blood cell containing products among injured patients of childbearing age: Single center experience and narrative literature review. Hematology. 2021 1;26(1):321-327.
[Crossref] [Google Scholar] [PubMed]
- Al-Dughaishi T, Al Harrasi Y, Al-Duhli M, Al-Rubkhi I, Al-Riyami N, Al-Riyami AZ et al. Red cell alloimmunization to Rhesus antigen among pregnant women attending a tertiary care hospital in Oman. Oman Med J. 2016 ;31(1):77.
[Crossref] [Google Scholar] [PubMed]
- Moinuddin I, Fletcher C, Millward P. Prevalence and specificity of clinically significant red cell alloantibodies in pregnant women: A study from a tertiary care hospital in Southeast Michigan. J Blood Med. 2019;10:283.
[Crossref] [Google Scholar] [PubMed]
- Kumpel, B. M. Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti‐D immunoglobulin: More questions than answers. Vox Sang. 2007: 99-111.
[Crossref] [Google Scholar] [PubMed]
- Urgessa F, Tsegaye A, Gebrehiwot Y, Birhanu A. Assessment of feto-maternal hemorrhage among rhesus D negative pregnant mothers using the Kleihauer-Betke Test (KBT) and Flow Cytometry (FCM) in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. 2014;14(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Van Dijk BA, Dooren MC, Overbeeke MA. Red cell antibodies in pregnancy: there is no ‘critical titre’. Transfu Med. 1995;5(3):199-202.
[Crossref] [Google Scholar] [PubMed]
- Osaro E, Ladan MA, Zama I, Ahmed Y, Mairo H. Distribution of Kell phenotype among pregnant women in Sokoto, North Western Nigeria. Pan Afr Med J. 2015;21(1).
[Crossref] [Google Scholar] [PubMed]
- Kulinska R. Haemolytic disease of the fetus and newborn/HDFN/timing in pregnant women and prophylaxis. Akush Ginekol (Sofiia). 2014 1;53(2):58-63.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9.
[Crossref] [Google Scholar] [PubMed]
- Vandenbroucke JP, Von ElmE, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE):Explanation and elaboration. PLoS Med. 2007;4(10):297.
[Crossref] [Google Scholar] [PubMed]
- Wallace BC, Schmid CH, Lau J, Trikalinos TA .Meta-Analyst: Software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol.2009;9(1):1–2.
[Crossref] [Google Scholar] [PubMed]
- Gottvall T, Filbey D. Alloimmunization in pregnancy during the years 1992–2005 in the central west region of Sweden. Acta Obstet Gynecol Scand. 2008;87(8):843-848.
[Crossref] [Google Scholar] [PubMed]
- Hassan MN, Noor NH, Noor SR, Sukri SA, Mustafa R, Aster HV. Hemolytic disease of fetus and newborn due to maternal red blood cell alloantibodies in the Malay population. Asian J Transfus Sci. 2014;8(2):113.
[Crossref] [Google Scholar] [PubMed]
- Rath ME, Smits-Wintjens VE, Lindenburg IT, Folman CC, Brand A, van Kamp IL, et al. Postnatal outcome in neonates with severe Rhesus c compared to Rhesus D hemolytic. Hemat Neon Alloi Hemol Disease. 2013:151.
[Crossref] [Google Scholar] [PubMed]
- Mandal S, Kaur D, Negi G, Basu S, Chaturvedi J, Maji M, et al . Irregular erythrocyte antibodies among antenatal women and their neonatal outcome at a tertiary care hospital in Northern India. Postgrad Med J . 2021 21.
[Crossref] [Google Scholar] [PubMed]
- Koelewijn JM, Vrijkotte TG, Van Der Schoot CE, Bonsel GJ, de Haas M. Effect of screening for red cell antibodies, other than anti‐D, to detect hemolytic disease of the fetus and newborn: a population study in the Netherlands. Transfusion. 2008;48(5):941-52.
[Crossref] [Google Scholar] [PubMed]
- Karim F, Moiz B, Kamran N. Risk of maternal alloimmunization in Southern Pakistan: A study in a cohort of 1000 pregnant women. Transfus Apher Sci. 2015 1;52(1):99-102.
[Crossref] [Google Scholar] [PubMed]
- Yang EJ, Shin KH, Song D, Lee SM, Kim IS, Kim HH, et al. Prevalence of unexpected antibodies in pregnant Korean women and neonatal outcomes . J Blood Trans. 2019 30;30(1):23-32.
[Crossref] [Google Scholar] [PubMed]
- Chatziantoniou V, Heeney N, Maggs T, Rozette C, Fountain C, Watts T, et al. A descriptive single‐centre experience of the management and outcome of maternal alloantibodies in pregnancy. Transfus Med. 2017;27(4):275-285.
[Crossref] [Google Scholar] [PubMed]
- Bollason G, Hjartardottir H, Jonsson T, Gudmundsson S, Kjartansson S, Halldorsdottir AM. Red blood cell alloimmunization in pregnancy during the years 1996‐2015 in Iceland: A nation‐wide population studyx. Transfusion. 2017 ;57(11):2578-2585.
[Crossref] [Google Scholar] [PubMed]
- Dajak S, Stefanovic V, Capkun V. Severe hemolytic disease of fetus and newborn caused by red blood cell antibodies undetected at first‐trimester screening (CME). Transfusion. 2011;51(7):1380-1388.
[Crossref] [Google Scholar] [PubMed]
- Chandrasekar A, Morris KG, Tubman TR, Tharma S, McClelland WM. The clinical outcome of non-RhD antibody affected pregnancies in Northern Ireland. Ulster Med. 2001;70(2):89.
[Crossref] [Google Scholar] [PubMed]
- Pan J, Zhan C, Yuan T, Chen X, Ni Y, Shen Y, et al. Intravenous immunoglobulin G in the treatment of ABO hemolytic disease of the newborn during the early neonatal period at a tertiary academic hospital: a retrospective study. J Perinatol. 2021;41(6):1397-1402.
[Crossref] [Google Scholar] [PubMed]
- Vaughan JI, Manning M, Warwick RM, Letsky EA, Murray NA, Roberts IA. Inhibition of erythroid progenitor cells by anti-Kell antibodies in fetal alloimmune anemia. N Engl J Med. 1998 19;338(12):798-803.
[Crossref] [Google Scholar] [PubMed]
- Gassner C, Doescher A, Drnovsek TD, Rozman P, Eicher NI, Legler TJ, et al. Presence of RHD in serologically D-, C/E+ individuals: A European multicenter study. Transfusion. 2005;45(4):527-538.
[Crossref] [Google Scholar] [PubMed]
- Velkova E. Correlation between the amount of anti-D antibodies and IgG subclasses with severity of haemolytic disease of foetus and newborn. Open Access Maced J Med Sci. 2015 15;3(2):293.
[Crossref] [Google Scholar] [PubMed]
Citation: Carmine H, Jackson DE (2023) Relative Risk of Haemolytic Disease of the Foetus and Newborn Based on Maternal Alloantibody Specificity: Systematic Review and Meta-Analysis. J Blood Disord Transfus. 14:543.
Copyright: © 2023 Carmine H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.