Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 0, Issue 0
Prevalence and Associated Risk Factors of Ovine Lungworm Infestation in and Around Kemissie Town Oromo Special Zone Ethiopia
Essa Yusuf and Shimeles Abegaz*Received: 05-Sep-2023, Manuscript No. JCRB-23-22884; Editor assigned: 07-Sep-2023, Pre QC No. JCRB-23-22884 (PQ); Reviewed: 21-Sep-2023, QC No. JCRB-23-22884; Revised: 29-Sep-2023, Manuscript No. JCRB-23-22884 (R); Published: 09-Oct-2023, DOI: 10.35248/2155-9627.23.S16.001
Abstract
The present study was conducted from May, 2022 to January, 2023 in and around Kemisse, Oromo special zone of the Amhara Regional State Ethiopia, with the objectives of two determining the prevalence of ovine lungworm infestation, and species identification and the associated risk factors which provoke the distribution of the disease. Of the total 384 clinically sick and healthy animals examined cooprologically 151 (39.3%) were found to be positive. Age groups, sexes, body conditions, respiratory signs and clinically sick and healthy animals were used as risk factors. The analysis of putative risk factors indicated that there was a significant difference in lung worm positivity based on the study area (χ2=8.913, P=0.030), presence or absence of respiratory sign (χ2=10.70, P=0.001), treatment practice (χ2=24.885, P=0.000) and body condition score (χ2=24.691, p=0.000). Whereas, there was no significant difference (p>0.05) on isolation rate between different age groups (χ2=0.219, P=0.896), and sexes (χ2=0.321, P=0.322). The lungworm parasites identified were include D. filarial, M. capillaries, Protostrongylus rufescens and mixed infection with prevalence of 77 (20.1%), 29 (7.6%), 26 (6.8%), 19 (4.5%) respectively. The prevalence was higher in animals showing clinical signs 88 (22.9%), and those animals not treated earlier 130 (33.9%), and lower in animal without clinical signs 63 (16.4%) and treated 21 (5.5%), and shows a statistically significant difference (P<0.05). The relationship between clinical sign and treatment measure are positively correlated with the development and distribution of the disease among the ovine species. In conclusion, this study indicated that seasonal de-worming approach based on the information obtained from the animal owners has effective measurement for combating the development and distribution of ovine lung worms and generally lungworm as one of respiratory disease complex and important internal parasites in the study area which impair the production and productivity of sheep, implying the need for control intervention.
Keywords
Oromo special zone; Lung worm; Faecal; Prevalence; Ovine; Risk factor
Introduction
Livestock production constitutes one of the principal means of achieving improved living standards in many regions of the developing world. In Sub-Saharan African countries, livestock plays a crucial role both in national economies and livelihood of rural communities. It provides drought power, milk, and meat, input for crop production and soil fertility and raw material for industry. Sheep are important domestic animals in tropical livestock production systems. They play a great role in food supply, a source of income and foreign currency. Small ruminants provide as much as 30% of meat and milk consumed in sub-Saharan Africa and is found on small holdings throughout the continent. They are especially important in the more extreme climates of the world [1].
Ethiopia is known by having the highest number of livestock population. According to the recent estimate of livestock population of the country, Ethiopia is a home for about 59.5 million cattle, 30.7 million sheep, 30.2 million goats, 12.22 million equines and 56.5 million poultry [2]. About 99.8% of the sheep and nearly all goat population of the country are local breeds. Of the total sheep population, 75% are raised in highlands with altitudes above 1,500 meter above sea level [3]. Sheep are among the most important livestock species kept by highlanders’ in the country; used for meat production and immediate cash income next to poultry. Providing up to 63% of cash income and 23% of food substance value obtained from livestock production [4].
In spite of the large population and importance of sheep, morbidity and mortality are high in the traditional agro-pastoral production system which leads to low productivity. This low productivity is a reflection of diseases, poor nutrition, poor animal production system and general lack of veterinary care. About half of all sheep mortality and morbidity in Ethiopian highlands are caused by pneumonia and endo-parasitism including lungworms [5,6]. Sheep lungworm infection which is caused by helminth parasites is among the causes of substantial productivity losses in sheep production of the country by causing respiratory distress resulting in great economic concern in the highlands of Ethiopia where sheep are important livestock units [7]. The production loss is a direct result of clinical and sub-clinical helminth infections resulting in low productivity due to stunted growth, insufficient weight gain, poor feed utilization and mortality and indirect losses associated with treatment and control costs lungworm disease is one of the major respiratory diseases widely distributed throughout the world but is particularly common in countries with temperate climate and in the high land of tropical and sub-tropical countries of the world, providing nearly perfect condition for their survival and development [8]. Lung worm infection is also called Verminous Bronchitis or Verminous Pneumonia which caused by the three economically important species of lungworm of sheep and goat namely; Dictyocaulus fiaria, Protostrongylus rufescens and Muellerius capillaries [9]. Among ovine lung worm parasites Dictyocaulus filarial is the most predominant lungworm species, followed by Mulleries capillaries and Protostrongylus refescens [10]. It lives in the lumen of the bronchial tree and commonly associated with chronic bronchitis and localized occlusion of the bronchial tree with atelectasis [11].
The prevalence of lungworm infection depends on different factors like, the climate, altitude, intermediate hosts and favorable ecological conditions such as rain fall, humidity, temperature, and marshy area for grazing, and management system for the development of lungworm species. Even though many anthelmentics are applied for the treatment of lung worm or Verminious pneumonia this can’t be as safety as control and prevention measures so that great control measures should be taken before sheep become infected with this respiratory disease. Control of these parasites is essential, for releasing the potential of sheep production. Lung worm is one of the respiratory tract diseases of sheep that cause direct and indirect economic loss. For proper control the knowledge of parasitic diseases and rules for their control must be applicable to all regions by doing further researches and investigating the current status of the disease. Study of each parasitic disease should not be limited to small areas [12].
Objectives
In order to investigate a sound lungworm control strategy at local and regional level, further and detailed investigation on epidemiology and importance of lungworm infections with respect to its temporal distribution is necessary. Hence, the current study was conducted with the following objectives:
• To determine the prevalence and risk factors of ovine lungworm in the study area.
• To identify the species of ovine lungworms in the study area.
• To assess the control and prevention methods of ovine lungworm in the study area.
Materials and Methods
Study area description
The study will be conducted from May, 2022 to December, 2022 in and around kemisse town, which is Oromo especial zone of Amhara region, Ethiopia. The town is situated at north east central part of Ethiopia at a distance of about 340 km from Addis Ababa on dessie line. It has a latitude and longitude of 10°43′N 39°52′E with an elevation of 1424 meters above sea level. Based on the 2007 national census conducted by the Central Statistical Agency of Ethiopia (CSA) this town has a total population of 19,420 (Figure 1).
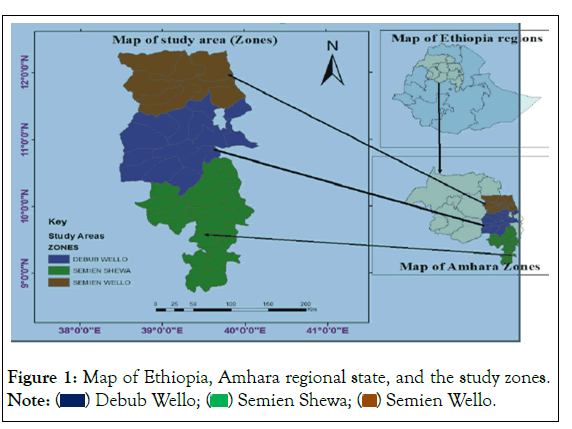
Figure 1: Map of Ethiopia, Amhara regional state, and the study zones. Note: ( ) Debub Wello; (
) Debub Wello; ( ) Semien Shewa; (
) Semien Shewa; ( ) Semien Wello.
) Semien Wello.
Study population
The study population will comprise all indigenous breed of sheep which are rearing all under extensive and semi extensive management system. Study animals were selected randomly from different Peasant Areas (PA’s) (Weladi, Kachur, Kalo, Shakla,) by considering the difference in altitude and size of populations. During sampling, sex, age, body condition, sheep those have clinical respiratory sign and apparently health and sheep those has been treated with antihelmentics and those has not been treated were recorded. The age of every sampled sheep was categorized in to young and adult (below six months and above respectively) using dentition and information from the owners. The body condition score of study animals were divided into good (fat), medium (moderate or average) and poor (thin or emaciated).
Study design and sampling method
A cross-sectional study was conducted to determine the prevalence of ovine lung worm infection, and to assess the associated risk factors responsible for the occurrence of ovine lungworm in and around kemissie town. Simple random sampling technique was used to collect a fecal sample. Different peasant association areas were selected by considering the difference in altitude, and households randomly selected. Then a fecal sample from sheep that were selected from each selected house hold of Peasant Area (PA) was taken and examined with different sample size by depending up on their animal population (by assuming 50% prevalence of ovine lung worm in the area.
Sample size determination
The sample size will be determined according to the formula given by Thrusfield as follows [13]:
n=1.962*pexp*(1-pexp)/d2
Where: n=is required sample size, pexp=is expected prevalence and d=is desired absolute precision (0.05).
1.962=Z-value for the 95% confidence interval.
Thus, the sample size was determined by considering previous study with prevalence of 55.1%. Therefore, using the above formula n=384. But for this study about 5% of the above sample size additionally was taken to reduce risk of sample loss during laboratory test and to increase precision of result. Thus, the total sample size in this study was 384.
Sample collection and laboratory analysis
Faecal samples were collected directly from the rectum of selected animals in universal bottle. While collecting faecal sample; sex, age, body condition, previous treatment and respiratory signs are properly recorded on the prepared format. Specimens were transported to Wollo University Veterinary Laboratory and Kombolcha regional veterinary laboratory in universal bottles. In the laboratory, faecal sample examination for the presence of L1 larvae was conducted using modified Baermann technique. Briefly 5 gm to 10 gm of fecal material was wrapped in double layered gauze and suspended in conical flask containing warm water using a clip wire. The whole apparatus was left for 24 hr overnight. So, that the larvae left the faeces and migrated through the gauze and settled at the bottom of the glass. Then after, the wrapped feaces were removed and the supernatant was discarded from the conical flask gently a small amount (3-5 ml) of sediment was transferred to a watching glass using Pasteur pipette for examination of L1 under the microscope. All larvae were identified morphologically as described by previous workers.
Data management and analysis
The data was analysed in relation to sex, age, body condition, clinical sign, deworming history and lung worm species. The data obtained from examination of faecal sample was coded for the above variables and entered into Microsoft excel spread sheet.
Statistical analyses was done on Statistical Package for Social Science (SPSS 20 version) software descriptive statistics was used to express prevalence and chi square (χ2) test was used to compare the association among variables. In all analysis the confidence level was held at 95% confidence interval and the desired absolute precision was set 5%. Using the SPSS statistical software the differences were considered as significant when p-value is less than 0.05 and non- significant if 0.05 is greater than that of p-value.
Results
Of total 384 faecal sample examined, 151 (39.3%) were found to be positive for lung worms nematodes of the lungs, as shown in Table 1.
| Number of animals Examined | Number of positive for lung worm | Frequency |
|---|---|---|
| 384 | 151 | 39.30% |
Table 1: Overall prevalence of lung worm infestation.
Prevalence of lungworm infection based on the study area
The prevalence of lungworm in different localities of the study area showed that 43 (11.2%), 27 (7.03%), 40 (10.4%), 41(10.7%) from Kallu, Kachur, Shikla, and Wolledi respectively. The highest prevalence was observed in Kallu (11.2%) and the lowest prevalence was observed in Kachur (7.03%). There was a significant difference χ2=8.913, P=0.030 in the prevalence of lung worms infestation among the kebeles (Table 2).
| Risk factors and Variables | No. of samples |
Prevalence (%) | Dictyocaulusfilaria | Protostrongylusrufescens | Muellerius capillaris |
Mixed infection | |
|---|---|---|---|---|---|---|---|
| Area | Kalu | 80 | 43 (11.2%) | 28 (7.3%) | 1 (0.3%) | 9 (2.3%) | 5 (1.3%) |
| Kachur | 74 | 27 (7.03%) | 14 (3.6%) | 2 (0.5%) | 6 (1.6%) | 5 (1.3%) | |
| Shikla | 111 | 40 (10.4%) | 18 (4.7%) | 9 (2.3%) | 11 (2.9%) | 2 (0.5%) | |
| Woledi | 119 | 41 (10.7%) | 17 (4.4%) | 14 (3.6%) | 3 (0.8%) | 7 (1.8%) | |
| Total | 384 | 151 (39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=34.819, p=0.000 | ||||||
Table 2: Prevalence of lung worm in the study area.
Coprological examination
Coprological examination in the present study revealed the existence of 3 different lung worm species from the family Dictyocaulidae and The Metastrongylidae. The dictyocaulidae include D. filarial and the metastronglidae include Protostrongylus rufescens and Muellerius capillaries with different prevalences. These include: Dictyocaulus filarial, 77 (20.1%). Muellerius capillaries 29 (7.6%) P. rufescens 26 (6.8%) and mixed infection by both families are 19 (4.9%) as shown in Table 3.
| Risk factor and Variables | No. of samples | Prevalence (%) | D. fillaria | p.rufisence | M.caplaris | Mixed infection | |
|---|---|---|---|---|---|---|---|
| Sex | Male | 190 | 72 (18.8%) | 37 (9.6%) | 13 (3.4%) | 13 (3.4%) | 9 (6.8%) |
| Female | 194 | 79 (20.6%) | 40 (10.4%) | 13 (3.4%) | 16 (4.2%) | 10 (2.6%) | |
| Total | 384 | 151 (39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=0.477,p=0.976 | ||||||
| Age | Adult | 255 | 102 (26.6%) | 52 (13.5%) | 13 (3.4%) | 21 (5.5%) | 16 (4.2%) |
| Young | 85 | 33 (8.6%) | 17 (4.4%) | 10 (2.6%) | 5 (1.3%) | 1 (0.3%) | |
| Old | 44 | 16 (4.2%) | 8 (2.1%) | 3 (0.8%) | 3 (o.8%) | 2 (0.5%) | |
| Total | 384 | 151 (39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=14.862, p=0.021 | ||||||
| B/c | Moderate | 236 | 81(21.1%) | 33 (8.6%) | 17 (4.4%) | 20 (5.2%) | 11(2.9%) |
| Poor | 102 | 60 (15.6%) | 39 (10.2%) | 6 (1.6%) | 7 (1.8%) | 8 (2.1%) | |
| Good | 46 | 10 (2.6%) | 5 (1.3%) | 3 (0.8%) | 2 (0.5%) | 0 (0.0) | |
| Total | 384 | 151 (39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=37.984,p=0.000 | ||||||
| Treatment | Deworm | 108 | 21(5.5%) | 13 (3.4%) | 2 (0.5%) | 2 (0.5%) | 4 (1.0%) |
| Not deworm | 276 | 130 (33.9%) | 64 (16.7%) | 24 (6.2%) | 27 (7.0%) | 15 (3.9%) | |
| Total | 384 | 151 (39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=26.904,p=0.000 | ||||||
| Res. sign | Yes | 184 | 88 (22.9%) | 47 (12.2%) | 15 (3.9%) | 19 | 7 |
| No | 200 | 63 (16.41%) | 30 (7.8%) | 11 (2.9%) | 10 | 12 | |
| Total | 382 | 151(39.3%) | 77 (20.1%) | 26 (6.8%) | 29 (7.6%) | 19 (4.9%) | |
| χ2, P-value | χ2=15.052, p=0.005 | ||||||
Table 3: Distribution of lung worm species with respect to different risk factor.
This result shows a significant difference χ2=34.819, p=0.000 of parasite species distribution in the study area.
Occurrence of lung worm associated with the risk factors
The analysis of putative risk factors indicated that there was a significant difference in lung worm positivity based on study area (χ2=8.913, P=0.030), presence or absence of respiratory sign (χ2=10.70, P=0.001), treatment practice (χ2=24.885, P=0.000)and body condition score (χ2=24.691, p=0.000). Whereas, there was no significant difference (p>0.05) on isolation rate between different age groups (χ2=0.219, P=0.896), and sexes (χ2=0.321, P=0.322) (Table 4).
| Risk factor and Variables | No. of samples | Prevalence (%) | χ2 | P=value | |
|---|---|---|---|---|---|
| Res. Sign | yes | 184 | 88 (22.9%) | χ2=10.70 | P=0.001 |
| No | 200 | 63 (16.4%) | |||
| Total | 384 | 151 (39.3%) | |||
| Dewarm | Yes | 108 | 21(5.5%) | χ2=24.885 | P=0.000 |
| No | 276 | 130 (33.9%) | |||
| Total | 384 | 151 (39.3%) | |||
| Sex | Male | 190 | 72 (18.8%) | ||
| Female | 194 | 79 (20.6%) | χ2=0.321 | P=0.322 | |
| Total | 384 | 151(39.3%) | |||
| Age | Adult | 255 | 102 (26.6%) | ||
| young | 85 | 33 (8.6%) | χ2=0.219 | P=0.896 | |
| Old | 44 | 16 (4.2%) | |||
| Total | 384 | 151 (39.3%) | |||
| B/c | Moderate | 236 | 81 (15.6%) | ||
| Poor | 102 | 60 (21.1%) | χ2=24.691 | P=0.000 | |
| Good | 46 | 10 (2.6%) | |||
| Total | 384 | 151 (39.3%) | |||
| Study site | Kalo | 80 | 43 (11.2%) | ||
| Kachur | 74 | 27 (7.0%) | χ2=8.913 | P=0.030 | |
| Shekla | 111 | 40 (10.4%) | |||
| Woledi | 119 | 41 (10.7%) | |||
| Total | 384 | 151 (39.3%) | |||
Table 4: Prevalence of lung worm infestation in relation to different risk factors in three study districts.
The prevalence of ovine lungworm was higher in female 79 (20.6%) than male 72 (18.8%), however, no significant (p>0.05) difference was observed in prevalence rate of lungworms between sex.
Age specific prevalence shows, adult age groups of sheep are more exposed for parasitic infestation than young and old groups and the variation was statistically not significant, (P>0.05). Concerning clinical observation those who have a clinical signs of difficulty of breathing have high prevalence comparing with asymptomatic sheep and shows statistically highly significant difference p<0.05). Those animals having treatment with anthelmintics have a low infestation than untreated and shows a statistically highly significant difference (p<0.05) between animals having treatment and untreated (χ2=24.885, P=0.000) Table 4.
Discussion
Lungworm was widely distribute throughout the world but are particularly common in countries with temperate climate, and in the high lands of tropical and subtropical countries. The species of importance in ovine belongs to two different families; The Dictyocaulidae and The Metastrongylidae. The Dictyocaulidae include Dictyocaulus filarial, The Metastrongylidae are represented by protostrongylus rufescens, muellerius capillaries and the last one which is not recover in our studies is Cystocaulus ocreatus.
To assess the present of the aforementioned lung worm parasites conducting a research is indispensable in our area to solve the problem of the farmers losing their animals due to respiratory problems. Based on the problem existed in the study area, a study was carry out and the conducted study result indicated that lungworm infection was one of the most common respiratory diseases of sheep with an overall prevalence of 39.3%. This result is in line with the findings of in Dessei and Kombolcha, in Ambo District who reported the prevalence of 40.4% and 34.90% respectively, however, the result of the current study was higher than the results reported by, around Bahir Dar, in and around Debre Berhan town, in Minjar Shenkora woreda, North Shoa, 20.2%,18.3%,15.9% respectively, but the prevalence of the current study is lower than the previous work reported by different researchers done in different place at different time, 57.1% of prevalence by in Tiyo district, Arsi, 56.5% by in Goba district, Bale, 57.1% prevalence by 58.8% and 55.10% in and around Assela. And far lower than the result reported, in Asella province, Central Ethiopia 72.44%, in Debra Birhan, 73.75%, in Wollo district, 71.3%.
In the current study researchers confirmed the present of 3 different types of Ovine Lungworm parasites with a prevalence rate of 77 (20.1%), 29 (7.6%), 26 (6.8%), due to D. filaria, M. capillaries, P. rufescens, respectively , and mixed infection 19 (4.9%), with two or three species of lungworm,. It was observed that D. filarial was the most predominant species in the area followed by M. capillaries, and P. rufescens, found to be the least in prevalence rate in the study area. This is in agreement with the findings in other parts of Ethiopia, who reported D. filarial to be the most prevalent in their study. In contrast to these findings, in Bahir Dar and in Dessie zuria district reported that M. capillaries were the most prevalent in their studies. The present study indicated that polyparasitism to be the major problem in the area. The presence of more than one parasite in sheep in these study areas may be related with lack of control measures against helminth parasites. Specifically lack of regular deworming approach tends that might have attributed to the incidence of poly-parasitism. The climatic condition of the study area where rainfall is infrequent and temperature is mild also favours the development and survival of infective larvae for most part of the years. Owing to the huge sheep population in the study area considerable contamination to the communal pasture grazing system could be the other factor which favours poly-parasitism.
In other way round the possible reason for the predominance of D. filaria in the study area might be attributed to the difference in the life cycles of the parasites. Thus, D. filaria has a direct life cycle and requires shorter time to develop to an infective stage while M. capillaries has an indirect life cycle which needs an intermediate host snail for completing its life cycle. Thus, require longer time to develop to infective stage. In the present study area, the environment may not favourable to the intermediate host that makes M. capillaries and P. rufescens lower in distribution. After ingestion the larvae D. filaria parasites can be shed with faeces within five weeks. Compared with D. filaria, the transmission of P. rufescens and M. capillaries is epidemiologically complex event involving host, parasite and intermediate host. Hence, M. capillaries and P. rufescens in sheep require slugs or snails as intermediate host which must be eaten for infection to occur; this might make them low prevalent in the present study area than D. filaria.
In addition the distribution of lung worm infection varies from place to place have different reasons. The possible explanation for such variations in the prevalence of lung worm infection in the current study areas could be due to variation in agro-ecology which favours or inhibits the survival of parasite’s larvae in general and/ or the presence or absence of snail intermediate host in case of M. capillaris and P. rufescence in the study areas. The occurrence of lungworms infestation is associated with time of sampling, level of immunity of sampled animal’s management practice of the animal and expansion of facilities like veterinary services.
This study showed that, the prevalence of lungworm infections was observed higher in the adult animals (26.6%) as compared to young animal (8.6%) and in old (4.2%). The difference was statistically insignificant (p>0.05). This might be associated with the frequent grazing behaviour of adult animals those graze continuously following weaning from suckling of their dam by which natural immunity obtained from their mother becomes reduced and the appropriate environmental climatic conditions can also contribute to higher rate of infection when sheep are sold to or bought from different agro-climatic conditions. This finding is however disagree within North and South Gondar zones and in and around Bahir Dar that they have founded the prevalence of lungworm infection is higher in ages of younger sheep than those of other age groups.
In the current study an attempt was made to see the influence of sex on the prevalence of infection in the study area. Sex was found as a major risk factors (P=.000) in the prevalence of ovine lungworm infection. The prevalence rate in female was (20.6%) that is higher than male sheep (18.8%). This finding agrees with the results in Wogera District Northern Ethiopia, in Asella province, in Jimma town around Bahir Dar, in North Ethiopia, and in and around Debre Berhan town who reports higher prevalence in female than in male. This higher prevalence rate of lung worm infection in female animal could be due to the fact that the resistance to infection is abolished at the time of parturition and during early lactation in female animals. But this contradicts with in Minijar Shenkora woreda, North Shoa, who reported higher prevalence of lung worm infection in males than females. These variations may be due to the improper distribution of sample selection between the two sexes. Other possible reason for this can be because of great care of owners to their female sheep than to their male sheep.
With regard to assess the influence of body condition, on variation of prevalence of lungworm infection, it was found that 21.1%, 15.6%, and 2.6% in poor, moderate, and good body condition animals respectively. Hence, the prevalence was higher in poor body condition sheep than moderate and good body condition sheep; the variation among body condition was statistically significant <0.05. This finding agrees with study reported, who reported the variation among body condition was statistically significant, however, disagrees with the study reported, who said the differences between body conditions are insignificant. The current prevalence of lung worm infestation agreed with they reported that high prevalence was found in animals which had poor body condition. This might be associated with the nutritional management of animals. Poor body condition occurred as a result of lack of feed or nutritional management. This may lead to lack of resistance to infection and contribute for increased prevalence rate in poorly conditioned animals. Evidently, the infection with a parasite by itself might results in progressive emaciation of the animals.
In this study, attempts were made to know if there is variation in lung worm infection between treated and untreated animals. With regards to treatment in the prevalence of lungworm infection, questionnaire survey findings were tried to associate anthelmintics usage with the faecal examination results. Higher prevalence (33.9%) of the parasites was recorded in sheep with non-dewormed than dewormed (5.5%) and it is statistically significant (p<0.05). The observation noted in this study agreed with study reported in Asella province, in Debre-tabor Awraja, in Debra Berhan, and in Wollo districts. In the current study, even if the infection is low (5.5%) in deworming sheep comparing with non-deworming, why these sheep harbour the parasites, this needs explanation and, the reason why dewormed sheep shows infection and this might be either due to the anthelmintic used in the area for the treatment only temporarily suppress egg production of the adult worms or parasite may develop resistance to the given anthelmintics. It may also be related to the poor quality of anthelmintic used in the study area. In contrast, some of none dewormed animals were not infected by lungworm; this also might need explanation, it might be due to the development of acquired immunity from previous exposure and it may also be due to none exposure to the infective stages of D. filaria or to intermediate host of the other species of the lungworms of sheep throughout their life.
With regard to know the influence of symptoms of respiratory sign on the prevalence of lungworm infection, questionnaire survey findings were tried to associate the manifestation of respiratory sign with the faecal examination results. Higher prevalence (22.9%) of the parasites was recorded in sheep with the respondents that said yes (shows clinical respiratory signs) than that said no (did not show the signs) (16.4%). The variation was statistically significant (P<0.05). This finding agreed with the study reported in Chilalo areas, Arsi Zone, in Asella province and in different parts of the country. In those mentioned authors, their findings indicate that the prevalence of the parasite was found high in animals which showing symptoms of respiratory signs than apparently healthy. In the current finding, even though apparently healthy sheep show low infection compare to those showing clinical respiratory signs, some of them were infected with lungworm. The reason why apparently health sheep appeared with lungworm might be due to; the parasites were in pre-patent stage; due to small adult worm burden in sheep which couldn’t produce eggs and hence larvae; or as a result of immunity developed due to exposure to a few lungworms which is not associated with clinical sign but animal shed larvae. And 33.3% of those animals manifesting respiratory signs were appeared negative on coproscopic examination; this might be, due to bacterial or viral diseases that causes occurrence of respiratory signs.
Conclusion
The result of the present study indicated that lungworm is one of the major parasitic helminthosis of sheep in and around Kemissie town. The major lungworm species identified in the study area were: D. filarial, M. capillaries, and P. rufescens. D. flarial was found as the dominant lungworm species in the study area than other lungworm species. Coproscopic examination and questionnaire survey revealed that, adult, female, none dewormed, clinically diseased sheep, and sheep with poor body conditions were more infection than their counter parts. It can also be concluded that the infections caused by lungworms are significantly common in the study area and are important health problems of sheep which is speculated to cause heavy economic loss.
Recommendations
• Therefore, in line with the above conclusion the following recommendations have been forwarded:
• Attention should be given for the prevention and control of lungworm infections.
• Regular deworming of sheep with broad-spectrum anthelmintic should be practiced before and after rainy season.
• Sheep that shows a respiratory sign should get treatment immediately
• Newly introduced animal to the flock should be dewormed before entering into the flock.
• Emphasis should be given to the control and prevention of lung worm in order to reduce the prevalence
• Animal health professionals should create awareness for animal owners to practice regular deworming of their animal.
• The district administrative bodies should enforce application of rotational and/or zero grazing system.
• Studying and knowing the epidemiology of the lungworm in relation to study area should be done to enable the development of appropriate control strategy.
• Veterinary service provider in the area should have the facility to diagnose and treat ovine lung worm infected animals to mitigate production losses.
References
- Mokhtaria K, Ammar SS, Ameur AS, Mohammed HS, Samia M, Fadhéla S, et al. Lungworm Infections in Goats Slaughtered in Algeria. Glob Vete. 2013;11(3):293-296.
- CSA (Central Statistical Agency): Agricultural sample survey 2016/2017. Report on livestock and livestock characteristics. Addis Ababa, Ethiopia, Statistical Bulletin. 2017;3(585):9-20.
- CSA (Central Statistical Agency): Report on Livestock and livestock characteristics (Private peasant holdings). Statistical Bulletin, Addis Ababa. 2015;2:570.
- Ibrahim N, Godefa Y. Prevalence of ovine lung worm infection in Mekelle town, North Ethiopia. Internet Journal of Veterinary Medicine. 2012;9(1):6.
- Alemu S, Leykun EG, Ayelet G, Zeleke A. Study on small ruminant lungworms in northeastern Ethiopia. Vete Parasitol. 2006;142(3-4):330-335.
[Crossref], [Google Scholar], [Pubmed]
- ILRI (International Livestock Research Institute): Hand book of livestock statistical for developing countries socio-economic and policy research working paper 26. ILRI, Nairobi, Kenya. 2000: Pp:299.
- Basaznew B, Ayalew E, Achenef M. Ovine lungworm infection: prevalence, species composition and associated risk factors in Dessie Zuria District, northeastern Ethiopia. African J Basic Appl Sci. 2012;4(3):73-76.
- Ayalew A, Debebe T, Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. J Parasitol Vector Biol. 2011;3(5):75-81.
- Rahmeto A, Mulugeta M, Solomon M. Lungworm infection in small ruminants in and around Wolaita Soddo town, Southern Ethiopia. J Vet Sci Technol. 2016;7(2):22-34.
- Kebede S, Menkir S, Desta M. On farm and Abattoir study of Lungworm infection of small ruminants in selected areas of Dale District, Southern Ethiopia. Int J Curr Microbiol App Sci. 2014;3:1139-1152.
- Taylor MA, Coop RL, Wall RL. Veterinary parasitology. Third edition Wiley. 2007:94-97.
- Gebrekidan M, Kemal K, Abdurahaman M. Prevalence of ovine lung worm in and around Jimma, South West Ethiopia. International Journal of Research Studies in Biosciences.2008:24-32.
- Thrusfield M. Veterinary epidemiology. 3rd ed. Black Well Science Ltd. Cambridge, USA. 2007:Pp: 225-228.
Citation: Yusuf E, Abegaz S (2023) Prevalence and Associated Risk Factors of Ovine Lungworm Infestation in and Around Kemissie Town Oromo Special Zone Ethiopia. J Clin Res Bioeth. S15:001.
Copyright: © 2023 Yusuf E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

