Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 10, Issue 2
Predicting the Possible Mechanism of Aphrodisiac Action of Pheonix Dactylifera L
Ben Enoluomen Ehigiator1*, Agnes Eno Cobhams1, Elias Adikwu2 and Inemesit Okon Ben32Department of Pharmacology and Toxicology, Niger Delta University, Bayelsa State, Nigeria
3Department of Pharmacology and Toxicology, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana
Received: 28-Jan-2022, Manuscript No. JTD-22-15569; Editor assigned: 01-Feb-2022, Pre QC No. JTD-22-15569(PQ); Reviewed: 15-Feb-2022, QC No. JTD-22-15569; Revised: 21-Feb-2022, Manuscript No. JTD-22-15569(R); Published: 28-Feb-2022, DOI: 10.35841/2329-891X-22.10.312
Abstract
Background: There are several methods and remedies available for managing sexual dysfunction, a major issue man, among such is the use of aphrodisiacs. There is still the search for aphrodisiac agents, especially those from natural or plant-based sources and Pheonix dactylifera (P. dactylifera) has a potential aphrodisiac effect. This study was designed to investigate the possible mechanism of its aphrodisiac action using the molecular docking technique, which was to dock compounds unto target proteins that contribute to the management of erectile dysfunction. The key targets used for this study are; phosphodiesterase-5 (PDE-5, arginase II, aromatase, angiotensin-converting enzyme ACE, and alpha-1 adrenoceptors).
Methods:About 49 compounds were obtained from Pubchem library, by virtual screening of articles related to P. dactylifera, ligand library generated and docked against various targets of interest, concerning the penile erection and erectile dysfunction.
Results:Rutin, pelargonin, procyanidins, quercetin, and procyanidins showed good docking scores on some of the targets considered for the study.
Conclusion: Rutin, pelargonin, procyandins may be potential drugs for ED
Keywords
Phoenix dactylifera; Aphrodisiac; Molecular docking; Erectile dysfunction
Introduction
Sexual feelings are an inevitable part of life. Sexual intercourse is one of the most cherished, indispensable, and integral parts of every individual and can as well be a cradle of pleasure and satisfaction [1]. A satisfying sexual relationship is dubbed as one of the most important parts of a sexual or marital relationship [2]. When sexual difficulties become persistent or recur frequently and cause marked distress and interpersonal difficulties, then, such a person may be said to have sexual dysfunction. Sexual dysfunction occurs in different forms in men. It may be acute or situational, as it may be due to response to the environment, loss of loved one, or job. It may also be persistent or chronic due to an underlying disease condition. However it occurs, sexual dysfunction may readily depreciate the quality of a sexual relationship and the general wellbeing of the person affected. The inability to maintain a healthy sexual and reproductive life has been indicated in depression, nervousness, anxiety, fear, and decline in quality of life. Interestingly, a good number of divorce cases that occur annually, have some bearing with sexual dysfunctions [3]. Erectile dysfunction, formerly referred to as “impotence”, is defined as the persistent inability to achieve or maintain an erection for satisfactory sexual performance over a period of three months. It is one of the prevalent forms of sexual dysfunction. It may be managed with the help of aphrodisiacs [4].
Aphrodisiacs are substances or agents (food, drug, scent, or device) that stimulate the erotic instinct, induce venereal desire, and surge pleasure and performance [4]. The study of aphrodisiacs is needed for effective corroboration of traditional medicine practice with a scientific approach on information, collection, preparation, side effects, efficacy, safety, and standardization of some of the plant parts. Interestingly, many effective herbal aphrodisiacs are accessible and have slight or no side effects [5]. This study attempts to give a predictive explanation of the likely mechanism of aphrodisiac action of Pheonix dactylifera.
Pheonix dactylifera or Date Palm Pollen (DPP), the male reproductive dust of palm flowers, belongs to the family Arecaceae (angiosperms, monocotyledon). It is a subtropical fruit tree, native to Iraq and other countries of the Middle East and West Africa, and it is the only phoenix species grown for its edible fruits. It is commonly known as Palmera, Tamara, Palmadatilrira, Datilero, Hurma [6] and its names among some ethnic groups in Nigeria include dabino (Hausa), Esoanobi (Yoruba), ubeokpoko (Igbo). DPP is widely cultivated in Saudi Arabia and it is fascinating to point that approximately 1000 tons of DPPs are reproduced every year by millions of palm trees grown in the Arabic regions [7]. The use of date palm in folk treatment of erectile dysfunction and infertility in males by the people of western Africa (Nigeria) has been reported [8]. Phytochemical analysis reveals that date palm pollen contains a variety of compounds including flavonoids, saponins, sterols, glycosides, phenolic acids, amino acids, fatty acids. However, since estrogen has been reported to contribute to the control of spermatogenetic stem cells and male reproductive tissues with estrogen receptors, date palm pollen which holds estrogenic gonad stimulating compounds, estrogen, sterols, and other useful micro- nutrients, can help treat erectile dysfunction. This study was designed to investigate the mechanism of the aphrodisiac effect of the phytochemicals constituents of P. dactylifera on some enzymes and receptors involved in sexual functions such as PDE-5, aromatase, and arginase II using virtual and molecular docking techniques.
Materials and Methods
In silico studies
Identified secondary metabolites of P. dactylifera employed for this study were determined from published literature and were used in the creation of the ligand library. Fifty (50) secondary metabolites were retrieved from NCBI PubChem library, in Standard Database Format (2D) as described by [9]. Docking of the structures was carried using Maestro software (Maestro Version 12.5.139, MMshare Version 5.1.139, Release 2020-3, Platform Windows-x64), following the steps of preparing the compounds and proteins before the docking, [10]. Human arginase II (1.8 Å), PDE-5 (2.3 Å), aromatase (2.75 Å), ACE (1.8 Å) bound with ligands were retrieved from the Protein Data Bank according to [11]. With the PDB ID: 4I06, 2H42, 5JKV, 1UZE respectively, and modeled doxazosin bound with alpha-1 adrenoceptor were retrieved from GPCRdb [12]. QikProp was used for the ADMET predictions using the Force Field of OPLS3e.
Results and Discussion
Erectile dysfunction (ED) has been predicted to likely affect over 322 million men worldwide by the year 2025, an increase from 152 million men in 1995 [13]. ED may also influence equally the quality of life of female partners of men with ED by depriving them of sexual satisfaction. Aphrodisiacs are substances or agents (food, drug, scent, or device) that stimulate the erotic instinct, induce venereal desire, and surge pleasure and performance [4]. The adverse effects resulting from the use of orthodox aphrodisiacs to enhance sexual satisfaction in males with erectile dysfunction could be major setbacks in their applications. It is as a result of the adverse effects that the use of non-convention medicines from the traditional origin is employed to checkmate erectile dysfunction in males. P. dactylifera is traditionally used as an aphrodisiac and fertility enhancer. It is used in the Middle East as a natural drug for the treatment of male infertility and the promotion of fertility in women. P. dactylifera fruit suspension has been documented to improve sperm count, motility, morphology, and DNA quality [14]. This study aimed to establish the possible mechanisms of aphrodisiac action of P. dactylifera has an abundance of photochemical substances (Table 1) [7,15-23]. Researchers have attributed the male aphrodisiac action of P. dactylifera to the presence of alkaloids, flavonoids, and saponins in the plant [24]. To predict the potential aphrodisiac mechanism of action, therefore, the identified compounds of P. dactylifera were obtained by virtual screening and docked with enzymes or targets related to erectile dysfunction including PDE-5, aromatase, Human arginase II, ACE, and alpha 1a adrenoceptor.
| Phytochemicals | Reference |
|---|---|
| Rutin, Apigenin, Luteolin, Quercetin, Caffeic acid, Coumaric acid, Gallic acid, Chlorogenic acid, Isochlorogenic acid. | Mohammad et al. [7] |
| Isoflavones, Lignans, Neoxanthins, Violaxanthin, Antheraxanthin, Β-Sitosterol, Stigmasterol, Campesterol, Isoflucosterol, Formononetin, Daidzein, Genistein, Matairesinol, Lariciresinol, Pinoresinol, Secoisolariciresinol, Coumestrol, Ρ-Hydroxybenzoic acid, Protocatechuic acid, Vanillic acid, Isorhamnetin, Syringic acid, Ο-Coumaric acid, Ρ-Coumaric acid, Ferulic acid, Sinapic acid, 5-Ο-Caffeoyl Shikimic acid, L-caffeoyl-beta-D-glucose, Salicylic acid, flavan-3-ol. | Al-Alawi et al.[15] |
| Diosmetin | Michael et al. [16] |
| Kaempferol | Borochov-Neori et al. [17] |
| Pelargonin, | Karasawa et al. [18] |
| Procyanidins, | Faqir et al. [19] |
| Lutein, β-Carotene | Thomas et al. [20] |
| Cinnamic acid | Ali et al., 2016. [21] |
| Coumaroylquinic acid | Mansouri et al. [22] |
| Vanillin acid, Chrysoeriol | Farid et al. [19] |
| β-resorcylic acid | Sabri et al. [23] |
Table 1: Ligands and sources mined from PubChem.
In this study, rutin, iso-quercetin, pelargonin, chrysoeriol, isoharmnetin, chlorgenic acid, and flavan-3-ols present in P. dactylifera showed aphrodisiac potential by inhibiting PDE-5, Arginase II, Aromatase, ACE, Alpha 1 receptor (Fig 1-3) which are pertinent to erectile dysfunction. According to [25], aphrodisiacs may be expected to act in either or all of these three ways; Increase libido via the endocrine system. Improve erection through their effects on neurotransmitters and some enzymes involved in sexual function. They may enhance sexual pleasure as a result of their effects on the psychologically-mediated pathway of sexual function. Studies showed that the down regulation of PDE-5 is crucial to the penile erectile process. PDE-5 catalyzes the breakdown of cyclic guanosine monophosphate (Basu and Ryder) in [26]. And also lowers the levels of NO in the endothelial cells, thereby reducing signaling. Aphrodisiac inhibits the hydrolyzing activity of PDE- 5 which increases active cGMP concentration causing dilation of corpus cavernosum tissues, vasodilation of blood vessels, and penile erection [26].
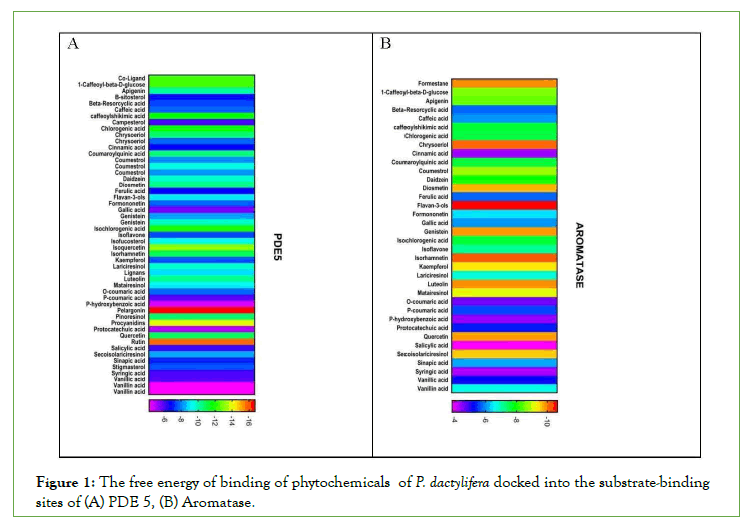
Figure 1: The free energy of binding of phytochemicals of P. dactylifera docked into the substrate-binding sites of (A) PDE 5, (B) Aromatase.
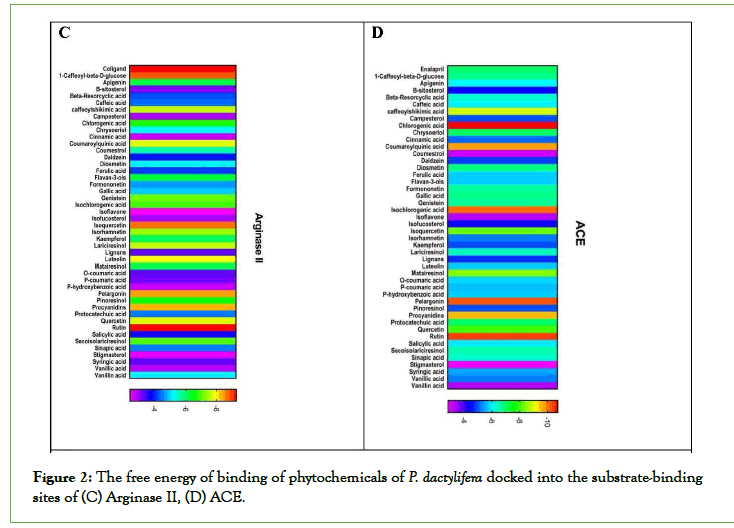
Figure 2: The free energy of binding of phytochemicals of P. dactylifera docked into the substrate-binding sites of (C) Arginase II, (D) ACE.
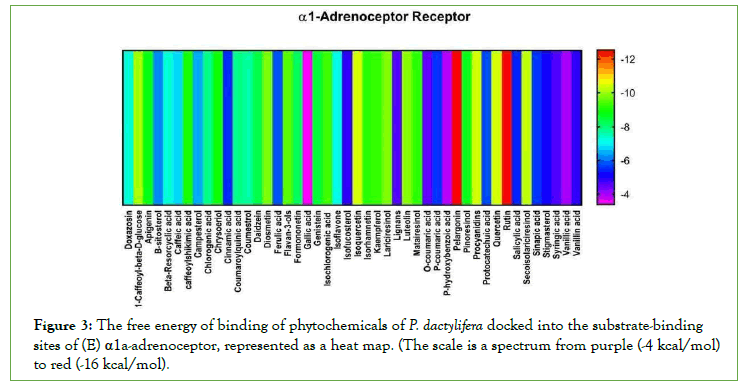
Figure 3: The free energy of binding of phytochemicals of P. dactylifera docked into the substrate-binding sites of (E) α1a-adrenoceptor, represented as a heat map. (The scale is a spectrum from purple (-4 kcal/mol)
to red (-16 kcal/mol).
Arginase II is an enzyme, which mobilizes the hydrolysis of L-Arginine to produce L-Ornithine and urea. Hence, the inhibition of arginase activity can be an additional target for aphrodisiac which could increase the bioavailability of L-arginine. This will contribute to the production of NO via a reaction catalyzed by NOS, thereby enhancing erection in males [27].
Aromatase is required for the production of estrogen, it acts by mobilizing the conversion of testosterone (androgen) to estradiol (estrogen). Decreased levels of testosterone and increased levels of estrogen have been indicated in the increased occurrence of ED. Therefore, inhibition of this enzyme is of paramount importance in promoting aphrodisiac effects and penile erection subsequently [28]. The inhibition of penile α-1 receptors could be employed to assist erection response in males with ED by inhibiting vasoconstriction of penile arteries specifically, cavernous artery, and inducing vasodilation of the arteries.
RAAS (renin-Angiotensin-Aldosterone system) which has been associated with hypertension is linked with the pathophysiology of ED [1]. Angiotensin converting enzyme converts angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor that modulates muscle contraction in penile corpus cavernosum tissues. The downregulation of ACE could be a target in the control of hypertension and hypertension-induced erectile dysfunction, it also accounts for increased NO and bradykinin levels which are vital biological molecules in the erectile process [9]. Inhibition of ACE activity has been reported to further reduce the angiotensin II levels and improve erectile function in ED patients.
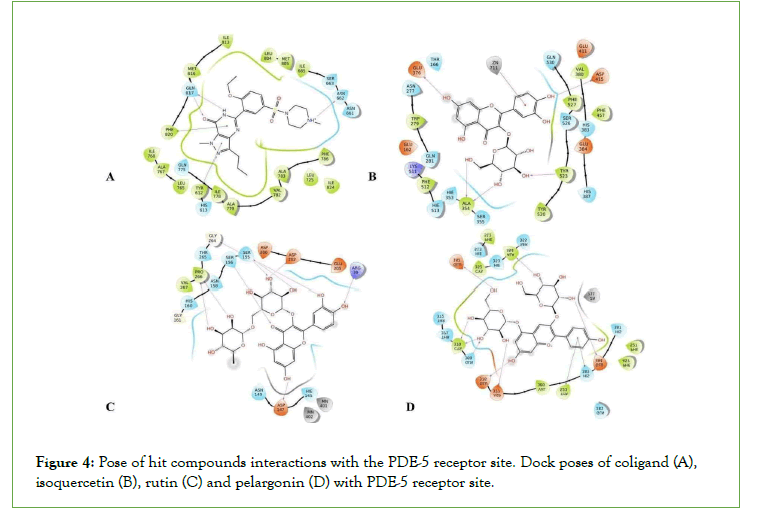
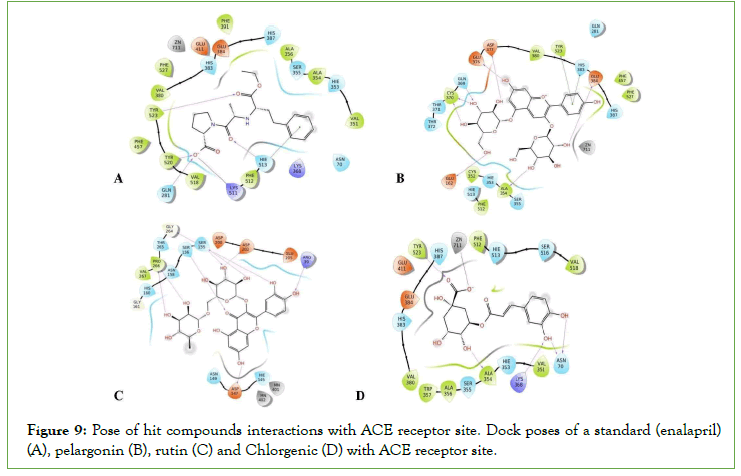
The observation in this research shows that rutin pelargonin and quercetin present in P. dactylifera produced the best effect on all the targets involved in the erectile process except aromatase (Fig 4–9). However, rutin and pelargonin are not potential oral drug candidates, as they violated Lipinski’s rule of five (Table 2). Furthermore, the combination of rutin and quercetin has been reported to manifest some synergistic action in vitro studies as reported by [29-31].
Figure 4: Pose of hit compounds interactions with the PDE-5 receptor site. Dock poses of coligand (A), isoquercetin (B), rutin (C) and pelargonin (D) with PDE-5 receptor site.
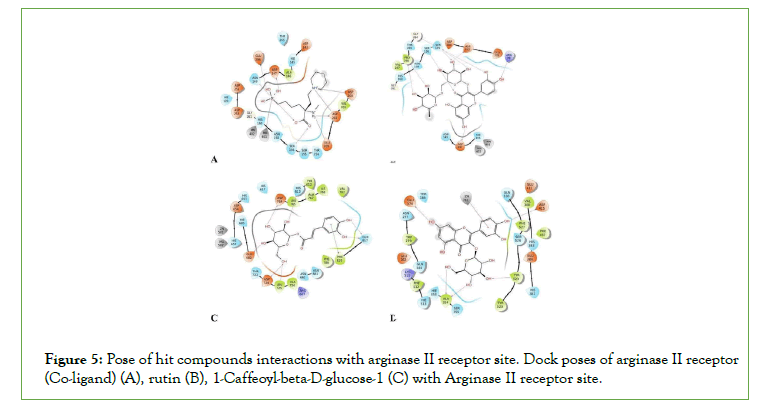
Figure 5:Pose of hit compounds interactions with arginase II receptor site. Dock poses of arginase II receptor (Co-ligand) (A), rutin (B), 1-Caffeoyl-beta-D-glucose-1 (C) with Arginase II receptor site.
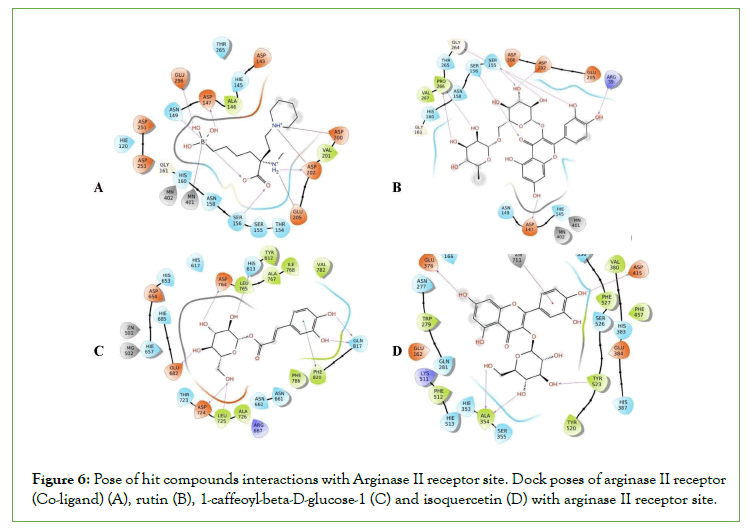
Figure 6: Pose of hit compounds interactions with Arginase II receptor site. Dock poses of arginase II receptor (Co-ligand) (A), rutin (B), 1-caffeoyl-beta-D-glucose-1 (C) and isoquercetin (D) with arginase II receptor site.
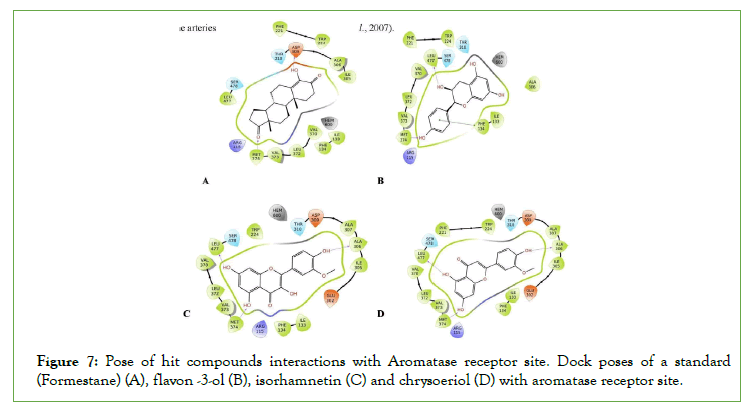
Figure 7: Pose of hit compounds interactions with Aromatase receptor site. Dock poses of a standard (Formestane) (A), flavon -3-ol (B), isorhamnetin (C) and chrysoeriol (D) with aromatase receptor site.
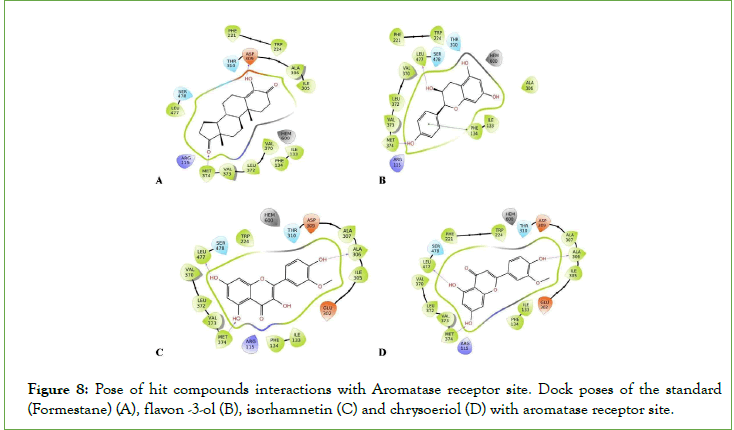
Figure 8: Pose of hit compounds interactions with Aromatase receptor site. Dock poses of the standard (Formestane) (A), flavon -3-ol (B), isorhamnetin (C) and chrysoeriol (D) with aromatase receptor site.
Figure 9: Pose of hit compounds interactions with ACE receptor site. Dock poses of a standard (enalapril)(A), pelargonin (B), rutin (C) and Chlorgenic (D) with ACE receptor site.
| S/N | Entry name | Molecular weight MW | Donor HB | Acceptor HB | QP1ogPo/w | HOA | ROF |
|---|---|---|---|---|---|---|---|
| 0 | Co-Ligand PDE5 (2H42) Formestane Aromatase | 474.577 | 1 | 11.75 | 1.947 | 3 | 0 |
| 0 | (3EQM) | 286.413 | 0 | 4 | 3.041 | 3 | 0 |
| 0 | Co-Ligand Arginase II (4106) | 318.412 | 5 | 5 | -0.708 | 1 | 0 |
| 0 | Enalapril (ACE) | 376.452 | 2 | 8.5 | 0.196 | 2 | 0 |
| 0 | Doxazosin (al-AR) | 302.413 | 1 | 4.75 | 2.628 | 3 | 0 |
| 1 | 1-Caffeoyl-beta-D-glucose | 342.302 | 6 | 12 | -1.478 | 2 | 1 |
| 2 | Antheraxanthin | 584.881 | 1 | 4.4 | 10.536 | 1 | 2 |
| 3 | Apigenin | 270.241 | 2 | 3.75 | 1.607 | 3 | 0 |
| 4 | B-carotene | 536.882 | 0 | 0 | 16.706 | 1 | 2 |
| 5 | B-sitosterol | 414.713 | 1 | 1.7 | 7.621 | 1 | 1 |
| 6 | Beta-Resorcyclic acid | 224.256 | 2 | 2.5 | 2.271 | 3 | 0 |
| 7 | Caffeic acid | 180.16 | 3 | 3.5 | 0.545 | 2 | 0 |
| 8 | caffeoylshikimic acid | 336.298 | 5 | 8.9 | 0.064 | 1 | 0 |
| 9 | Campesterol | 400.687 | 1 | 1.7 | 7.301 | 1 | 1 |
| 10 | Chlorogenic acid | 354.313 | 6 | 9.65 | -0.27 | 1 | 1 |
| 11 | Chrysoeriol | 300.267 | 2 | 4.5 | 1.761 | 3 | 0 |
| 12 | Cinnamic acid | 148.161 | 1 | 2 | 1.897 | 3 | 0 |
| 13 | Coumaroylquinic acid | 338.313 | 5 | 8.9 | 0.399 | 2 | 0 |
| 14 | Coumestrol | 268.225 | 2 | 4.5 | 1.305 | 3 | 0 |
| 15 | Daidzein | 254.242 | 2 | 4 | 1.792 | 3 | 0 |
| 16 | Diosmetin | 300.267 | 2 | 4.5 | 1.766 | 3 | 0 |
| 17 | Ferulic acid | 194.187 | 2 | 3.5 | 1.371 | 3 | 0 |
| 18 | Flavan-3-ols | 274.273 | 4 | 4.7 | 1.122 | 3 | 0 |
| 19 | Formononetin | 268.268 | 1 | 4 | 2.63 | 3 | 0 |
| 20 | Gallic acid | 170.121 | 4 | 4.25 | -0.578 | 2 | 0 |
| 21 | Genistein | 270.241 | 2 | 3.75 | 1.695 | 3 | 0 |
| 22 | Isochlorogenic acid | 354.313 | 6 | 9.65 | -0.34 | 1 | 1 |
| 23 | Isoflavone | 238.242 | 0 | 4.5 | 1.839 | 3 | 0 |
| 24 | Isofucosterol | 412.698 | 1 | 1.7 | 7.542 | 1 | 1 |
| 25 | Isoquercetin | 464.382 | 7 | 13.75 | -1.377 | 1 | 2 |
| 26 | Isorhamnetin | 316.267 | 3 | 5.25 | 1.2 | 3 | 0 |
| 27 | Kaempferol | 286.24 | 3 | 4.5 | 1.042 | 3 | 0 |
| 28 | Lariciresinol | 360.406 | 3 | 6.4 | 2.671 | 3 | 0 |
| 29 | Lignans | 414.411 | 1 | 8.45 | 2.493 | 3 | 0 |
| 30 | Lutein | 568.881 | 2 | 3.4 | 10.681 | 1 | 2 |
| 31 | Luteolin | 286.24 | 3 | 4.5 | 0.927 | 3 | 0 |
| 32 | Matairesinol | 358.39 | 2 | 6 | 2.84 | 3 | 0 |
| 33 | Neoxanthin | 600.88 | 2 | 5.15 | 9.877 | 1 | 2 |
| 34 | 0-coumaric acid | 164.16 | 2 | 2.75 | 1.469 | 3 | 0 |
| 35 | P-coumaric acid | 164.16 | 2 | 2.75 | 1.425 | 3 | 0 |
| 36 | P-hydroxybenzoic acid | 138.123 | 2 | 2.75 | 0.578 | 3 | 0 |
Table 2: Show the pharmacological properties of P. dactyfera compounds, derived by ADME/Tox.
Conclusion
In conclusion, the effective activity, inhibition/stimulation of targets linked with the management of ED by phytonutrients in P. dactylifera, could be associated with the PDE-5, ACE, alpha adrenoceptor, and arginase II inhibitory properties of phytochemicals, such as: rutin, pelargonin, and to a lesser extent, procyanidins, which are present in P. dactylifera. Compounds like rutin and pelargonin may not be seen as perfect oral drugs, because they seem to violate Lipinski’s oral drug rule of five, making them more viable as potential parenteral agents. However, useful compounds can’t and shouldn’t be discarded without getting a fair experimental hearing.
Acknowledgements
We are immensely grateful to Dr. Idowu Omotuyi, Mr. Niyi Adelakun, Mr. Olumide Inyang, and the staff at the Centre for Biocomputing and Drug Development, Adekunle Ajasin University.
Competing Interests
Authors have declared that no competing interests exist.
REFERENCES
- Erhabor JO, Idu M. Aphrodisiac potentials of the ethanol extract of aloe barbadensis mill root in male wistar rats. BMC Complement Altern Med. 2017;17(1):1-10.
[Crossref] [Google Scholar] [Indexed]
- McCarthy BW. Marital style and its effects on sexual desire and functioning. J Fam Psychother. 1999; 10(3):1-2.
[Crossref] [Google Scholar] [Indexed]
- Amato PR, Previti D. People's reasons for divorcing: Gender, social class, the life course and adjustment. J Fam Issues. 2003;24(5):602-626.
- Malviya N, Jain S, Gupta VB, Vyas S. Recent studies on aphrodisiac herbs for the management of male sexual dysfunction-a review. Acta Pol Pharm. 2011;68(1):3-8.
- Indurwade NH, Kawtikwar PS, Kosalge SB, Janbandhie NV. Herbal plants with aphrodisiac activity. Indian Drugs. 2005; 42(2):67-72.
- Abdelmajid R. The date palm in Tunisia: I. The genetic patrimony. Bioversity International, International Plant Genetic Resources Institute. 2005; 2.
- Mohammad T, Mannan H, Roja R. The role of date palm pollen (P. dactylifera) infertility: a comprehensive review of current evidence. Evid Based Complementary Altern Med. 2015;21(4):320-324.
[Crossref] [Google Scholar] [Indexed]
- Abdi F, Roozbeh N, Mortazavian AM. Effects of date palm pollen on fertility: Research proposal for a systematic review. BMC Res Notes. 2017;10(1):1-4.
[Crossref] [Google Scholar] [Indexed]
- Ehigiator BE, Adesida AS, Omotuyi IO. Chicoric Acid, a phytochemical compound of Solenostemon monostachyus: Possible drug candidate for the relief of erectile dysfunction. Int J Eng Appl Sci Technol. 2020;4:509-518.
- Brooks WH, Daniel KG, Sung SS, Guida WC. Computational validation of the importance of absolute stereochemistry in virtual screening. J Chem Inf Model. 2008;48(3):639-645.
[Crossref] [Google Scholar] [Indexed]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank. Nucleic Acids Res. 2000;28(1):235-242.
[Crossref] [Google Scholar] [Indexed]
- Munk C, Isberg V, Mordalski S, Harpsøe K, Rataj K, Hauser AS, et al. GPCRdb: The G protein-coupled receptor database-an introduction. British J Pharmacol. 2016;173(14):2195-2207.
[Crossref] [Google Scholar] [Indexed]
- Reddy AK, Lulkovich K, Ottwell R, Arthur W, Bowers A, Al-Rifai S, et al. Evaluation of spin in abstracts of systematic reviews and meta-analyses focused on treatments of erectile dysfunction: A cross-sectional analysis. Sexual Medicine. 2021;9(1):100284.
- Bahmanpour S, Panjeh SM, Talaei T, Vojdani Z, Poust PA, Zareei S, et al. Effect of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Int J Mol Sci.2006; 31(4): 208-212.
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi JS, Al-Shihi BI, Baqi Y. Date palm tree (Phoenix dactylifera L.): natural products and therapeutic options. Front Plant Sci. 2017;8:845.
- Michael HN, Salib JY, Eskander EF. Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytotherapy Research. 2013;27(5):699-704.
[Crossref] [Google Scholar] [Indexed]
- Borochov-Neori H, Judeinstein S, Greenberg A, Volkova N, Rosenblat M, Aviram M. Antioxidant and antiatherogenic properties of phenolic acid and flavonol fractions of fruits of ‘Amari’and ‘Hallawi’date (Phoenix dactylifera L.) varieties. J Agric Food Chem. 2015; 63(12):3189-3195.
- Karasawa K, Uzuhashi Y, Hirota M, Otani H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J Agric Food Chem. 2011;59(20):11287-11293.
- Farid K, Robert WO, Irvila R, Andrea B. Characterization of phenolic compounds in mature Moroccan medjool date palm fruits (Phoenix dactylifera L.) by HPLC-DAD-ESI-MS. J Food Comp and Anal. 2018; 70:63-71.
- Thomas LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutrition and cancer. 2006; 54(2):184-201.
- Ali A, Naheed B, Muhammed T. Phytochemical and therapeutic evaluation of date (Phoenix dactylifera L.). A review. J Pharm Pharmacol. 2016; 9:2222-4857.
- Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food chemistry. 2005; 89(3):411-420.
- Elma SN, Badarusham K, Rosli D, Salvamani S, Hassan MS, Hashim R. Solvents extraction effects on bioactive compounds of Ajwa date (Phoenix dactylifera L.) flesh using mixture design. Chem Eng Trans. 2018.
- Kotta S, Ansari SH, Ali J. Exploring scientifically proven herbal aphrodisiacs. Pharmacognosy reviews. 2013; 7(13):1.
[Crossref] [Google Scholar] [Indexed]
- Basu A, Ryder RE. New treatment options for erectile dysfunction in patients with diabetes mellitus. Drugs. 2004; 64(23):2667-2688.
[Crossref] [Google Scholar] [Indexed]
- Chew KK, Stuckey BG, Thompson PL. Erectile dysfunction, sildenafil and cardiovascular risk. Med J Aust. 2000; 172(6):279-283.
[Crossref] [Google Scholar] [Indexed]
- Abedi A, Parviz M, Karimian SM, Rodsari HR. Aphrodisiac activity of aqueous extract of Phoenix dactylifera pollen in male rats. Adv Sexual Med. 2013;3: 28-34.
[Crossref] [Google Scholar] [Indexed]
- Anjum FM, Bukhat SI, El-Ghorab AH, Khan MI, Nadeem M, Hussain S, et al. Phytochemical characteristics of date palm (Phoenix dactylifera) fruit extracts. Pak J Food Sci. 2012; 22(3):117-127.
- Adefegha SA, Oboh G, Fakunle B, Oyeleye SI, Olasehinde TA. Quercetin, rutin, and their combinations modulate penile phosphodiesterase-5′, arginase, acetylcholinesterase, and angiotensinI-converting enzyme activities: a comparative study. Comp Clin Path. 2018; 27(3):773-780.
- Derek L. Broadening the field of opportunity for medicinal chemists. Med Chem Comm. 2021;6(11):1907-2044.
- Ehigiator BE, Okoli RI, Ndubueze NG, Ebong OO. In-vivo and Insilico Investigations on Goron Tula (Azanza garckeana F. Hoffm), a Booster of Female Sexual Arousal and Vaginal Lubrication. J Trop Dis doi. 2021; 10.
Citation: Ehigiator BE, Cobhams AE, Adikwu E, Ben IO (2022) Predicting the Possible Mechanism of Aphrodisiac Action of Pheonix dactylifera L. J Trop Dis. 10:312.
Copyright: © 2022 Ehigiator BE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.