Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 6, Issue 3
Portal and Severe Mesenteric Vein Thrombosis Associated with Acute Cytomegalovirus Infection: Case Report
Ali Ilgın Olut1*, Burak Şeker1, Hilal Küpeli1, İbrahim Erdinç2 and Selma Tosun12Department of Cardiovascular Surgery, Health Science University Izmir Bozyaka Educational and Research Hospital, Turkey
Received: 18-Nov-2019 Published: 24-Sep-2021, DOI: 10.35248/2576-389X.21.6.158
Introduction
Venous thromboembolism (VTE) is relatively a common and potentially fatal disease and is the third leading cause of cardiovascular mortality, with 5% of individuals have at least one VTE episode in their lifetime[1,2]. Idiopathic (unprovoked) venous thromboembolism (IVTE) is defined as VTE which occurs in the absence of triggering circumstances such as prolonged immobilization, a journey lasting for more than 6-8 h, fracture of a lower limb, major surgery, active cancer, antiphospholipid antibody syndrome, pregnancy or drug usage such as oral contraceptive etc[3]. The studies demonstrated that in almost 50% of first VTE, a thrombophilic factor could be identified and the incidence of IVTE is reported as 25-50% in different studies [1-3]. Generally, IVTE requires a special attention as the patient needs careful investigation and periodical monitorization, and in many cases should be treated for a lifetime. When assessing the etiology of IVTE, infectious causes such as cytomegalovirus (CMV) are rarely considered. However, in many recent reports, there is mounting evidence of infections as causes of VTE. A meta-analysis of existing data showed that between 2-9% of patients hospitalized with VTE had an acute CMV infection[4]. Here, we present an otherwise healthy 21 years old male presented with severe VTE in portal and in all branches of inferior and superior mesenteric veins whom diagnosed as having an acute CMV infection by serological and molecular methods.
Case Report
A 21-year-old male was referred to our emergency department with fever of 38.0 Co, acute severe abdominal pain, nausea and vomiting. On medical examination there was a general abdominal tenderness and abnormal laboratory results were as: WBC: 10.500 mm3, AST:191 U/l, ALT:61 U/l, CRP:153 pg/ml, ESR 59 mm/h, total bilurubin:3.8 mg/ml, urine bilirubin (+++). Abdominal CT of the patient revealed diffuse thrombosis of superior and inferior mesenteric veins including all branches and partial thrombosis of portal vein from distal to proximal sites and hepato-splenomegaly. The patient was hospitalized and immediate anti-thrombolytic treatment (enoksaparin 1 mg/kg x2 sc) was started. For investigation of infectious etiology of fever and hepato-splenomegaly, serological tests were performed. Toxoplasma, salmonella, brucella and HIV, hepatitis A, B, C, E, herpes simplex, Epstein–Barr, viruses were all negative and anti- CMV IgG and anti-CMV IgM antibodies were positive (ARCHITECT CMV ELISA-KIT Ireland). Hematologic investigation of patient’s coagulation profile was normal and screening for hereditary thrombophilia panel, protein C resistance, proteins C-protein S and lupus anticoagulant was negative, anti-thrombin activity was normal, and factor VIII activity was within normal range. CMV avidity testing a showed a very low result (<%10) and CMV-DNA was 1444 IU/ml. On detailed medical history he was a non-smoker, had no known chronic disease or drug usage but he donated kidney to his mother 16 months ago in our hospital and serological tests for CMV IgG-IgM were negative at that time. The patient was accepted as having an acute CMV hepatitis complicated by acute portal and mesenteric vein thrombosis. Though he was immunocompetent, due to the high viremia and the critical clinical condition of the patient, i.v ganciclovir therapy was started along with anti-thrombolytic treatment. After ten days of therapy, disappearance of fever and reduction of transaminases was observed, CMV-DNA returned to negative and doppler USG showed regression of thrombosis in both portal and mesenteric veins.
Literature Review
By using the words cytomegalovirus infection & venous thrombosis on 12 February 2019, we found 110 articles in PUBMED and no records in Turkish Medline with using the words sitomegalovirus & tromboemboli. When the search was constricted to only human studies in English literature, including case or cases of VTE in immunocompetent individuals, 32 articles were found, with 61 patient cases[4, 5-36]. Flow diagram presenting the number of studies screened, assessed for eligibility and included in the review are given in Figure 1.
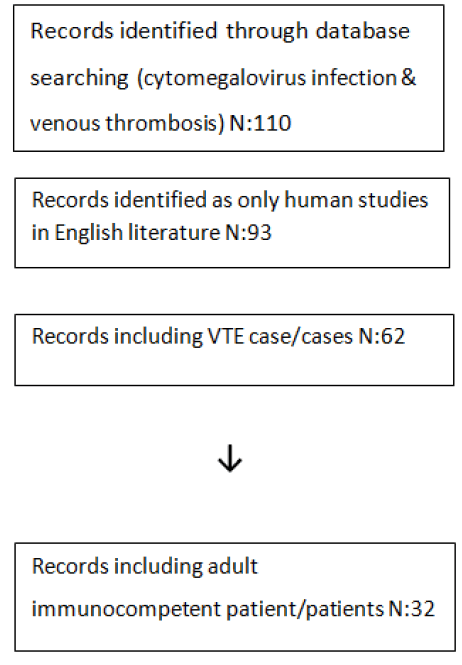
Figure 1: Flow diagram presenting number of studies screened, assessed for eligibility and included in the review.
Table 1: Flow diagram presenting number of studies screened, assessed for eligibility and included in the review.
In the analysis of cases, the mean age of patients was 37 years with an apparently female predominance (61%). The sites veins affected were as: 28 deep veins of lower extremities, 20 pulmonary, 15 portal, 7 mesenteric, one hepatic and one sinus vein.
Most of the patients (67%) had a concomitant risk factor -either hereditary or acquired such as oral contraceptive (OCP) use- and interestingly in one third of cases no thrombophilic factor other than CMV infection was present. In three cases, any additional risk factor was not sought. Table 1 explains the clinical characteristics of cases of VTE occurring in the course of acute CMV infection in immunocompetent adult patients.
| Article | Hypercoagulable | Extra-vascular | |||
|---|---|---|---|---|---|
| Age/sex | VTE location | state risk factors | manifestations | Treatment | |
| Inacio et al. (1997)[5] | 31 F | Mesenteric vein, PVT | OCP | Hepatitis | OA |
| Ofotokun et al. (2001[6] | 50 M | PVT | None | CMV viremia, splenomegaly | Ganciclovir |
| Abgueguen et al. (2003)[7] | 32 F | DVT, PE | FVL | NR | NR |
| 38 F | DVT, PE | None | CMV colitis | ||
| Youd et al. (2003[8] | 35 M | PE | NR | Hepatitis | NR |
| Rovery et al. (2005[9] | 33 M | DVT | DVT history, FVL | CMV viremia | NR |
| Yildiz et al. (2006[10] | 30-49 (37.5) | 6 DVT | All with congenital thrombophilic condition | 3 pharyngitis | Anticoagulants |
| 9 F, 1 M | 3 PE | 1 Hepatitis | (in 9 for 6 months, in one for 12 months) | ||
| 2 mesenteric | |||||
| Spahr L et al.(2006)[11] | 36 F | PVT+hepatic vein+Budd Chiari | OCP | CMV viremia | Heparin |
| Paran et al. (2007)[12] | 21 F | DVT | Anti-cardiolipin ab | NR | NR |
| 35 M | DVT | FVL | NR | NR | |
| 29 M | Bilateral DVT | OCP | NR | NR | |
| Squizzato et al. (2007)[13] | 34 M | PVT | None | Splenomegaly | OA |
| Ergas et al. (2008)[14] | 28 M | Mesenteric vein + PE | MTHFR | NR | NR |
| Ladd et al. (2009)[15] | 17 F | PVT, PE | OCP | NR | NR |
| Abgueguen et al. (2010) [17] | 32 F | PE | None | Ulcerative colitis | OA |
| 38 F | PE | FVL | None | OA | |
| 82 F | PE (bilateral) | None | None | OA | |
| Justo et al. (2011)[4] | 29 F | PE | OCP | NR | NR |
| 32 M | SVT | None | NR | ||
| 54 M | SVT | None | NR | ||
| Poon et al. (2011)[18] | 30 F | PE | NR | Splenic infarct | OA |
| Ticlear et al. (2011) [19] | 26 F | OCP | NR | ||
| 28 F | OCP | NR | |||
| 36 F | All with DVT | OCP | NR | NR | |
| 36 F | Surgery | CMV Viremia | |||
| 36 F | Pregnancy | NR | |||
| Kalaitzis et al. (2012)[20] | 40 M | Mesenteric vein | None | CMV viremia, | Enoxaparin |
| Small bowel necrosis | |||||
| Sherman et al. (2012)[21] | 70 M | Sinus vein thrombosis | None | NR | OA |
| Schimanski et al. (2012) [22] | 29 F | DVT + PE | Pregnancy | ||
| 31 F | DVT | OCP, F VIII | |||
| 38 F | DVT | OCP | |||
| 42 F | DVT | F VIII, FVL | NR | NR | |
| 46 F | DVT | None | |||
| 58 M | DVT | FVL | |||
| 61 F | DVT | F VIII | |||
| Pichenot et al. (2013)[23] | 39 M | PVT, PE | None | CMV viremia | OA, valgancyclovir OA |
| 40 F | PVT | OCP | CMV viremia | OA, ganciclovir | |
| 43 F | Bilateral DVT, PE | Heavy smoker | None | ||
| Galloula et al. (2014)[24] | 24 F | PVT | OCP | NR | OA |
| Nakayama et al. (2014) [25] | 19 M | DVT, PE | APL | Alveolar hemorrhage | OA |
| CMV viremia | |||||
| Rinaldi et al. (2014)[26] | 62 F | PVT | Heavy smoker | Hepatitis | OA, ganciclovir Enoxaparin |
| 20 F | PVT | None | Hepatitis | ||
| Vandamme et al. (2014) [27] | 30 M | Bilateral PE | None | Myo-pericarditis + Alveolar hemorrhage | NR |
| Bertoni et al. (2015)[28] | 39 M | Mesenteric vein | FVL | NR | OA |
| Wang et al. (2015)[29] | 61 M | PVT | None | None | OA |
| Chou et al. (2016)[30] | 78 M | PE | NR | CMV colitis | Heparin, ganciclovir |
| Bountouris et al. (2017)[31] | 25 M | DVT, PE | None | NR | rivaroxaban |
| Vael et al. (2017)[32] | 58 F | PVT | None | CMV colitis | Thrombolysis, |
| hemicolectomy, heparin | |||||
| Kelkar et al. (2017)[33] | 46 M | PVT | None | CMV viremia, Hepatitis, CMV colitis | Heparin + OA |
| Puccia et al. (2017)[34] | 30 M | PVT | None | CMV viremia, Hepatitis | Heparin + ganciclovir |
| Salembier et al. (2018)[35] | 35 M | Mesenteric vein, PVT | Hereditary Thrombophilia | Hepatitis | Heparin |
| Ngu et al. (2018)[36] | 27 M | DVT | None | CMV viremia, Hepatitis | OA |
Table 1: Clinical characteristics of cases of venous thromboembolism occurring in the course of acute cytomegalovirus infection in immunocompetent patients.
Discussion
CMV infection was first suspected to be a cause of venous thromboembolism (VTE) at 1974, when Vorlicky et al. reported a case of an infant with congenital CMV infection and renal vein thrombosis[37]. By then, many cases of CMV related thrombosis has been reported in immunocompromised patients and in immunocompetent individuals. Reports have described CMV-associated thrombosis in many different anatomical sites, such as the lower limbs as DVT’s, splanchnic vein thrombosis (SpVT), portal vein thrombosis (PVT), mesenteric vein thrombosis (MVT), splenic vein thrombosis (SVT), pulmonary embolism (PE), and the Budd-Chiari syndrome (BCS) [11]. The first documented case of thrombosis in the course of acute CMV infection in an immunocompetent patient was documented by Inacia et al. at 1997, whom reported a case of a heavy smoker 31-year-old woman that was using oral contraceptive pills and developed acute portal vein thrombosis during the course of an acute CMV infection[5]. The authors suggested a relationship between endothelial cell-damaging effects of the virus and thrombosis. At 2001, Otofokun et al. reported a previously healthy adult with acute CMV infection that was complicated by extensive mesenteric arterial and venous thrombosis. This was the first reported case of VTE in an immunocompetent individual that had no predisposing risk factors for thrombosis[6].
To determine the incidence of thrombosis in acute CMV infection, the first cohort study was performed by Atzmony et al. at 2010 whom retrospectively analyzed the incidence of venous as well as arterial thromboses among 140 patients with acute CMV infection and 140 matched controls. They found the incidence of thrombosis as 6.4% in case and %0 in control group[16]. Later at 2012, Schimanski et al. reported a prospective study among 166 hospitalized venous thrombosis patients and stated the incidence of acute CMV infection as: 4.3% of all venous thrombosis and 7.4% of unprovoked venous thrombosis patients[22]. In a case control study by Ticlear et al., among 258 DVT and 139 control patients, authors reported five cases of acute CMV infection and viremia in case group: all were females age below 37 and they stated that as 31 of 258 patients with VT (12%) were younger than 37 years, 16% of all VT patients younger than 37 years had an active cytomegalovirus infection[19]. A retrospective study by Yildiz et al. at 2016 also suggested that among VTE patients, VTE’s with acute CMV infection are comparatively younger (37.5 years’ vs 56.6 years, P = 0.0088) with female predominance (90% vs 56%; p = 0.026) a similar finding with the analysis of our literature review[10].
To explain the pathophysiology of thrombophilia in CMV infection, several theories were suggested such as; the virus infects endothelial cells and enhances the expression of adhesion molecules that triggering platelet adhesion and aggregation on vessel walls, activation of factor X by the virus that leads thrombin formation, the capacity of the virus to increase circulatory levels of Von-Willebrand factor and factor VIII[38,39]. But up to date, the most accepted theory is that, acute CMV infection is associated with transient appearance of anti-phospholipid antibodies and causes a hypercoagulable status. This theory has been demonstrated in vitro and in vivo in several studies[40,41].
In, Mandell, Dolin and Bennett’s Principles & Practice of Infectious Diseases (2015) and in the Red Book (The Authority on Pediatric Infectious Diseases from the American Academy of Pediatrics - 2018) thrombosis is not mentioned as a complication of CMV disease, neither in immunocompetent nor in immunodeficient patients[42,43]. Actually, it seems thrombophilia associated with acute CMV infection is not as rare as thought and the role of CMV in vasculopathy and venous thrombosis has been underestimated. In our case, we believe that severe splanchnic VTE in an immunocompetent, 21- year old, non-smoker male without any chronic disease or drug usage, with no documented hereditary thrombophilic condition and serologically tested negative for CMV IgG-IgM (as he was a kidney donor) in recently, is a strong evidence for association of acute CMV infection with the patient’s condition.
By this case report and literature review, principally, we hope to increase the awareness of association with acute CMV infection and thrombosis/thromboembolic events especially in patients with idiopathic thromboses. Secondly, as the results of the studies clearly indicated that patients who suffered from a first unprovoked VTE have an 8-10% annual risk of recurrence, long term and as in some VTE guidelines (such as American Thoracic Society 2016) lifelong anticoagulant treatment is advised at the expense of bleeding risk, costs and inconvenience to the patients[4,44]. In case of VTE’S with transient or reversible causes such as CMV related VTE, identification of such a subset of patients may prevent lifelong anticoagulation and potentially harmful complications.
REFERENCES
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64.
- Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21: 23–9.
- Cosmi B. Management of idiopathic venous thromboembolism. Expert Rev Cardiovasc Ther. 2016;14(12):1371-84.
- Justo D, Finn T, Atzmony L, et al. Thrombosis associated with acute cytomegalovirus infection: a meta-analysis. Eur J Intern Med 2011;22: 195–9.
- Inacio C, Hillaire S, Valla D, Denninger MH, Casadevall N, Erlinger S. Case report: cytomegalovirus infection as a cause of acute portal vein thrombosis. J Gastroenterol Hepatol. 1997;12:287-8.
- Ofotokun I, Carlson C, Gitlin SD, et al. Acute cytomegalovirus infection complicated by vascular thrombosis: a case report. Clin Infect Dis 2001;32:983–6.
- Abgueguen P, Delbos V, Chennebault JM, et al. Vascular thrombosis and acute cytomegalovirus infection in immunocompetent patients: report of 2 cases and literature review. Clin Infect Dis 2003;36:E134–9.
- Youd P, Main J, Jackson E. Cytomegalovirus infection and thrombosis: a causative association? J Infect 2003;46:141–3.
- Rovery C, Granel B, Parola P, et al. Acute cytomegalovirus infection complicated by venous thrombosis: a case report. Ann Clin Microbiol Antimicrob 2005;4:11.
- Venous thromboembolism associated with acute cytomegalovirus infection: epidemiology and predisposing conditions. Yildiz H, Zech F, Hainaut P. Acta Clinica Belgica. 2016;71:231–234.
- Spahr L, Cerny A Morard I, Rubbia-Brandt L, Schrenzel J. Acute partial Budd-Chiari syndrome and portal vein thrombosis in cytomegalovirus primary infection: a case report. BMC Gastroenterol. 2006: 10;6:10.
- Paran Y, Halutz O, Swartzon M, et al. Venous thromboembolism and cytomegalovirus infection in immunocompetent adults. Isr Med Assoc J 2007;9:757–8.
- Squizzato A, Ageno W, Cattaneo A, et al. A case report and literature review of portal vein thrombosis associated with Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 2007;44:13–6.
- Ergas D, Herskovitz P, Skurnik Y, et al. Superior mesenteric vein thrombosis with pulmonary embolism: a rare presentation of acute cytomegalovirus infection. Isr Med Assoc J 2008;10:235–6.
- Ladd AM, Goyal R, Rosainz , et al. Pulmonary embolism and portal vein thrombosis in an immunocompetent adolescent with acute Cytomegalovirus hepatitis. J Thromb Thrombolys 2009;28:496–9.
- Atzmony L Halutz O, Avidor B, Finn T, Zimmerman O, Steinvil A, Zeltser D, Giladi M, Justo D. Incidence of cytomegalovirus-associated thrombosis and its risk factors: a case-control study. Thromb Res. 2010;126:439-43.
- Abgueguen P, Delbos V, Ducancelle A, Jomaa S, Fanello S, Pichard E. Venous thrombosis in immunocompetent patients with acute cytomegalovirus infection: a complication that may be underestimated. Clin Microbiol Infect. 2010;16:851-4.
- Poon ML, Tang JW, Chee YL. Case report: cytomegalovirus-induced thrombosis in an immunocompetent patient. J Med Virol 2012;84:116–8.
- Tichleaar VY, Sprenger HG, Makelburg AB, Niesters BG, Kluin-Nelemans HC, Lijfering WM. Active Cytomegalovirus infection in patients with acute venous thrombosis: a case-control study. Am J Hematol 2011;86:510-2.
- Kalaitzis J, Basioukas P, Karzi E, et al. Small-bowel necrosis complicating a cytomegalovirus-induced superior mesenteric vein thrombosis in an immunocompetent patient: a case report. J Med Case Rep 2012;6:118.
- Sherman S, Justo D, Engel T, et al. Cytomegalovirus-associated cerebral sinus vein thrombosis. J Med Virol 2012;84:1934–6.
- Schimanski S, Linnemann B, Luxembourg B, Seifried E, Jilg W, Lindhoff-Last E, Schambeck CM. Ann Hematol. Cytomegalovirus infection is associated with venous thromboembolism of immunocompetent adults--a case-control study. 2012;91:597-604.
- Pichenot M, Morell-Dubois S, Flateau C, et al. Acute Cytomegalovirus infection as a transient risk factor for thrombosis: report of three cases and focus on specific coagulation pathways. Thromb Res 2013;132: 145–7.
- Galloula A, Rossi A, Gautier V, et al. Portal vein thrombosis associated with an acute cytomegalovirus infection. J Mal Vasc 2014;39:224–30.
- Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol 2014;2014:271548.
- Rinaldi F, Lissandrin R, Mojoli F, et al. Acute cytomegalovirus infection as a cause of venous thromboembolism. Mediterr J Hematol Infect Dis 2014;6;2014041.
- Vandamme YM, Ducancelle A, Biere L, et al. Myopericarditis complicated by pulmonary embolism in an immunocompetent patient with acute cytomegalovirus infection: a case report. BMC Res Notes 2014;7:193.
- Bertoni M, et al. Asymptomatic superior mesenteric vein thrombosis as unusual complication of acute cytomegalovirus infection: a case report. Italian J Med 2015;10:147–50.
- Wang T, Kuttikat A, Pulsalkar P, et al. Cytomegalovirus-associated portal vein thrombosis in an immunocompetent patient: an underestimated complication. Oxf Med Case Rep 2015;294–6.
- Chou JW, Cheng KS. Pulmonary embolism in an immunocompetent patient with acute cytomegalovirus colitis. Intest Res 2016;14:187–90.
- Bountouris I, Moris D, Tsilimigras DI, et al. Karaolanis G pulmonary embolism in a young immunocompetent adult infected with cytomegalovirus. Are novel oral anticoagulants an efficient alternative? In Vivo 2017;31:1193–5.
- Vael A, Degryse H, Bracke P. Acute cytomegalovirus infection as a rare cause of portal vein thrombosis with small bowel infarction in an immunocompetent patient. J Belgian Soc Radiol 2017;101:16.
- Kelkar AH, Jacob KS, Yousif EB, Farrell JJ Medicine (Baltimore). Venous thromboembolism related to cytomegalovirus infection: A case report and literature review 2017;96:9336
- Puccia F, Lombardo V, Giannitrapani L, Licata A, Mazzola G, Soresi M. Case report: acute portal vein thrombosis associated with acute cytomegalovirus infection in an immunocompetent adult. J Ultrasound. 2017;20:161-165.
- Salembier A, Verhamme M, Verhamme P, Van Moerkercke W. Acute non-cirrhotic portal vein thrombosis : review. Acta Gastroenterol Belg. 2018;81:318-322.
- Ngu S, Narula N, Jilani TN, Bershadskiy A. Venous Thrombosis Secondary to Acute Cytomegalovirus Infection in an Immunocompetent Host: Consideration for New Screening Guidelines. Cureus. 2018;5;10:2742.
Citation: Olut AI(2021) Portal and severe mesenteric vein thrombosis associated with acute cytomegalovirus infection: Case report. J Infect Dis Diagn.6:156
Copyright: © 2021 Olut AI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

