Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 4
Phytochemical Screening of Bark of Ficus Elastica and Their Antioxidant Activity
Zubia Gulzar1*, Zaheen Tara1 and Fozia Bibi22Department of Political Science, Rawalpindi Women University, Lahore, Pakistan
Received: 17-Feb-2023, Manuscript No. JPPM-23-21551; Editor assigned: 22-Feb-2023, Pre QC No. JPPM-23-21551(PQ); Reviewed: 08-Mar-2023, QC No. JPPM-23-21551; Revised: 18-May-2023, Manuscript No. JPPM-23-21551(R); Published: 16-Jun-2023, DOI: DOI:10.35248/2157-7471.23.14.685
Abstract
Bark of Ficus elastica was dried in the shade for twenty days and ground into powder. After sieving, the powder was stored in a desiccator and soaked it in methanol for 72 hours at ambient conditions. After filtration, methanol was eliminated by distillation at reduced pressure, yielding a dark brown-colored extract with 9.8% dissolved solids. Its pH was 5.3. Preliminary phytochemical screening of Ficus elastica bark extract revealed the presence of secondary metabolites flavonoids, saponins, tannins and terpenoids. The primary metabolites identified were proteins (0.825%), total sugars 28.57%, of which reducing sugars (20%), non-reducing sugars (8.57%). The antioxidant activity of Ficus elastica extract against DPPH and ascorbic acid was evaluated using 5, 10, 15, 20 and 25 μL extract. The maximum antioxidant activity of the bark extract was 87.118% while the antioxidant activity of ascorbic acid was 100%.
Keywords
Ficus elastic; Metabolites; Saponins; Tannins; Proteins; Antioxidant; DPPH
Introduction
Ficus elastica belongs to the Moraceae family. As a result of the plant's ability to survive extreme environmental conditions such as high water levels and high temperatures, it thrives under extreme conditions. In tropical and subtropical forests worldwide, Ficus consists of about 800 species and 2000 varieties of woody trees, shrubs and vines. Ficus elastica grows in the canopy of tropical rainforests, woodlands, shrublands and light tropical forests In addition, they can be grown indoors or in greenhouses elsewhere. Moreover, it is grown in Nicaragua in gardens and sporadically in parks. It grows in the Himalayan foothills, lower forests and coasts of Ecuador, New South Wales, and Australia as well as in urban areas as a street tree throughout the Mediterranean and roadside in the Dominican Republic. The species was also discovered in Kawainui Marsh, Hawaii, near a former nursery (Smithsonian Herbarium Collection). South Asia and India are the native habitats of Ficus elastica. A global compendium of weeds lists the flora of Pakistan and West Punjab as environmental weeds, garden weeds and naturalized weeds. Trees of this species produce dense shade that restricts growth underneath, as well as being tolerant of shade, drought, and a variety of soil types. It is extremely difficult to control since seeds can germinate in trees and grow downward, making it extremely difficult to control. Seeds require a specific pollinator, which may have prevented this species from spreading and establishing itself in newly introduced areas [1].
The phytochemical study was conducted on Ficus elastica because there are no previous studies in Pakistan on this topic. A metabolite is an essential component of a cell, which is isolated to be used in organic synthesis. Microorganisms require primary metabolites in order to grow properly. Secondary metabolites are formed near the end of the stationary phase of growth and do not contribute to growth, development, or reproduction. These metabolites can be used in industrial microbiology to obtain amino acids; to develop vaccines and antibiotics. Saponins are a class of chemical compounds, one of many secondary metabolites. It is notable for its ability to foam when dissolved in water. Saponins are a group of chemicals with detergent-like properties that plants produce to help them resist microbial pathogens such as fungi and certain bacteria and viruses [2].
Materials and Methods
Saponin is found in liquid soaps, jewelry polishes, laundry detergents, dermatitis cures, insecticides, pet shampoos, human shampoos, household cleaners, surfactants, wetting agents, nutrient adsorption, sprinklers and stickers, antimicrobial, antimalaria, lowering cholesterol, lowering blood pressure, killing nematodes, bone health, fighting cancer, supporting the immune system, removing parasites (ticks, fleas), cleaning cars. In the dietary supplement and neutraceutical industries, saponins are heavily marketed. As hemolytics, expectorants, antiinflammatory agents, and immune stimulators, saponins have a wide range of properties. Research has shown that lowering blood cholesterol levels, maintaining bone health, preventing cancer and boosting immunity are all possible benefits [3].
Polyphenols in plants called tannins bind to proteins and precipitate them or shrink them. They inhibit the growth rate of digestive enzymes, which has a major effect on animal nutrition. Topical applications of tannins help remove any irritants from the skin. Their anti-inflammatory properties can be used to treat burns and other illnesses [4].
Plant pigments with a wide range of uses include flavonoids. A class of plant metabolites known as flavonoids is considered to have positive impacts on health via activating antioxidant and cell signaling pathways. It has actions against viruses, cancer, inflammation, and allergies. The terpinoids, also known as isoprenoids, are a broad and diversified class of organic compounds that are produced naturally. Terpenoids are essential for most organisms' survival because they exert metabolic regulation and mediate interactions between different species, such as plant pollination and defence. Antioxidants have an important role in lowering oxidative stress, which can affect and destroy biological molecules. Oxidative stress is defined as a disproportion between oxidants and antioxidants that favours oxidants, potentially causing damage [5].
Plant material
Plants are powerful biochemists and have been used in phytomedicine since time immemorial; man can extract a remarkable variety of industrial chemicals from them. Natural ingredients derived from plants can be dried from any part of the plant, including the bark, leaves, flowers, roots, fruits, seeds and so on. In many laboratories, comprehensive screening of plant species with the goal of discovering new bioactive components is a routine operation. A logical path is followed by scientific analysis of plant components. Plants are collected at random or by following leads provided by local healers in geographic locations where the plants can be found. The extraction of secondary plant components can be conducted using either fresh or dried plant material. Ficus elastica bark was collected from PCSIR's garden [6].
Preparation of extract
The stem bark of Ficus elastica were air dried and cut to increase the surface area. The bark finely grounded was percolated with methanol at room temperature for three days, than filtered.
Solvent is evaporated by steam distillation and extract was preserved for analysis.
Chemicals
Methanol, DMC (Dimethyl Carbonate), ferric chloride, potassium hydroxide, hydrochloric acid, chloroform, potassium sulfate, copper sulfate, selenium dioxide, ferrous sulfate and hydrochloric acid, all the chemicals used were standard and of analytical grade.
Phytochemical screening
Extract was analyzed chemically to investigate classes of compounds in the stem of Ficus elastica using standard procedure to identify the constituents.
Detection of tannins
2 mL of distilled water was added to 1 mL of extract in a test tube, which was then heated over a water bath. Then 2 drops of ferric chloride were added. Changing the color of the solution to a dark green color indicated the presence of tannins [7].
Detection of saponins
1 ml test sample was taken up with 5 ml distilled water and then shaken well. This solution was heated to boiling. The velvety foam indicates the saponins.
Detection of flavonoids
1 ml of extract was taken and added 0.1 M KOH then added few drops of dilute HCl. The change in color from dark brown to yellow indicated flavonoids.
Detection of terpenoids
1 ml extract was placed in a test tube. 2 ml of chloroform was added to the test sample. To this mixture was then added 3 drops of concentrated H2SO4, forming a layer. The appearance of red color in the solution indicates the presence of terpenoids.
Results and Discussion
Quantitative estimation of proteins
Proteins in the extract were determined by the KJELDAHL Method. 1ml extract of bark was poured in a digestion flask. Digestion mixture (K2SO4 90 g, CuSO4 5 g, SeO2 0.2 g, FeSO4 10 g) was added with 20 ml H2SO4 and proceed for digestion. Place the digestion flask on the heat source for boiling. Continued boiling till clear liquid (decolonize mixture from brick red to clear). Cool it and transferred the digested sample to the volumetric flask and marked the volume with distilled water. Took 2% boric acid solution in100 ml of beaker and connected it with tip of the condenser of distillation unit. Pipette 5 ml digested sample in distillation flask. Added 15 ml of sodium hydroxide and started distillation. Continued distillation for 3 more minute until colour of boric acid solution changed. Titrate the collected distillate with standard HCL 70/N solution. Take two concise readings to determine the volume [8].
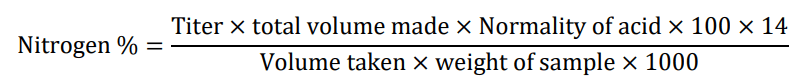
Protein %=%Nitrogen × Protein factor
Proteins=0.825%
Hydrolyze: Took 5 ml sample in 250 ml flask then added 5 ml distilled water to dilute and 1 ml 6 N HCl, after that the mixture was heated for 1 minute. Allowed to cool at room temperature
Neutralize: Put the mixture in a beaker added few drops of 40% NaOH to neutralize the mixture up to pH 7.
Dilution: In a 50 ml volumetric flask took the sample and added distilled water up to graduation mark.
Benedict’s test: Took 5 ml Benedict’s reagent in a beaker added distilled water in it. Heat the mixture till it starts boiling.
Titration: The sample extract was titrated with benedict’s reagent, at a specific volume blue color of the sample mixture turned greenish brown which indicates the presence of sugars [9].
Reducing sugars
Take 10 ml of sample extract and prepared dilution of 50 ml in a volumetric flask. In a beaker took 5 ml of benedicts solution diluted it up to 40 ml and started heating till boiling. Than added the sample extract drop by drop into the benedict’s solution and noted the change in colour occurred at a specific volume of the sample extract. Change in color from blue to brick red indicates the presence of sugars (Table 1) [10].
| Secondary metabolites | |||
|---|---|---|---|
| S.No | Groups | + Present -Not Identified |
Solvent |
| 1 | Flavonoids | + | Methanol |
| 2 | Terpinoids | + | MeOH |
| 3 | Tannins | + | MeOH |
| 4 | Saponins | + | MeOH |
Primary metabolites
Table 1: Phytochemical analysis of bark extract.
Antioxidant activity
DPPH radical scavenging activity of extract was determined by using DPPH method described by with some modification. Extract was taken 5, 10, 15, 20, 25 μl/ml. One milliliter of DPPH solution (30 ml in methanol) then was mixed with each concentration. The mixed solution was incubated in dark at 20°C for 40 minutes. Absorbance was measured at 517 nm using UV spectrophotometer with methanol as blank. Experiment was performed in triplicates at each concentration. The percentage scavenging of DPPH by the extract was calculated by the following formula (Figure 1) [11].
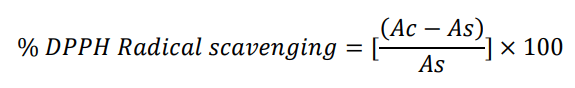
Here: Ac= absorbance of blank as=absorbance of sample
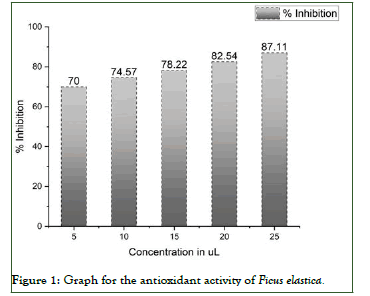
Figure 1: Graph for the antioxidant activity of Ficus elastica.
In the study of Ficus elastica we have investigated metabolites like saponins, tannins, flavonoids terpenoids, sugars, proteins. Antioxidant study suggested that these metabolites are favorable for antioxidant activity. The primary metabolites identified were proteins (0.825%), total sugars 28.57%, of which reducing sugars (20%), non-reducing sugars (8.57%). The antioxidant activity of Ficus elastica extract against DPPH and ascorbic acid was evaluated using 5, 10, 15, 20, and 25 μL extract. The maximum antioxidant activity of the bark extract was 87.118%while the antioxidant activity of ascorbic acid was 100% (Table 2) [12].
| S/N | Concentration in µl | Absorbance | % Inhibition |
|---|---|---|---|
| 1 | 5 | 0.354 | 70 |
| 2 | 10 | 0.3 | 74.57 |
| 3 | 15 | 0.257 | 78.22 |
| 4 | 20 | 0.206 | 82.54 |
| 5 | 25 | 0.152 | 87.11 |
Table 2: Antioxidant activity using DPPH assay.
Conclussion
This study has explored the various phytochemicals, including glycosides, phenols and flavonoids, present in the bark of F. auriculata for the first time. The Antioxidant efficacy of methanol and chloroform extract, being equivalent to standard ascorbic acid at concentration of 0.1 mg/ml, indicates that this plant can has great scope for isolation and identification of important antioxidant molecules which can be formulated to make antioxidant dosage forms. On top of that, these natural antioxidants can have potential advantages among various diseases with oxidative stress. This plant also showed promising antibacterial activity even though it was comparatively weaker than standard antibiotics. If we can purify and isolate the antibacterial Biomolecules then definitely they would give equivalent antibacterial activity to that of standard antibiotics. So, further study is necessary to get maximum benefit from this plant. It can play a pivotal role to change the traditional system of medicine into a scientific and standard medication system. So this study confirming the presence of antioxidant and antibacterial activity of this plant has indicated the further necessary study on this plant for isolation and identification phytochemicals of such property.
References
- Iqbal E, Salim KA, Lim LB. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Uni Sci. 2015;27(3):224-232.
- Benedict SR. A reagent for the detection of reducing sugars. J Bio Chem. 1909;5(5):485-487.
- Robert D. Simoni, Robert L. Hill, Martha Vaughan. Benedict's solution, a reagent for measuring reducing sugars: The clinical chemistry of Stanley R. Bened J Biol Chem. 2006;277(16):10-11.
- Ayoola GA, Coker HB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and antioxidant activity of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res. 2008;7(3):1019-1024.
- Banso A, Adeyemo S. Phytochemical screening and anti-mmalarial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemitri. 2016;18:39-44.
[Crossref]
- Sofowora A. Medicinal Plants and traditional Medicines in Africa. Jhon Wiley and Sons Limit, Hoboken, 1982, pp. 64-79.
- Farhat MB, Landoulsi A, Chaouch-Hamada R, Sottomayor JA, Maria JJ. Characterization and qualification of phenolic compounds and antioxidant properties of different salvia species growing in different habitats. Indus Crops Prod. 2006;49:904-914.
- Das K, Tiwari RKS, shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current meths and future trends. J Med Plant Res. 2010;4(2):104-111.
- Buzzini P, Arapitsas P, Goretti M, Branda E, Turchetti B, Pinelli P, et al. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev Med Chem. 2008;8(12):1179-1187.
[Crossref] [Google Scholar] [PubMed]
- Bennick A. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. 2009;13(2):184-196.
[Crossref] [Google Scholar] [PubMed]
- Lockyer B, Islam S, Rahman A, Dickerson J, Pickett K, Sheldon T, et al. Understanding misinformation and vaccine hesitancy in context: Findings from a qualitative study involving citizens in Bradford, UK. Health Expect. 2021;24(4):1158-1167.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Hameed ESS. Total phenlicocntent and free radical scavanging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271-1277.
Citation: Gulzar Z, Zaheen Tara Z, Bibi F (2023) Phytochemical Screening of Bark of Ficus elastica and Their Antioxidant Activity. J Plant Pathol Microbiol. 14:673.
Copyright: © 2023 Gulzar Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

