Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Pharmacokinetics and Pharmacodynamics of Unfractionated Heparin and Ferric Pyrophosphate Citrate Co-administration during Hemodialysis: No DrugDrug Interaction
Raymond D Pratt*Received: 30-Aug-2021 Published: 20-Sep-2021, DOI: 10.35248/0975-0851.21.s4.004
Abstract
Background: Ferric Pyrophosphate Citrate (FPC) for intravenous administration is approved to maintain hemoglobin in patients receiving Chronic Hemodialysis (HD). The aim of this study is to investigate the coadministration of intravenous (IV) FPC with Unfractionated Heparin (UFH) as an admixture via the HD-machine syringe pump.
Methods: Open-label, randomized, 3-period crossover study. Three (3) treatments in randomized sequence: Treatment A: FPC 6.75 mg IV via the post dialyzer blood line and continuous infusion of UFH pre-dialyzer via the HD-machine infusion pump; Treatment B: FPC 6.75 mg IV mixed with UFH via the pre dialyzer heparin line; Treatment C; IV UFH via the on machine syringe pump x 3 h. anti-Xa activity, activated prothrombin time (aPTT), Thrombin time (TT) and serum iron parameters were measured. Pharmacokinetics and dynamics were determined using non-compartmental methods and comparisons of Cmax and AUC were calculated using a standard bioequivalence approach.
Results: Mean anti-Xa, aPTT, and TT concentrations were comparable across all timepoints at baseline, and throughout the study. The concentration-time profiles for iron and TSAT were the same between the FPC/UFH admixture and FPC/UFH administered by separate routes. Results on the visual clotting scale were similar across all treatments. FPC and UFH were well tolerated with no reported adverse events.
Conclusion: No clinically relevant drug-drug interaction between FPC and UFH on the anticoagulation effects of UFH (as assessed by anti-Xa activity, aPTT, and TT) or on the ability of FPC to deliver iron when these agents are coadministered as a single admixture. No new safety concerns were identified.
Keywords
Anticoagulation; Anti-Xa; Unfractionated Heparin; Pharmacokinetics; Iron; Hemodialysis; Bioequivalence
Introduction
Ferric pyrophosphate citrate injection (Triferic AVNU, FPC), an iron-replacement product, is an iron complex in which iron (III) is bound to pyrophosphate and citrate [1]. Unlike traditional intravenous (IV) iron, FPC does not require processing by macrophages; it donates iron directly to transferrin for optimal utilization in erythropoiesis, avoiding sequestration within reticuloendothelial system macrophages [2].
FPC at 2 μM iron (110 μg Fe/L) final concentration in hemodialysate provides 6.75 mg iron per dialysis and is the approved dialysate concentration in the United States. In addition to administration in the dialysate, FPC at a dose of 6.75 mg can be administered as a slow continuous IV infusion over 3 to 4 hours via the pre dialyzer infusion line, the postdialyzer infusion line, or a separate connection to the venous blood line during hemodialysis.
Hemodialysis requires some form of anticoagulation to prevent clotting in the dialyzer circuit. Unfractionated Heparin (UFH) has been the long-time standard to provide anticoagulation [3]. Unfractionated heparin is typically administered as a bolus dose of 25 to 50 IU/kg at the initiation of hemodialysis and then by a continuous infusion of 600 to 3000 IU per hour via the onmachine syringe (UFH) pump [4].
Alternately, UFH or Low Molecular Weight Heparin (LMWH) can be administered as a pre dialysis single IV bolus, which can be followed by an additional bolus if needed. This mode of administration does not require use of the on-machine syringe pump. Anticoagulation within the dialysis circuit is monitored by visual observation of the dialyzer and drip chambers.
The dose of UFH is individually determined and may be variable depending on underlying comorbidities [5-7].This study was conducted to investigate the ability of an admixture of FPC plus UFH to maintain adequate anticoagulation of the dialyzer circuit when mixed with FCP, and to assess the impact of coadministration of UFH on iron delivery of FPC in patients with Hemodialysis Dependent Chronic Kidney Disease (HDD-CKD).
Methods
The study objectives were to investigate if there is a drug-drug interaction between FPC and UFH to anti-coagulate the dialyzer blood circuit for plasma by measuring anti-factor Xa (anti-Xa) activity and to assess the overall safety profile of the FPC plus UFH admixture.
The study was a prospective, single-center, open-label, 3 period, crossover trial investigating three treatments: Treatment A, Administration of IV FPC and UFH separately in the postdialyzer and pre-dialyzer blood line; Treatment B, the coadministration of IV of FPC and UFH in the same syringe predialyzer and Treatment C, administration of IV UFH predialyzer alone.
The study was listed on ClinicalTrials.Gov as NCT0404224. The protocol and informed consent was reviewed by an external IRB (IntegReview, Austin Tx). Twelve subjects with HDD-CKD received each of three treatments in a randomized sequence, at 3 successive hemodialysis sessions (Days 1, 3 and 5).
A follow-up visit was performed (either in person or by telephone) within 1 week after the last hemodialysis session to assess for any adverse events that occurred or any concomitant medications that were administered after the last hemodialysis treatment. The study design is presented in Figure 1.
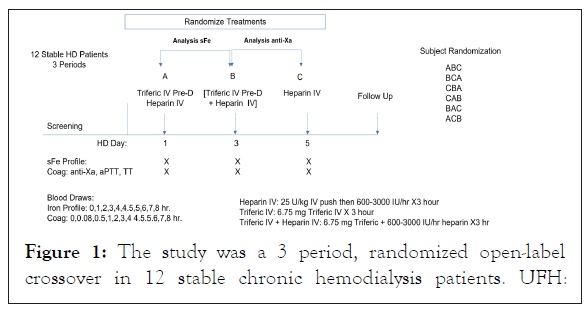
Figure 1: The study was a 3 period, randomized open-label crossover in 12 stable chronic hemodialysis patients. UFH:
Unfractionated Heparin; FPC: Ferric Pyrophosphate Citrate; IV: Intravenous; Pre-D: Pre-dialyzer blood line; Post-D: Post-dialyzer bloodline.
The open-label, randomized, crossover design was an appropriate study design for an assessment of potential drugdrug interactions between FPC and UFH because the primary objective (pharmacokinetics/pharmacodynamics) would not be affected by the investigator’s or subject’s knowledge of the treatment.
Randomization was used to avoid bias in the assignment of subjects to a treatment sequence and to increase the likelihood that known and unknown subject attributes (e.g., demographics, other baseline characteristics) would be evenly balanced across treatment sequences.
The 1 day interval between hemodialysis sessions was sufficient to prevent carryover effects of UFH and FPC given their short half-lives.
The dose of FPC was fixed at 6.75 mg (Treatment A and Treatment B). The 6.75 mg dose also represents the approved dose of FPC when administered as a slow continuous IV infusion over 3 to 4 hour [8]. No adjustments in the FPC dose were allowed.
The dose of UFH (25-50 IU/kg IV push, then 600 3000 IU/ hour) was fixed at the dose that each subject usually received to maintain circuit anticoagulation (Treatments A, B, and C). No patient required an adjustment in the UFH dose. The administration of each treatment is summarized in Table 1.
| Study treatment | Administration instructions |
|---|---|
| Treatment A (reference) FPC post-dialyzer UFH pre-dialyzer |
FPC for IV administration was drawn up into a syringe by connecting the syringe tip to the ampule luer connector and withdrawing the contents of the ampule. The syringe containing FPC was mounted on an infusion pump and programmed to deliver the 4.5mL contents over 3hours into the post-dialyzer blood line. The UFH was infused via the HD‑machine syringe pump into the pre‑dialyzer blood line during the first 3hours of hemodialysis. |
| Treatment B (test) FPC + UFH pre-dialyzer |
The UFH for continuous infusion was drawn up in a syringe. The needle was discarded, and the syringe tip was connected to the LDPE luer-lock FPC ampule. The entire contents (4.5 mL) were withdrawn. The syringe was inverted to mix the FPC and UFH and the volume noted. The syringe containing FPC + UFH was placed on the HD-machine syringe pump, which was programmed to deliver the entire volume over 3hours into the pre‑dialyzer blood line. |
| Treatment C (reference) UFH pre-dialyzer |
The UFH dose that was to be administered by continuous infusion was drawn up in a syringe and infused via the HD-machine syringe pump into the pre‑dialyzer blood line over the first 3hours of hemodialysis. |
Table 1: Study administration parameters.
Blood samples for coagulation studies (anti-Xa, aPTT, and TT) were obtained before the start of infusion (0 hour) and at 5 minutes (0.08 hours), 30 minutes (0.5 hours), and 1, 2, 3, 4, 5, 6, 7, and 8 hours after the start of infusion during each treatment period. Blood samples for a safety iron (sFe) profile, which included sFe, Total Iron-binding Capacity (TIBC), TSAT, ferritin, and transferrin, were obtained before the start of infusion (0 hour) and at 1, 2, 3, 4, 5, 6, 7, and 8 hours after the start of infusion during each treatment period. Blood samples were obtained from the arterial blood line port during hemodialysis and from a peripheral vein or via an indwelling needle after completion of hemodialysis. Samples were sent to the laboratory for analysis within 24 hours after collection.
Bioanalytical methods
Values for anti-Xa, aPTT, and TT were determined by the clinical laboratory using standard clinical assays. Values for sFe, TIBC, TSAT, ferritin, and transferrin were determined by the clinical laboratory using established methods. Pharmacokinetic and pharmacodynamic parameters for the coagulation and serum iron parameters were derived for each study treatment from the concentration time profiles, using noncompartmental methods in Phoenix WinNonlin®, version 8.1 (Certara USA, Inc, Princeton, New Jersey).All adverse events (nonserious and serious) that were observed by the investigators, reported in response to open-ended questioning, or spontaneously reported by the subjects from the time of screening (serious adverse events) or the first administration of the study treatment (nonserious adverse events) through the follow-up visit were recorded in the source documents, regardless of whether the events were considered related to the study drug or not.
Clotting in the dialyzer was evaluated at 1 hour intervals during each hemodialysis treatment using a Visual Clotting Scale (VCS). Clotting was classified according to the following scale: Grade 1 = no detectable clotting, Grade 2=minimal clot formation (fibrinous ring), Grade 3 = clot formation (up to 5 cm) but dialysis still possible, and Grade 4=complete occlusion of air traps or dialyzer rendering dialysis impossible. The determination of concentrations of total sFe, TIBC, TSAT, and transferrin in human plasma is consistent with the recommendations for measurement of iron analytics in the US FDA draft guidance for industry on bioequivalence [9]. The coagulation panel (anti-Xa, aPTT, and TT) represents tests that are routinely used to assess anticoagulation status in patients who receive UFH. Comparisons of Cmax and AUC to establish equivalence were performed according to US FDA Bioequivalence recommendations [10].
Results
The study population included 9 men (75.0%) and 3 women (25.0%) of mean age 57.0 years (range: 33-68 years). Eleven of the 12 subjects were black/African American (85.2%); none was of Hispanic or Latino ethnicity. Etiology of HDD-CKD was hypertension in all 12 subjects, with 4 subjects also having a diagnosis of type 2 diabetes. Seven of the 12 subjects had previously received treatment with IV iron. The demographics of the study participants are presented in Table 2.
| Characteristic | All subjects (N=12) |
|---|---|
| Sex, n (%) | |
| Male/Female | 9 (75.0)/3 (25.0) |
| Age, years | |
| Mean (SD) | 57.0 (9.8) |
| Median (Minimum, Maximum) | 61.0 (33, 68) |
| Race, n (%) | |
| Black/African American/Other | 11 (91.7)/1 (8.3) |
| Weight, kg | |
| Mean (SD) | 87.7 (15.5) |
| Median (Minimum, Maximum) | 88.9 (62.5, 113.5) |
| Etiology of CKD, n (%) | |
| Hypertension | 12 (100.0) |
| Type 2 diabetes | 4 (33.3) |
| C-reactive protein at screening, mg/L (reference range: <0.9 mg/L) | |
| Mean (SD) | 12.7 (15.3) |
| Ferritin at screening, ng/mL (reference ranges: males, 22-322 ng/mL; females, 10-291 ng/mL) | |
| Mean (SD) | 911.8 (359.2) |
| Median (Minimum, Maximum) | 957.3 (335, 1506) |
| Abbreviations: CKD: Chronic Kidney Disease | |
Table 2 : Demographic and disease-related characteristics at baseline.
Mean anti-Xa, aPTT, and TT concentrations were comparable across the 3 study treatments at baseline, throughout the 3-hour IV infusion, and during the subsequent post-infusion period (Figure 2).
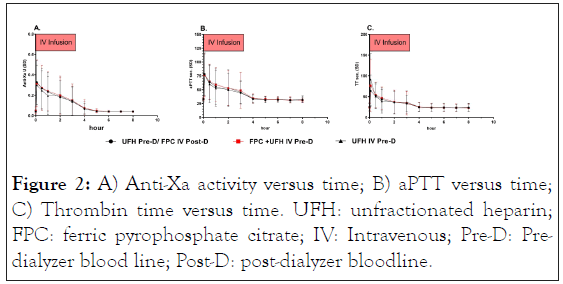
Figure 2: A) Anti-Xa activity versus time; B) aPTT versus time; C) Thrombin time versus time. UFH: unfractionated heparin; FPC: ferric pyrophosphate citrate; IV: Intravenous; Pre-D: Predialyzer blood line; Post-D: post-dialyzer bloodline.
Mean anti-Xa, aPTT, and TT values were maximal after the UFH loading dose, with values for all 3 parameters peaking at 0.08 hours (5 minutes, the first sampling time) after the bolus and start of the UFH infusion for all 3 study treatments. Mean values decreased to baseline levels by 4 hours (aPTT and TT) to 5 hours (anti-Xa) after the start of the UFH infusion.
Mean sFe and TSAT concentrations were comparable amongst the 3 study treatments the start of dialysis. Mean concentrations for iron parameter remained unchanged during infusion of the UFH alone (Treatment C). Mean sFe and TSAT concentrations showed the expected increases in sFe and TSAT during IV infusion of the FPC/UFH regimens (Treatments A and B), with nearly superimposable curves (Figure 3). Peak sFe and TSAT values were reached at the end of the 3-hour IV infusion for both Treatment A (reference) and Treatment B (test), and concentrations remained above baseline values at the end of the 8 hour sampling period for both treatments with the expected return to baseline values by 8 hours post dialysis. Mean concentrations of TIBC, ferritin, and transferrin were constant across all treatments (Figure S-1) indicating no need to correct for volume changes.
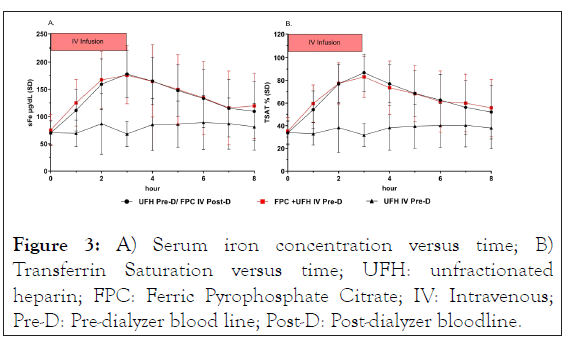
Figure 3: A) Serum iron concentration versus time; B) Transferrin Saturation versus time; UFH: unfractionated heparin; FPC: Ferric Pyrophosphate Citrate; IV: Intravenous; Pre-D: Pre-dialyzer blood line; Post-D: Post-dialyzer bloodline.
Exposure of sFe was comparable between the FPC/UFH regimens during hemodialysis (AUC0-4) and during the 8-hour sampling window (AUC0 t), regardless of whether FPC and UFH were co-administered by separate routes (Treatment A) or as a single admixture via the pre dialyzer line (Treatment B) (Table 3).
| Parameter | Statistic | Treatment A (Reference)a (N=12) |
Treatment B (Test) (N=12) |
Treatment C (Reference) (N=12) |
|---|---|---|---|---|
| Cmax (µg/dL) | Geometric mean (CV%) | 180 (20.9) | 174 (31.5) | 93.9 (55.0) |
| AUC0-t (h•µg/dL) | Geometric mean (CV%) | 1040 (31.1) | 1050 (43.3) | 604 (42.5) |
| AUC0-4 (h•µg/dL) | Geometric mean (CV%) | 548 (25.6) | 563 (34.4) | 287 (4.9) |
Table 3: Noncompartmental pharmacokinetic parameters for total serum iron.
Geometric mean Cmax values for anti-Xa were comparable (within 10% of each other) across the 3 study treatments (Table 4). Geometric mean AUC0-4 and AUC0-t values were comparable (within 5%) between the FPC/UFH treatments (Treatments A and B), but values were approximately 10% lower for UFH alone (Treatment C).
| Analyte | Parameter Geometric mean (CV%) |
Treatment A (Reference) (N=12) |
Treatment B (Test) (N=12) |
Treatment C (Reference) (N=12) |
|---|---|---|---|---|
| Anti-Xa | Cmax (sec) | 0.279 (62.7) | 0.256 (78.5) | 0.288 (59.0) |
| AUC0-t (h•sec) | 0.775 (58.3) | 0.798 (61.6) | 0.733 (62.4) | |
| AUC0-4 (h•sec) | 0.581 (75.7) | 0.600 (77.9) | 0.538 (81.0) | |
| aPTT | Cmax (sec) | 70.9 (46.70 | 70.5 (47.6) | 74.3 (45.5) |
| AUC0-t (h•sec) | 317 (27.5) | 328 (28.6) | 320 (29.1) | |
| AUC0-4 (h•sec) | 188 (37.7) | 196 38.5) | 188 (39.8) | |
| TT | Cmax (sec) | 46.6 (53.3) | 48.6 (94.1) | 71.3 (114) |
| AUC0-t (h•sec) | 160 (20.7) | 160 (22.4) | 167 (26.0) | |
| AUC0-4 (h•sec) | 97.6 (31.6) | 98.0 (34.6) | 104 (40.1) |
Table 4: Noncompartmental pharmacodynamic coagulation parameters (Anti-Xa, aPTT, and TT).
Geometric mean Cmax, AUC0-4, and AUC0-t values for aPTT were comparable (within approximately 5%) across the 3 treatments.
Geometric mean Cmax values for TT were comparable between the FPC/UFH treatments (Treatment A, 46.6 sec; Treatment B, 48.6 sec) and approximately 50% lower than for UFH alone (Treatment C, 71.3 sec).
Geometric mean AUC0-4 and AUC0 t values were comparable across the 3 study treatments.
Coadministration of the FPC/UFH admixture via the pre dialyzer line (Treatment B) was equivalent to separate administration as measured by Anti-Xa activity (Table 5).
| Parameter | Treatment | Geometric LSM |
Treatment Comparison | Ratio of Geometric LSM | 90% CI for Ratio |
|---|---|---|---|---|---|
| AUC0-4 | A (reference)a | 0.581 | B/C | 1.11 | (0.972, 1.28) |
| B (test)b | 0.600 | B/A | 1.03 | (0.900, 1.18) | |
| C (reference)c | 0.538 | ||||
| AUC0-t | A (reference)a | 0.775 | B/C | 1.09 | (0.977, 1.21) |
| B (test)b | 0.798 | B/A | 1.03 | (0.924, 1.15) | |
| C (reference)c | 0.733 | ||||
| Cmax | A (reference)a | 0.256 | B/C | 0.89 | (0.758, 1.04) |
| B (test)b | 0.279 | B/A | 0.918 | (0.782, 1.08) | |
| C (reference)c | 0.288 |
Table 5 : Bioequivalence anti-factor xa activity.
The upper and lower bounds of the 90% CI of the geometric mean ratio (Treatment B/Treatment C) for AUC0-t were within the 80% to 125% range for equivalence; the lower bound of the 90% CI of the geometric mean ratio was within and the upper bound of the 90% CI of the geometric ratio for AUC0 4 was slightly outside (128%) of the upper bound of the equivalence range.
The lower bound of the 90% CI for the geometric mean ratio for Cmax fell just outside the lower bound of the equivalence range.
The route of administration of FPC/UFH had no effect on the anticoagulation activity of UFH. The 90% CIs of the geometric mean ratio (Treatment B/Treatment A) for AUC0-4 and AUC0- t were within the 80% to 125% equivalence range (Table 5).
The lower bound of the 90% CI of the geometric mean ratio for Cmax was slightly outside the lower bound of the equivalence range.
Coadministration of FPC/UFH as a single admixture via the pre dialyzer line (Treatment B) had no effect on the aPTT activity of UFH (Table 6).
| Parameter | Treatment | Geometric LSM | Treatment Comparison | Ratio of Geometric LSM | 90% CI for Ratio |
|---|---|---|---|---|---|
| AUC0-4 | A (reference)a | 188 | B/A | 1.05 | (0.985, 1.11) |
| B (test)b | 196 | B/C | 1.04 | (0.983, 1.11) | |
| C (reference)c | 188 | ||||
| AUC0-t | A (reference)a | 317 | B/A | 1.03 | (0.992, 1.08) |
| B (test)b | 328 | B/C | 1.03 | (0.983, 1.07) | |
| C (reference)c | 320 | ||||
| Cmax | A (reference)a | 70.9 | B/A | 1.04 | (0.983, 1.11) |
| B (test)b | 70.5 | B/C | 0.948 | (0.863, 1.04) | |
| C (reference)c | 74.3 | ||||
Table 6: Bioequivalence activated partial thromboplastin time.
The upper and lower bounds of the 90% CIs of the geometric mean ratios (Treatment B/Treatment C) for AUC0-4, AUC0- t, and Cmax were within the equivalence range of 80% to 125%.
No effect of the route of coadministration of FPC/UFH (as a single admixture or via separate routes) was observed on aPTT. The upper and lower bounds of the 90% CIs of the geometric mean ratios (Treatment B/Treatment A) for all 3.
Co-administration of the FPC/UFH as a single admixture via the pre dialyzer line (Treatment B) had no impact on TT. The upper and lower bounds of the 90% CIs of the geometric mean ratios (Treatment B/Treatment C) for AUC0-4 and AUC0-t were within the equivalence range of 80% to 125%. No effect of the route of coadministration of FPC/UFH (as a single admixture, Treatment B, or via separate routes, Treatment A) was observed on TT (Table S-1). The VCS graded dialyzer circuit clots on a Scale of 1 to 4, with Grade 1- no clots observed; Grade 2- Fibrinous ring in air trap or blood stripes in <5% of dialyzer fibers; Grade 2 Clots on venous air chamber filter or blood stipes >5% of dialyzer fibers; and Grade 4- Clots in air trap or clots in dialyzer fibers. Responses on the VCS were consistent with laboratory findings of the anticoagulant effects of the 3 study treatments. Response on the VCS reflected responses of “no detectable clotting” (Grade 1), there were only a few minimal clotting (Grade 2 responses during the hours 1 through 3 of continuous heparin infusion. One Grade 4 response was reported at the end of dialysis in Treatment A (FPC+UFH) and one Grade 3 response reported at end of dialysis in each of Treatments A and C (UFH alone) (Figure S-2). The results are consistent with a continuous anticoagulant effect regardless of the route of administration. None of the subjects required an additional bolus dose of UFH during administration of Treatment A or B (FPC-containing treatments); 1 subject required an additional bolus dose of UFH during Treatment C (UFH) although there was no evidence of clotting on the VCS. All treatments were well tolerated with no adverse events reported.
Pharmacokinetic/pharmacodynamic conclusions
The results of the pharmacokinetic/pharmacodynamic analyses demonstrate that coadministration of the FPC/UFH admixture via the pre dialyzer line had no clinically relevant impact on the anti-Xa activity, aPTT or TT. The route of administration of FPC/UFH (as an admixture, Treatment B, or by separate routes, Treatment A) had no effect on the coagulation parameters. The FPC/UFH admixture (Treatment B) was associated with an approximate 30% decrease in TT Cmax values relative to UFH alone (Treatment C). The coagulation effect as measured by AUC0-4 and AUC0-t were within the equivalence range consistent with the results from the anti-Xa and aPTT. The Cmax values exhibited a higher variability than the other measurements of coagulation parameters. Plasma total sFe pharmacokinetic parameters were comparable between the FPC/UFH admixture (Treatment B) and FPC/UFH administered by separate routes (Treatment A). No adverse events were reported with any treatment. Responses on the VCS were consistent with laboratory findings of an anticoagulant effect of both FPC/UFH regimens.
Discussion
This study was conducted to investigate the ability of a FPC/UFH admixture to maintain adequate anticoagulation of the dialyzer circuit and to assess the impact of administration of the FPC/UFH admixture on the iron delivery of FPC in patients with HDD-CKD. FPC is an iron complex in which iron (III) is bound to pyrophosphate and citrate [1]. FPC does not require processing by macrophages; it donates iron directly to transferrin for optimal utilization in erythropoiesis, avoiding sequestration within reticuloendothelial system macrophages [2]. FPC is currently approved in the United States for the replacement of iron to maintain hemoglobin in adult patients with HDD-CKD. FPC may be administered in the dialysate or as a slow continuous IV infusion over 3 to 4 hours via the pre dialyzer infusion line, via the post-dialyzer infusion line, or via a separate connection to the venous blood line during hemodialysis. Administration of iron to patients with HDDCKD typically involves slow administration of macromolecular iron-carbohydrate nanoparticles over 5 to 10 minutes to avoid rate-related adverse reactions to iron. Iron sucrose or iron dextran can be co-administered in combination with UFH via the on-machine infusion pump and pre dialyzer heparin line [11,12]. In-vitro drug-drug interaction studies have demonstrated that FPC can be admixed with unfractionated heparin and retain its pharmacodynamic effect for up to 24 hours (Figure S-3 and Tables S-2 and S-3). Conversely, UFH does not have any impact on FPC when incubated for up to 24 hours (Table S-4). Due to the rapid donation of iron from FPC to transferrin, no free FPC or Non-Transferrin Bound Iron (NTBI) can be detected in plasma, so the in-vitro studies support the clinical and laboratory observations in this study [13]. Coadministration of the FPC/UFH admixture via the pre dialyzer line had minimal impact on the anti Xa activity of UFH. The 90% CIs extended just above (AUC) or just below (Cmax) the equivalence bounds, however the overall anticoagulant effect as measured by AUC0-4 and AUC0-t were within equivalence bounds. The FPC/UFH admixture (Treatment B) had no impact on aPTT values relative to UFH alone (Treatment C) or FPC/UFH administered by separate routes or on the AUC values for TT as compared with FPC/UFH administered by separate routes (Treatment A) or with UFH alone (Treatment C).Thrombin time reflects the conversion of fibrinogen to fibrin but is also sensitive to the presence of inhibitors that may be present in the plasma (e.g., heparin). Thrombin cleaves fibrinogen, releasing fibrinopeptide A and fibrinopeptide B. Although not well understood, iron may bind to fibrinogen and accelerate coagulation [14,15]. This may explain the observation that TT is shorter when iron is co-administered during hemodialysis as compared to Treatment C in which no iron was administered. The observation that the differences in TT were only observed on Cmax may reflect a transient effect of FPC iron on TT. There was no concurrent effect on aPTT, anti-Xa activity, or visual clotting in the dialyzer circuit. Thrombin time is a clotbased plasma assay and, historically, has been used by some laboratories to monitor patients who receive UFH. The assay is performed by adding a known concentration of thrombin to platelet-poor plasma and measuring the time to clot formation. Under the appropriate assay conditions, heparin produces a dose-dependent prolongation of TT, which is semi logarithmic. Thrombin Time is too sensitive to monitor heparin anticoagulation due to lack of standardization for this purpose. Therefore, the aPTT is used to monitor UFH anticoagulation during hemodialysis if needed.
The study confirms the results of a previous study which used a population PK model for anti-Xa activity to confirm the effective anti-coagulation dose of UFH [16]. This study differs in that it shows the effect of UFH over the duration of dialysis including the rapid decay in anti-coagulation effects once the UFH infusion is terminated approximately 1 hour prior to the end of dialysis. While there was high variability in individual responses, all patients achieved an adequate anti-coagulation effect as measured by Anti Xa and aPTT. Plasma total sFe pharmacokinetic parameters were comparable between the FPC/UFH admixture (Treatment B) and FPC/UFH administered by separate routes (Treatment A). The concentration-time profiles for sFe and TSAT were equivalent between the FPC/UFH admixture (Treatment B) and FPC/UFH administered by separate routes (Treatment A). No differences in transferrin, ferritin, or TIBC concentrations were observed between the study treatments or over time. This study confirmed the clinical observations from the FPC clinical trials that FPC had no effects on access bleeding or vascular thrombosis during long term chronic administration [17,18]. Intravenous administration over 3 hours of FPC (6.75 mg) +UFH as an admixture was well tolerated by HD patients. No safety concerns were identified.
Conclusion
The results of this study demonstrate no clinically relevant drug interaction between FPC and UFH on the anticoagulation effects of UFH (as assessed by anti-Xa activity, aPTT, and TT) or on the ability of FPC to deliver iron (as assessed by sFe) when these agents are co-administered as a single admixture. No safety concerns were identified. The presentation of FPC for IV use was designed for simple withdrawal of the drug from the luer ampule and has been demonstrated to be stable for up to 24 hours under ambient conditions. Admixture of FPC with heparin is easily accomplished by removing the needle used to remove the heparin from the multi-dose vial and connecting the syringe to the luer adapter on the FPC ampule. The resulting volume is then programmed to be administered by the syringe pump, ceasing approximately one hour prior to the end of the dialysis procedure. The admixture of FPC with UFH is free of drug-drug interactions and can provide safe and effective anticoagulation along with the delivery of FPC iron.
Acknowledgement
The author would like to acknowledge the contributions of Innovative Analytics, Kalamazoo MI USA for data management. Nuventra Inc. Raleigh NC USA for PK and Equivalence analysis. Hemostasis Laboratory Ontario CA for in-vitro coagulation studies. BioScreen Testing Services, Torrence, CA for Analytical Support. The clinical study was conducted at Orlando Clinical Research Center, Orlando FL USA. The author would like to thank the OCRC staff and especially the patients who volunteered to participate in this trial.
REFERENCES
- Gupta A, Pratt R, Mishra B. Physicochemical characterization of ferric pyrophosphate citrate. Biometals. 2018; 31 (6): 1091-1099.
- Pratt R, Handelman GJ, Edwards TE, Gupta A. Ferric pyrophosphate citrate: interactions with transferrin. Biometals. 2018; 31 (6): 1081-1089.
- Farrell PC, Ward RA, Schindhelm K, Gotch F. Precise anticoagulation for routine hemodialysis. J Lab Clin Med. 1978; 92 (2): 164-176.
- Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: Still the best option. Semin Dial. 2010; 23 (5): 510-515.
- Ward RA. Heparinization for routine hemodialysis. Adv Ren Replace Ther. 1995; 2 (4): 362-370.
- Kessler M, Moureau F, Nguyen P. Anticoagulation in chronic hemodialysis: Progress toward an optimal approach. Semin Dial. 2015; 28 (5): 474-489.
- Kaneva K, Bansal V, Hoppensteadt D, Cunanan J, Fareed J. Variations in the circulating heparin levels during maintenance hemodialysis in patients with end-stage renal disease. Clin Appl Thromb Hemost. 2013; 19 (4): 449-552.
- Inc. RM. Triferic AVNU US Package Insert. 2020.
- FDA U. Draft Guidance on Iron Sucrose. 2013.
- FDA U. Statistical Approaches to Establishing Bioequivalence. In: CDER, editor. 2001.
- Granolleras C, Zein A, Oules R, Branger B, Fourcade J, Shaldon S et al. Continuous administration of intravenous iron during haemodialysis. Nephrol Dial Transplant. 1997; 12 (5): 1007-1008.
- Davis P, Bednarz D, Briglia A, Paganini EP. A protocol for coadministration of iv iron dextran and heparin in chronic hemodialysis patients. ANNA J. 1998; 25 (5): 533-538.
- Pratt RD, Swinkels DW, Ikizler TA, Gupta A. Pharmacokinetics of ferric pyrophosphate citrate, a novel iron salt, administered intravenously to healthy volunteers. J Clin Pharmacol. 2017; 57 (3): 312-320.
- Nielsen VG, Jacobsen WK. Iron modulates the alpha chain of fibrinogen. Biometals. 2016; 29 (2): 235-238.
- Nielsen VG, Henderson J. Sonoclot (R)-based method to detect iron enhanced coagulation. J Thromb Thrombolysis. 2016; 42 (1): 1-5.
- Brunet P, Simon N, Opris A, Faure V, Lorec-Penet AM, Portugal H, et al. Pharmacodynamics of unfractionated heparin during and after a hemodialysis session. Am J Kidney Dis. 2008; 51 (5): 789-795.
- Fishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, Guss CD, et al. Ferric pyrophosphate citrate (Triferic) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015; 30 (12): 2019-2026.
- Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int. 2015; 88 (5): 1187-1194.
Citation: Pratt RD (2021) Pharmacokinetics and Pharmacodynamics of Unfractionated Heparin and Ferric Pyrophosphate Citrate Coadministration During Hemodialysis: No Drug-Drug Interaction. J Bioequiv Availab. S4: 004.
Copyright: © 2021 Pratt RD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : The study was funded by Rockwell Medical, Inc, USA

