Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 17, Issue 6
Pharmacokinetic Bridging Study To Support The Use Of Nemolizumab Prefilled Pen For The Treatment Of Atopic Dermatitis And Prurigo Nodularis
Luca Loprete1*, Jabbar Lopez Z1, Piketty C1, Rasmussen S2, Silverberg J3, Stander S4, Duval V5, Machu JL6, Ulianov L6 and Wagner N62Department of Dermatology, George Washington University School of Medicine and Health Sciences, Washington, USA
3Department of Dermatology, University Hospital, Munster, Germany
4Department of Certara Strategic Consulting, Princeton, USA
5Department of Pharmacology, Galderma R and D, Lausanne, Switzerland
6Department of Galderma R and D, Dallas, USA
Received: 25-Nov-2025, Manuscript No. JBB-25-30374; Editor assigned: 27-Nov-2025, Pre QC No. JBB-25-30374 (PQ); Reviewed: 11-Dec-2025, QC No. JBB-25-30374; Revised: 18-Dec-2025, Manuscript No. JBB-25-30374 (R); Published: 25-Dec-2025, DOI: 10.35248/0975-0851.25.17.643
Abstract
Background: Nemolizumab is a monoclonal antibody that targets the receptor alpha of the neuro-immune cytokine IL-31. This clinical PK bridging study was conducted to support the marketing application of the prefilled dual chamber pen. Methods: This was a phase 1, randomized, multicenter, open-label, single-dose, parallel-group study in healthy adult subjects. Participants (N=192) were randomized by device (pen or syringe) and by injection site (abdomen, thigh, arm) and received one subcutaneous 60 mg nemolizumab dose. The primary endpoint pharmacokinetic parameters were analyzed using a linear mixed-effect model. Safety data were summarized descriptively. Results: Mean age was 41.5 years (19 to 65 years), mean body weight was 72.76 kg (45.4 to 107.4 kg) and 61% of participants were female. The geometric least-square mean ratio comparing pen versus syringe was 105.90% for Cmax and 97.69% for AUC0-∞. The associated 90% confidence intervals fall within the 80.00%-125.00% bioequivalence range. No treatment-emergent anti-drug antibodies were detected with the pen. Treatment emergent adverse events were experienced by 56% and 43% of subjects in the syringe and the pen groups, respectively and were primarily mild or moderate in severity. Conclusions: Single dose of nemolizumab administered as new formulation presentation (pen) was bioequivalent to the prefilled dual chamber syringe used in Phase 3 clinical studies. Nemolizumab delivered subcutaneously by pen or syringe was well tolerated, and no new safety risks were identified with the pen. Hence, results from this PK bridging study support the pen presentation as the commercial drug product
Keywords
Nemolizumab; Pharmacokinetics; Healthy subjects; Prefilled pen; Dual chamber syringe
Introduction
IL-31 is a naturally occurring cytokine that is involved in induction and maintenance of pruritus, epidermal dysregulation, dermal inflammation and fibrosis. Nemolizumab, a humanized anti-human Interleukin (IL)-31 Receptor Alpha (RA) monoclonal antibody, inhibits the binding of IL-31 to IL-31 RA and subsequent signal transduction, including the release of proinflammatory cytokines and chemokines. Nemolizumab showed statistically and clinically significant improvements in skin inflammation and itch in adults and adolescents with moderate-to-severe Atopic Dermatitis (AD) [1] and significantly reduced the signs and symptoms of Prurigo Nodularis (PN) [2].
Nemolizumab is approved for the subcutaneous treatment of moderate to severe AD inadequately controlled by topical therapies in patients ≥ 12 yo and for the treatment of adults with PN. AD is a chronic inflammatory skin disease estimated to occur in 10% to 20% of the population [3] and up to 25% of children [4]. The disease is characterized by pruritus (itching), xerosis (skin dryness), and eczematous lesions whose features include erythema, infiltration/papulation, oozing with crusting, excoriations, and lichenification. PN is characterized by symmetrically distributed, multiple, highly pruritic, hyperkeratotic, erosive or crusted nodules and papules [5]. This leads to an impaired quality of life and a high burden due to severe itching, chronic skin lesions, and lack of treatment options [6].
The efficacy, safety, tolerability, Pharmacokinetic (PK), pharmacodynamics, and immunogenicity of nemolizumab were extensively assessed during Phase 1, 2, and 3 studies in healthy volunteers, in subjects with AD, and in subjects with PN. Similar nemolizumab systemic exposure was observed in subjects with AD and subjects with PN who received the same dose. During part of the clinical development, this nemolizumab formulation presentation used in clinical studies was a single-dose, single-use dual-chamber syringe prefilled with a lyophilized powder (nemolizumab) and water for injection in 2 separate chambers. The nemolizumab pen was later introduced as the intended commercial presentation [7,8].
A clinical PK bridging study was conducted to demonstrate bioequivalence between the syringe and the pen. This study supports the marketing approval of the pen and its subsequent use in future clinical trials.
The PK bridging study is part of the multidisciplinary approach described in the Food and Drug Administration (FDA) draft guidance “bridging for drug-device and biologic-device combination products” [9]. This multidisciplinary approach combines comparative analytical (structural and functional) characterization, human factor studies and a clinical PK bridging study.
Methods
Study design and procedures
The study was conducted at each site according to the protocol SPR.201590 (ClinicalTrials.gov identifier NCT05405985). All subjects received a detailed description of the study and provided written Informed Consent (ICF) prior to enrolment. The protocol and ICF were reviewed and approved by Advarra Institutional Review Board (IRB), a duly constituted IRB in United States, before subjects were screened for entry. The study was conducted in accordance with the accepted version of the declaration of helsinki and/or all federal regulations, as set forth in Parts 50, 56, 312, Subpart D, of Title 21 of the United States (US) Code of Federal Regulations, European Union 536/2014, Annex 1, D, 17 (a), in compliance with International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice (GCP) guidelines and according to the appropriate regulatory requirements in the countries where the study was conducted.
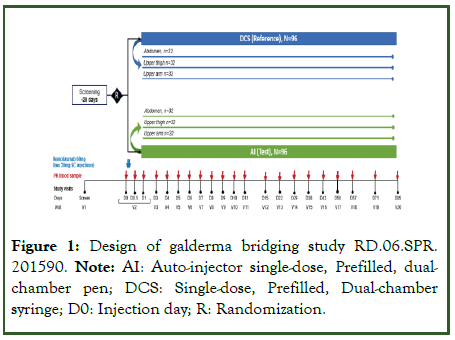
This was a Phase 1, randomized, multicenter, open-label, singledose, 2-treatment, parallel-study in healthy adult subjects at 2 sites in the USA-Celerion, Lincoln, Nebraska and Celerion, Tempe, Arizona. The study was designed according to the EMA and FDA guidelines for bioequivalence studies with therapeutic proteins [10-14]. The study consisted of an up to 28-day screening period and a 12 week PK evaluation period. The maximum study duration for each participant was set at 113 days, which includes the screening period (Figure 1). At baseline, subjects who met eligibility criteria were randomized 1:1 to receive a single 60 mg dose of nemolizumab delivered with either a syringe or a pen. The 60 mg dose was selected as the highest used in phase 3 studies. Subjects were further randomized 1:1:1 to receive injection in 1 of 3 injection sites (i.e., abdomen, front upper thigh, or outer upper arm).
Figure 1: Design of galderma bridging study RD.06.SPR. 201590. Note: AI: Auto-injector single-dose, Prefilled, dualchamber pen; DCS: Single-dose, Prefilled, Dual-chamber syringe; D0: Injection day; R: Randomization.
Subjects received a 60 mg dose of nemolizumab via 2 Subcutaneous (SC) injections of 30 mg nemolizumab. Injections were to be administered at the same location (i.e., abdomen, front upper thigh, or outer upper arm) and the same side of the body, with injection sites at least 1 inch (2.5 cm) apart. Blood samples were collected before and after nemolizumab administration for up to 12 weeks post dose for determining the complete serum PK profile of nemolizumab. Subjects were to be confined to the study site for up to 2 nights, from the evening prior to nemolizumab dosing or from nemolizumab dosing (Day 0) to the morning after nemolizumab dosing (Day 1).
The syringe was single-dose, single-use and prefilled with a lyophilized powder (biological product nemolizumab) and water for injection in 2 separate chambers with assembled finger rest. The syringe was intended to be used for SC injection after reconstitution. The lyophilized nemolizumab powder was in chamber 1 and water for injection as a reconstitution liquid was in chamber 2 of the syringe.
The pen was fully disposable, single-use, and single-dose with a prefilled dual-chamber cartridge intended for SC injection after reconstitution of the lyophilized nemolizumab powder with water for injection. The pen was equipped with a simple Twist and Mix and Needle Isolation Technology® intended for SC injection. After reconstitution, each syringe and pen contained 61.5 mg/mL of nemolizumab to deliver a dose of 30 mg with nominal injection volume of 0.49 mL (for the 60-mg dose, 2 injections using 2 syringes were needed).
Subjects
Healthy adult volunteers aged 18-65 years, with a body weight ≥ 45 kg and a body mass index of ≥ 18.0-<30.0 kg/m2, were enrolled in the study. All volunteers were in good physical health, as assessed through medical history, full physical examination, vital signs measurement and clinical laboratory assays, according to the study inclusion criteria. No subjects had a history of drug, alcohol or substance abuse within 6 months of the screening visit. Female subjects of childbearing potential (i.e., fertile, following menarche and until becoming postmenopausal unless permanently sterile) agreed to either to be strictly abstinent throughout the study and for 12 weeks after the study drug injection, or to use an adequate and approved method of contraception throughout the study and for 12 weeks after the study drug injection. Male subjects were not required to use contraception, and there was no restriction on sperm donation.
Exclusion criteria included history or presence of significant diseases, any cutaneous infection within 1 week before the baseline visit or any infection requiring treatment with oral or parenteral antibiotics, antivirals, antiparasitics, or antifungals within 2 weeks before the baseline visit, any confirmed or suspected COVID-19 infection within 2 weeks before the screening or baseline visit, positive serology results, active or untreated latent tuberculosis infection, known or suspected immunosuppression, history of lymphoproliferative disease or history of malignancy of any organ system within the last 5 years, history of hypersensitivity or allergic reactions to an immunoglobulin product or to any of the study drug excipients. All prescription medications were not allowed during the study. Subjects were not enrolled if they had previous treatment with nemolizumab or received a live-attenuated or non-live vaccine within 4 weeks before the baseline visit or were expected to be vaccinated during the study or during the 12 weeks after the last study drug injection, except for non-live seasonal vaccinations, COVID-19, and/or emergency vaccinations. Subjects were notenrolled if they had participated in other clinical trials in the past 8 weeks or donated blood in the past 3 months.
Blood sampling
Blood samples were to be collected from each subject to assess: nemolizumab serum concentrations for PK analysis and ADA for immunogenicity evaluation.
A total of 21 blood samples were to be collected for nemolizumab PK assessment, before and after nemolizumab SC injection (i.e., pre-dose and 12 hours, 24 hours, and 3, 4, 5, 6, 7, 8, 9, 10, 11, 15, 22, 29, 36, 43, 50, 57, 71 and 85 days post-dose).
A total of 3 blood samples were collected for ADA assessment before and after nemolizumab SC injection (i.e., pre-dose, 29 and 85 days post-dose).
For both PK and ADA assessments, blood was collected into SST tubes, allowed to clot for at least 30 mins at room temperature and then centrifuged at 1300 ± 20 g for 10 mins also at room temperature and no more than 60 min after collection. Serum was transferred to polypropylene cry vials, taking care not to transfer any red blood cells. and then stored frozen at either -80°C ± 10°C or -20°C ± 5°C if a -80°C freezer was not available at site.
Bioanalytical assays
All methods used for the bioanalytical assays were developed and validated according to the requirements of the international guidelines for bioanalytical method validation [15,16].
The concentration of nemolizumab in serum was determined by Syneos Health Bioanalysis (Quebec site) using a validated Enzyme- Linked Immunosorbent Assay (ELISA). The method had a Lower Limit of Quantification (LLOQ) of 100 ng/mL and an Upper Limit of Quantification (ULOQ) of 64000 ng/mL and adhered to the regulatory requirements for selectivity, sensitivity, precision, accuracy, dilution linearity, hook effect, parallelism in disease matrices, and stability.
The ADA were determined using a validated Electrochemiluminescence Immunoassay (ECLIA). The presence of ADA in serum was to be confirmed using a multi-tiered approach composed of screening, confirmatory, and titer assays. The samples confirmed to be ADA-positive were then characterized by Neutralizing Antibody (NAb) assays using a validated cell-based assay.
Pharmacokinetic parameters
Pharmacokinetic parameters were determined or calculated with a Non-Compartmental Analyses (NCA), from the serum nemolizumab concentration-time data using the validated software Phoenix® WinNonLin® Version 8.3.5 (Certara, Inc). Actual sample times were used in the calculations.
The primary study outcome measures were serum peak concentration (Cmax) and area under the concentration-time curve extrapolated to infinity (AUC0-∞), calculated using the linear trapezoidal rule. The primary endpoint of the study was the evaluation of the similarity of the two administration devices in terms of rate (Cmax) and extent (AUC0-∞), of serum nemolizumab concentration. The following nemolizumab pharmacokinetic parameters were also calculated: and area under the concentration–time curve up to the last sampling point (AUC0–t) and from time 0 to 4 weeks (AUC0-4weeks), time to Cmax (tmax), terminal volume of distribution (Vz/F), total clearance (Cl/F) and half-life (t1/2) for an extravascular administration.
Immunogenicity
Presence of Anti-Drug Antibody (ADA) was evaluated using a first screening assessment followed by confirmatory assessment on positive ADA samples. Titer and neutralizing potential were determined on confirmed positive ADA samples. Treatmentemergent ADAs were defined according to ADA classification described in Shankar, et al [17]. Incidence of treatment-emergent ADA is the sum of treatment inducted ADA (ADA developed de novo following nemolizumab SC injection) treatment boosted ADA (pre-existing ADA that were boosted to a higher titer level following nemolizumab SC injection).
Safety
The safety profile of nemolizumab was assessed by evaluating treatment-emergent Adverse Events (AEs), physical examination, ECG recording, laboratory tests and vital signs checks. Vital signs (systolic and diastolic blood pressure, body temperature and heart rate) were measured at screening, at baseline and at days 8, 43 and 85 (final visit). A 12-lead resting ECG was recorded at screening and at final visit. Blood and urine samples were collected for routine hematology, blood chemistry, and urinalysis at screening and at final visit. Virology assessments were performed at screening. AEs were assessed throughout the study and were coded using MedDRA version 25.0. A full physical examination was performed by the investigator at screening, at baseline and at days 8, 43 and 85.
Sample size and statistical analyses
A total of 192 healthy subjects were included in the study, i.e., 96 subjects per arm (pen or syringe). The sample size was defined based on a 90% power of claiming the bioequivalence between pen and syringe administration within the range of 80.00 to 125.00% for the primary PK parameters (AUC0-∞ and Cmax) with the expected Geometric Mean Ration (GMR) of 0.95. The statistical analyses were performed using SAS® software version 9.4 (SAS Institute, Cary, NC).
For the primary PK endpoint analyses, a linear model assessed the log-transformed PK parameters of nemolizumab, with delivery method (pen [test] or syringe [reference]), injection site (abdomen, arm, or thigh), and interaction between delivery method and injection site as fixed effects. The AUC0-∞ and Cmax geometric mean ratio between pen and syringe were provided, by injection site and overall, after back transformation and the corresponding 90% Confidence Intervals (CI) and p-values. AUC0-t, AUC0-4weeks, and t1/2 were also analyzed using the methods applied for the primary PK parameters.
Bioequivalence was concluded if the 90% CIs for the PK parameter(s) geometric mean ratio(s) expressed as a percentage of test versus reference were entirely within the 80.00% to 125.00% reference interval.
Tmax was analyzed using the non-parametric Wilcoxon rank sums test for 2 independent samples
Results
Subjects
Of the 192 randomized subjects, 191 subjects completed the study. One subject discontinued the study for personal reasons after Day 4. As only 2 post-dose samples were collected for this subject, the derived PK parameters were restricted to Cmax, Tmax, and AUC0-t. The overall safety and PK sets included all the 192 treated subjects.
Overall, the majority of subjects were female (61%) and White (84%). Hispanic or Latino represented 54% of subjects overall. The mean age was 41.5 years (range: 19 to 65 years) and the mean body weight was 72.76 kg (range: 45.4 to 107.4 kg). Demographic and baseline characteristics were similar between the two groups (Table 1).
| Pen (test) (N=96) |
Syringe (reference) (N=96) |
Overall (N=192) |
|
|---|---|---|---|
| Sex, n (%) | |||
| Male | 41 (42.7%) | 34 (35.4%) | 75 (39.1%) |
| Female | 55 (57.3%) | 62 (64.6%) | 117 (60.9%) |
| Race, n (%) | |||
| American Indian or Alaska Native | 1 (1.0%) | 1 (1.0%) | 2 (1.0%) |
| Asian | 0 | 2 (2.1%) | 2 (1.0%) |
| Black or African American | 9 (9.4%) | 8 (8.3%) | 17 (8.9%) |
| Multiple | 4 (4.2%) | 5 (5.2%) | 9 (4.7%) |
| White | 82 (85.4%) | 80 (83.4%) | 162 (84.4%) |
| Age (years) | |||
| Mean (SD) | 40.9 (11.71) | 42.1 (12.52) | 41.5 (12.11) |
| Minimum, maximum | 19, 65 | 19, 65 | 19, 65 |
| Body mass index (kg/m2) | |||
| Mean (SD) | 25.76 (2.743) | 26.10 (2.777) | 25.93 (2.758) |
| Minimum, maximum | 18.22, 29.64 | 18.87, 29.86 | 18.22, 29.86 |
| Height (cm) | |||
| Mean (SD) | 166.6 (9.98) | 168.0 (11.16) | 167.3 (10.58) |
| Minimum, maximum | 146, 188 | 149, 193 | 146, 193 |
| Weight (kg) | |||
| Mean (SD) | 71.6 (10.70) | 74.0 (12.66) | 72.8 (11.75) |
| Minimum, maximum | 45.4, 98.6 | 49.8, 107.4 | 45.4, 107.4 |
Table 1: Demographic and baseline characteristics (safety population).
All subjects were in good physical health, based on physical examination, medical and surgical history.
Pharmacokinetics
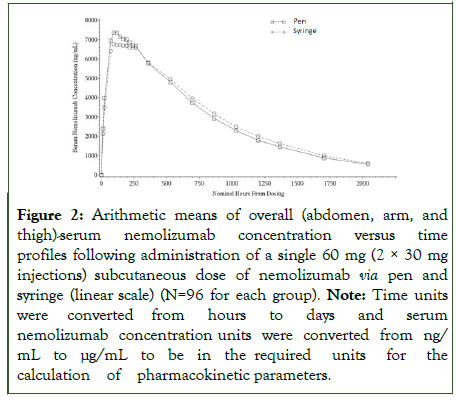
The mean ± Standard Deviation (SD) serum concentration-time profiles obtained after nemolizumab SC administration by the pen and the syringe are shown in Figure 2.
Figure 2: Arithmetic means of overall (abdomen, arm, and thigh)-serum nemolizumab concentration versus time profiles following administration of a single 60 mg (2 × 30 mg injections) subcutaneous dose of nemolizumab via pen and syringe (linear scale) (N=96 for each group). Note: Time units were converted from hours to days and serum nemolizumab concentration units were converted from ng/mL to μg/mL to be in the required units for the calculation of pharmacokinetic parameters.
The main serum pharmacokinetic parameters data (mean ± SD) and the results of their statistical comparisons are presented in Table 2.
| Pharmacokinetic parameter (unit) | Pen (test) (N=96) |
Syringe (reference) (N=96) |
Geometric LSM ratio (%) |
90% CI (%) | Inter-subject CV% |
|---|---|---|---|---|---|
| Cmax (µg/mL) | 8.00±2.34 | 7.51±2.31 | 105.90 | 98.01 - 114.41 | 33.29 |
| AUC0-∞ (µg•day/mL) | 270±85.8 | 279±89.7 | 97.69 | 90.39 - 105.59 | 33.36 |
| Tmax (day) | 4.99 (1.00, 21.99) | 5.99 (1.00, 22.00) | |||
| AUC0-t (µg•day/mL) | 250±73.6 | 260±75.4 | 92.46 | 81.95 - 104.32 | 53.99 |
| AUC0-4weeks (µg•day/mL) | 157±37.9a | 155±39.8 | 101.69 | 95.33 - 108.48 | 27.52 |
| t1/2 (day) | 18.0±5.91a | 18.5±4.52 | 95.44 | 89.50 - 101.78 | 27.38 |
| CL/F (L/day) | 0.244±0.077a | 0.243±0.104 | - | - | - |
| Vd/F (L) | 5.98±1.76a | 6.08±1.58 | - | - | - |
Table 2: Summary of serum nemolizumab pharmacokinetic parameters following administration of a single 60 mg (2 × 30 mg injections) subcutaneous dose of nemolizumab via pen and syringe (pharmacokinetic population).
AUC0-∞=area under the concentration-time curve extrapolated to infinity; AUC0-4 weeks=area under the concentration-time curve from time 0 to 4 weeks after study drug administration; AUC0-t=area under the concentration-time curve from time 0 to last study drug administration; CL/F=oral clearance; Cmax=observed maximum serum concentration; CV: coefficient of variance; LSM: least square mean t1/2=elimination half-life; Tmax=time to maximum concentration; Vd/F=volume of distribution Note: All subjects received a single dose of nemolizumab 60 mg (2 × 30 mg injections). Tmax values were presented as median (minimum, maximum). Other parameters were presented as arithmetic mean (± SD). Time units were converted from hours to days and serum nemolizumab concentration units were converted from ng/mL to μg/mL to be in the required units for the calculation of pharmacokinetic parameters. For the statistical analysis, tested parameters (except Tmax) were ln-transformed prior to analysis. Geometric LSMs were calculated by exponentiation the least-squares means from analysis of variance. Geometric mean ratio=100 × (test/reference). Inter-subject CV%=100 x (square root (exp[MSE]-1), where MSE=residual variance from analysis of variance. an=95.
Nemolizumab serum concentrations peaked at 5 days (pen) or 6 days (syringe) post-dose, then declined gradually with a terminal half-life of 18-19 days. Maximum plasma concentration (Cmax) of nemolizumab was 8.0 ± 2.34 μg/mL for the pen (test) and 7.5 ± 2.31 μg/mL for the syringe (reference) administration. The apparent volume of distribution and apparent clearance were comparable between the two devices. Mean AUC parameters (AUC0–∞, AUC0–t and AUC0-4 weeks) were also similar between the treatments (Table 2) and among administration site (abdomen, thigh, and arm).
The statistical analysis results of PK parameters comparing test vs . reference comparison (overall and by injection site) are summarized in Table 2. Test/reference geometric Least Squares Means (LSM) ratios, 90% CIs, and inter-subject CV% for the tested PK parameters Cmax and AUC0-∞ (primary PK variables) and for AUC0-t, AUC0-4 weeks, and t½ (secondary PK variables) are shown.
When comparing the primary PK parameters of nemolizumab between the pen and the syringe presentations, the geometric least-square mean ratios were 105.90% for Cmax and 97.69 % for AUC0–∞. The associated 90% CI falls within the 80.00%-125.00% range, indicating a similar rate and extent of nemolizumab absorption between the two formulations. For all the primary PK parameters, no significant difference was observed between the 3 injection sites (abdomen, arm, and thigh), with p-values >0.05.
The 90% CIs for the test/reference ratios were also within the 80.00% to 125.00% range for the secondary PK parameters (AUC0-t, AUC0-4 weeks, and t½), while the Wilcoxon Signed Rank Test evidenced no statistically significant difference between test and reference for nemolizumab Tmax (p-value of 0.1105).
Immunogenicity
In the pen group, 2 of 95 subjects (2.1%) had ADAs at Day 85, with titers of 40 and 80. Neither of these subjects had treatmentemergent ADAs.
In the syringe group, 8 of 96 subjects (8.3%) had ADAs at Day 85, with titers ranging from 10 to 20. Among those 96 subjects, 3 (3.1%) subjects had treatment-emergent ADAs. Of the 3 subjects with treatment-emergent ADAs, none reported a Treatment-Emergent Adverse Event (TEAE) or had any identified impact on the PK profile of nemolizumab.
During the entire study, no subject tested positive for Neutralizing Antibodies (NAb).
Safety
Both tested treatments showed a good safety profile and no Serious AEs (SAE) or TEAE leading to study discontinuation were reported. A higher percentage of subjects in the syringe group compared with the pen group experienced at least 1 TEAE (56% versus 43%, respectively) and at least 1 study drugrelated TEAE (42% versus 22%, respectively) during the study. All subjects that experienced TEAEs had events considered mild or moderate in severity. One (1%) subject, each in the pen and syringe groups, experienced a treatment emergent AE of Special Interest (AESI), i.e., a COVID infection.
The most common TEAEs were headache (16%) and weight increased (6%). The incidence of headache was higher in the syringe group (23%) compared with the pen group (8%), while the incidence of weight increased was similar between the pen (7%) and syringe (5%) groups.
Summary of drug-related TEAEs experienced by ≥ 5.0% of subjects in any group is presented in Table 3.
| System organ class Preferred term |
Pen (test) (N=96) | Syringe (reference) (N=96) | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Abdomen N=32 n (%) |
Arm N=32 n (%) |
Thigh N=32 n (%) |
Overall N=96 n (%) |
Abdomen N=32 n (%) |
Arm N=32 n (%) |
Thigh N=32 n (%) |
Overall N=96 n (%) |
N=192 n (%) |
|
| Cardiac Disorders Palpitations |
0 | 0 | 0 | 0 | 0 | 0 | 2(6) | 2(2) | 2(1) |
| Gastrointestinal Disorders Nausea |
1(3) | 0 | 0 | 1(1) | 2(6) | 0 | 2(6) | 4(4) | 5(3) |
| Investigations Weight increased |
2(6) | 4(13) | 1(3) | 7(7) | 2(6) | 2(6) | 1(3) | 5(5) | 12(6) |
| Metabolism and Nutrition Disorders Decreased appetite |
0 | 0 | 0 | 0 | 0 | 0 | 2(6) | 2(2) | 2(1) |
| Musculoskeletal and Connective Tissue Disorders Back pain |
0 | 0 | 0 | 0 | 3(9) | 0 | 0 | 3(3) | 3(2) |
| Myalgia | 0 | 0 | 1(3) | 1(1) | 0 | 0 | 2(6) | 2(2) | 3(2) |
| Nervous System Disorders Headache |
2 (6) | 2 (6) | 2 (6) | 6 (6) | 8 (25) | 5 (16) | 7 (22) | 20 (21) | 26 (14) |
| Somnolence | 0 | 0 | 2 (6) | 2 (2) | 0 | 0 | 0 | 0 | 2 (1) |
| Skin and Subcutaneous Tissue Disorders Pruritus |
0 | 1 (3) | 0 | 2 (2) | 0 | 0 | 0 | 0 | 2 (1) |
Note: All subjects received a single dose of nemolizumab 60 mg (2 x 30 mg injections). Adverse events were classified according to the Medical Dictionary for Regulatory Activities Version 25.0. Although a subject may have had ≥2 adverse events, the subject was counted only once within a category.
Table 3: Drug-related treatment-emergent adverse events experienced by ≥ 5.0% of subjects in any group (safety population).
No clinically relevant effects on ECGs or laboratory parameters were observed. Two subjects in pen group experienced a mild increase in heart rate while one subject in syringe group experienced a mild rise in blood pressure. N=number of subjects in the treatment group; n=number of subjects who experienced the events; TEAE=treatment emergent adverse event.
Discussion
This was a Phase 1, open-label, randomized, single-dose, 2-treatment, parallel study in healthy volunteers which demonstrated bioequivalence after a single SC injection of nemolizumab as new formulation presentation (pen) and syringe.
In the Phase 3 studies, the nemolizumab presentation was a single use, single-dose, syringe pre-filled with a lyophilized powder (nemolizumab) and water for injection in 2 separate chambers [18].
In parallel to the conduct of the phase 3 clinical trials, a singledose, prefilled, dual-chamber pen containing 30 mg of nemolizumab lyophilized powder and diluent, water for injection was developed and introduced. The pen facilitates SC injections by patients or caregivers and is the presentation for commercial use. The pen is assembled around a dual-chamber cartridge, containing the same nemolizumab formulation as the syringe. After the reconstitution, both the syringe and the pen contain 61.5 mg/mL of nemolizumab to deliver a dose of 30 mg with nominal injection volume of 0.49 mL.
In accordance with the FDA draft guidance “bridging for drug device and biologicdevice-combination products,” a gap analysis was performed to identify all differences between the 2 combination products, the syringe and the pen. The difference in (1) primary and secondary container closure, in (2) manufacturing process and in (3) filling volume were addressed by Chemistry, Manufacturing, and Controls (CMC). Upon reconstitution with water for injection, the drug formulation is the same for the pen and the syringe. The shape of components was different, but materials used for the primary container closures in direct contact with the drug (i.e., barrel and plungers) were similar between the syringe and the pen.
The human factors engineering program supports the use of the pen in addressing the difference in user interfaces. The clinical PK bridging study evaluated how differences in drug delivery between the pen and syringe influence the PK profile of nemolizumab. Parameters examined included injection speed (unspecified for the syringe, ≤ 15 seconds for the pen), injection angle (45° for the syringe, 90° for the pen), and injection depth (13 mm for the syringe, 4.6-7.6 mm for the pen).
The study was designed based on various factors, including clinical context, safety, the PK profile of nemolizumab and regulatory recommendations [9-14]. Furthermore, bioanalytical assays were appropriate for their intended use and adequately validated as outlined in EMEA and FDA Guidelines on bioanalytical method validation [15,16]. The parallel design was considered necessary due to the long terminal half-life of nemolizumab (19 days). The study was designed with a singledose administration protocol, recognizing that single-dose PK evaluations typically provide greater sensitivity than steady-state studies when determining variations in the rate and extent of drug substance absorption from the product into systemic circulation. Furthermore, in the case of nemolizumab, repeateddose studies demonstrated a limited systemic accumulation over time, with median accumulation below 2 and steady-state concentrations reached after the loading dose [7,8]. This confirms that the PK profile of nemolizumab is not affected by the number of administrations and can be predicted from singledose PK parameters. In addition, nemolizumab has a low immunogenic potential and the PK profile of nemolizumab is not affected by ADA [19].
This study was powered for demonstrating bioequivalence; a total of 192 healthy male and female subjects were randomized in the study as planned. All subjects received the study drug dose by either AI or DCS and were included in the safety and PK analyses.
Bioequivalence was established between the pen and the syringe. The PK parameters were similar in all treatment groups across the administration sites-abdomen, thigh, or arm. The ADA incidence ranged from 0% for the pen to 3.1 % (3/96) for the syringe and no NAb were observed, confirming the low immunogenicity potential of nemolizumab. No influence of the injection site on the immunogenic profile was observed in either administration modality.
With regards to safety, a low percentage of subjects treated with the pen experienced at least 1 TEAE compared to (43% versus 56% with the prefilled syringe) and at least 1 study drug-related TEAE (22% versus 42% with the prefilled syringe); the lowest incidence of TEAEs and study-drug related TEAEs were observed in the abdomen and thigh with the pen. Overall safety data confirmed a favorable safety profile of nemolizumab administered as a single 60-mg SC dose with prefilled pen.
Conclusions
The PK profile of nemolizumab was adequately characterized in healthy volunteers after a single 60-mg SC dose from the 2 drug product presentations of nemolizumab (pen and syringe). Bioequivalence was established between the pen and the syringe. Nemolizumab SC delivered by pen or syringe was well tolerated, and no new safety risks were identified with the pen. Hence, results from this PK bridging were used to leverage safety and efficacy data gathered in the existing phase 3 clinical program with the syringe presentation and support the pen as the commercial drug product presentation.
References
- Silverberg JI, Wollenberg A, Reich A, Thaci D, Legat FJ, Papp KA, et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet. 2024;404(10451):445-460.
[Crossref] [Google Scholar] [PubMed]
- Shawn G et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023; 389:1579-1589.
[Crossref] [Google Scholar] [PubMed]
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109-22.
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-32.
[Crossref] [Google Scholar] [PubMed]
- Hyde JN, Montgomery FHA. A practical treatise on diseases of the skin: for the use of students and practitioners. JAMA. 1909:174-5.
- Warlich B, Fritz F, Osada N, Bruland P, Stumpf A, Schneider G, et al. Health-related quality of life in chronic pruritus: an analysis related to disease etiology, clinical skin conditions and itch intensity. Dermatology. 2015;231(3):253-9.
[Crossref] [Google Scholar] [PubMed]
- Nemluvio. Highlights of prescribing information these highlights do not include all the information needed to use nemluvio safely and effectively.
- Nemluvio. Summary of product characteristics.
- Guideline. U.S. Department of health and human services, food and drug administration. Draft guidance for industry: guideline bridging for drug-device and biologic-device-combination products. 2019.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues.
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Clinical Pharmacology Data to Support a demonstration of Biosimilarity to a Reference Product. 2016.
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs-General Considerations. 2022.
- Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials
- European Medicines Agency. Guideline on the investigation of bioequivalence.
- Guideline. European medicines agency; Committee for Medicinal Products for human use (CHMP). Guideline on Bioanalytical Method Validation. 2011.
- Guideline. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry: Guideline on Bioanalytical Method Validation. 2018.
- Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658-73.
[Crossref] [Google Scholar] [PubMed]
- Silverberg JI., Wollenberg A, Reich A, Thaçi D, Legat FJ, Papp KA, et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet. 2024;404(10451):445-460.
[Crossref] [Google Scholar] [PubMed]
- Wagner N, Loprete L. Absence of clinically impactful immunogenicity of nemolizumab in long-term treatment of patients with atopic dermatitis and prurigo nodularis. EADV poster. 2024.
Citation: Loprete L, Lopez JZ, Piketty C, Rasmussen S, Silverberg J, Stander S, et al. (2025). Pharmacokinetic Bridging Study to Support the Use of Nemolizumab Prefilled Pen for the Treatment of Atopic Dermatitis and Prurigo Nodularis. J Bioequiv Availab. 17:643.
Copyright: © 2025 Loprete L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.