Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 5
On-Station and Field Evaluation of Inactivated Fowl Cholera Vaccine Produced from Local Pasteurella multocida Isolates, Ethiopia
Zewde Tariku Wolde*, Gezahegn Mamo, Esayas Gelaye, Takele Abayneh and Jaleta ShukaReceived: 19-Oct-2022, Manuscript No. JBP-23-18473; Editor assigned: 24-Oct-2022, Pre QC No. JBP-23-18473 (PQ); Reviewed: 07-Nov-2022, QC No. JBP-23-18473; Revised: 18-Jul-2023, Manuscript No. JBP-23-18473 (R); Published: 16-Aug-2023, DOI: 10.35248/2155-9597.23.14.484
Abstract
Vaccination is one of the common preventive measures of fowl cholera. On-station and field study was carried out from November 2017 to April 2018 to evaluate the safety, immunogenicity and protective efficacy of inactivated fowl cholera vaccine developed from local Pasteurella multocida isolate at national veterinary institute, Ethiopia. A total of 60 chickens (8 weeks old; bovan brown breed) randomly divided into 3 groups, each 20 chick, were used for onstation study. The first and second groups were vaccinated twice (three weeks apart) with 1 ml and 0.5 ml of inactivated fowl cholera vaccine containing 2.5 × 108 cfu/ml, respectively both through the IM route. The third group was kept as unvaccinated control. Sera were collected from all groups at day 0, 14, 21, 28 and 35 and kept at -20°C until analysis using Indirect Haemagglutination Test (IHT). All groups were challenged using virulent isolate of Pasteurella multocida containing 1.67 × 108 cfu/ml 15 days after the booster vaccination. Moreover, field or on-farm evaluation was conducted on 200 layers kept in a separate compartment from a selected commercial poultry farm. The chickens were divided into two groups of each 100 chicken and vaccinated twice (three weeks apart) with 0.5 mL and 1 mL through IM route. Sera were collected at day 0, 21 and 35 and examined by indirect haemagglutination test. In the experimental evaluation, mean antibody titers of group I were found to be 1.6 ± 1.2, 211.3 ± 2.1, 244.5 ± 1.2, 319.8 ± 1.2 and 502 ± 1.2 on day 0, 14, 21, 28 and 35 respectively. In group II, mean antibody titers were 1.3 ± 1.2, 203.7 ± 3.0, 234.2 ± 1.2, 367.2 ± 1.2.5 and 452.9 ± 1.2 on day 0, 14, 21, 28 and 35 respectively. In group III, the mean antibody titer was similar on the respective days which were not different from the value on day 0, 1.4 ± 1.2. In case of protective efficacy, both 1 ml and 0.5 mL dose vaccinated chicken had survival rate of 87.5%. On field evaluation, the mean antibody titers were 4.2 ± 1.1, 310.4 ± 1.0 and 532.6 ± 1.0 on day 0, 21 and 35 respectively. Both on-station and farm (field) evaluation showed that formalin killed fowl cholera vaccine induced good immune response with significant increase in mean antibody titer after booster vaccination, which corresponded to significant (87.5%) protection as observed in the challenge experiment. No difference was found in the protective efficacy and immunogenicity between 1 ml and 0.5 ml dose rates of the vaccine suggesting that the use of 0.5 ml dose rate in two injections 3 weeks apart would be economical that could provide optimal protection under field condition.
Keywords
Chickens; Immunogenicity; Inactivated fowl cholera vaccine; Pasteurella multocida
Introduction
The poultry sector continues to grow and industrialize in many parts of the planet. An increasing population, greater purchasing power and urbanization are strong drivers of growth. The total numbers of chickens in Ethiopia were estimated at 56 million few years back and now has increased to 65.87 million with expected increase within the future indicating the sector’s importance. It occupies a singular position in terms of high quality protein food contribution to rural smallholder farming families in Africa and particularly in Ethiopia. Despite the very fact that poultry industry is growing fast in Ethiopia, the industry is facing many constraints among which are infectious diseases with significant impact on the event of poultry industry [1].
Fowl cholera is one of contagious bacterial disease of domesticated and wild avian species caused by infection with Pasteurella multocida. It typically occurs as a fulminating disease with massive bacteremia and high morbidity and mortality in older birds. Chronic infections also occur with clinical signs and lesions associated with localized infections. The pulmonary system and tissues related to the musculoskeletal system are often the seats of chronic infection. Common synonyms for animal disease are avian pasteurellosis and avian hemorrhagic septicemia. This disease has been reported as a crucial disease in domestic poultry for more than 200 years and caused devastating economic losses to poultry industry worldwide. In poultry, it's often associated with severe economic crisis due to production losses. The acute sort of fowl cholera is associated with high mortality while chronic infections and asymptomatic carriers result in the persistence of bacteria within flock. The bacterium is often disseminated within a flock and between houses by secretion and excretion that contaminate the environment and diagnosed clinically by excretion from mouth, nose and ears and cyanosis of comb and wattles [2].
Vaccination is taken into account as one of the common preventive measures worldwide to reduce the prevalence and incidence of disease. Many authors reported that the widespread distribution of the many diseases had negative impact on the chicken production performance in developing countries and found to alert the timely vaccination strategies. Both live and inactivated vaccines are attempted to control the disease. The live attenuated vaccines give good protection, having an extended duration of immunity and cross-protection against different serotypes of Pasteurella multocida. However, it's less used in many countries due to the side effects, reactions including the localization of the organisms within the joints and sometimes causing lung infection. The opposite principal problem of live fowl cholera vaccine is the lack of regular maintainable attenuation or its instability presenting a risk of regaining its virulence. The formalin inactivated vaccines had more comparative advantage over the attenuated live vaccines since they're safe and provide acceptable protection. Immune responses vary consistent with breed, age and rearing zone in such how that younger chickens (1-5 weeks of age) particularly of those vaccinated at 1 or 2 weeks of age appear to be consistent with the relatively low humoral antibody response. Efficacy of a vaccine depends on many factors including the immunogenic characteristic of the vaccine strain. It’s widely accepted that a local strain having immunogenic value should be selected as the ideal vaccine strain to prepare effective vaccine to control a particular disease like fowl cholera [3].
All veterinary vaccines found to be effective in controlled condition should be tested for safety and if possible, for efficacy within the field, before being authorized for general or commercial use. Field studies are designed to demonstrate efficacy under working conditions and to detect unexpected reactions, including mortality which will not have been observed during the development of the product. Under field conditions there are many uncontrollable variables that make it difficult to get good efficacy data, but demonstration of safety is more reliable. Although inactivated fowl cholera vaccine is available elsewhere, the poultry enterprises in Ethiopia have experienced problems with the disease thanks to unavailability of the vaccine. The supply of an effective locally produced vaccine will reduce the dependence on imported vaccine, exchange of the country and maximize the vaccine production per year. To deal with this problem a prototype in-activated vaccine against fowl cholera had been developed at the National Veterinary Institute (NVI), Ethiopia where alum adjuvated prototype vaccine formulation given IM was found to supply effective protection under experimental condition but awaiting scaling-up and performance evaluation of the first batch of vaccine produced on large scale. This study was administered to scale-up the production the prototype inactivated fowl cholera vaccine developed at NVI and subsequently evaluate its safety and immunogenicity and protective efficacy both at on-station and field levels [4].
Materials and Methods
Study animals and their management
A total of 60 chickens (8-week-old Brown Bovans) were purchased for vaccine evaluation at the station. Chickens were examined and chickens without fowl cholera antibodies were included in the study. Chickens were fed the supplied formulated feed and drinking tap water adlibtum. The type of feed used was starter feed, hen feed and layer feed, which varied according to the age of the chicken during the experiment. All chickens used for the experiment were handled according to standard ethical guidelines after approval by the animal ethics committee of the college of veterinary medicine, Addis Ababa university. A total of 200 randomly selected laying hens (bovans brown breed) from a poultry farm in Bishoft were used for the field evaluation of the vaccine. For the purpose of the study, the chickens were kept in a separate compartment, although in the same house as the rest of the flock. The farm has a total population of 3000 chickens (including those used for the study) raised under the same feeding and management system [5].
Vaccine production
The vaccine was prepared according to NVI/SOP. Chemicals except glucose and serum used for fowl cholera vaccine production media were mixed together and the pH was measured using a pH meter and adjusted to 7.4 and then sterilized in an autoclave. After sterilization, prefiltered glucose and serum were added to the production medium. Sterilization of glucose and serum was performed with a pressure filter with a pore size of 0.22 μm in a class II biosafety cabinet. The total volumes of media used for the production of this vaccine were 32 liters.
Preparation of inoculum and production of fowl cholera
To prepare the inoculum, freeze-dried bacteria were diluted and allowed to grow on tryptose agar plates. After 24 hours of incubation at 37°C, clear colonies were grown on the agar plate. One colony was transferred to a 2 ml hemolytic tube containing tryptose medium and then incubated for 7 hours at 37°C and then checked for purity by gram staining. They were then transferred to 1 liter of fowl cholera inoculum medium and incubated overnight.
The purity of fowl cholera inoculum was aseptically checked by gram staining and inoculated into a vial of fowl cholera production medium, then incubated for 24 hours under agitation at 80 rpm. Bacterial growth was checked by examination of smears, turbidity and culture pH. The pH of the culture was between 5.2-5.8, which is the optimum pH value for inactivation purposes.
Culture inactivation
Pasteurella multocida suspensions produced with optimal growth were inactivated using a 40% formaldehyde solution (0.5% final concentration) at 37°C and maintained for 48 hours. The anaculture was then placed at 37°C for 72 h to complete inactivation and further processed for sterility, inactivation and safety tests [6].
Anaculture quality control
The anaculture was checked for purity and sterility by gram staining and culturing on sterility test media such as tryptose agar, tryptose broth, VF broth and Sabouraud agar medium. All test media were incubated at 37°C except Sabouraud agar, which was incubated at room temperature. Uninoculated medium from each type was also incubated as a negative control. All these inoculated media were observed for seven days for any microbial growth. The safety of anaculture was performed on laboratory animals. Three rabbits were injected intramuscularly with 1 mL of inactivated culture from each anaculture vial and observed for 14 days for any adverse reaction.
Vaccine adjuvant
In this experiment, aluminum potassium sulfate (Alum) was used as an adjuvant, and the ratio between culture and aluminum potassium sulfate was 10%. The vaccine was then divided into 50 ml vials according to NVI standard operating procedure. Finally, the quality control of the inactivated fowl cholera vaccine was evaluated using the same method as for anaculture [7].
Experimental design
The current research was conducted from November 2017 to April 2018 in NVI, Ethiopia. The study involved two phases, the first phase was the large-scale production of the first batch of fowl cholera vaccine according to the SOP developed during the development of the trial vaccine and the subsequent evaluation of vaccine safety, immunogenicity and protective efficacy against NVI in the workplace. In the vaccine evaluation at the station, the chickens were randomly divided into three groups (each group consisted of 20 chickens). Group I and II were inoculated with 0.5 ml and 1 ml of vaccine, respectively, while group III was kept as an unvaccinated control. Identification of each chicken was done using wing tags. The second phase was a field evaluation of the safety and immunogenicity of the vaccine at the Addis Ababa university college of veterinary medicine poultry farm on randomly selected 200 laying hens, reared in a separate area on the farm, grouped into two groups of 100 hens. One of the groups was vaccinated while the other was left as an unvaccinated control.
Vaccination-challenge experiment at the station: In the station experiment, chickens were acclimatized for one week in the NVI animal experimental facility, after which two groups were vaccinated with two different doses (0.5 ml and 1 ml) of the vaccine intramuscularly, while the third group was left as an unvaccinated control. Primary vaccination was given on day zero, followed by a booster dose on day 21 after the primary vaccination. Blood was collected from all groups (vaccinated and non-vaccinated controls) by exsanguination from the wing vein just before primary vaccination (day zero), on days 14, 21, 28 and 35 after primary vaccination for determination of serum antibody levels. About 2 ml of anticoagulant-free blood was collected with a 3 ml syringe from the wing vein of all experimental chickens and the syringe was held at an angle and the blood was allowed to clot at room temperature for one hour, after which the serum was collected. The serum was then centrifuged at 2000 rpm for 10 minutes and stored at -20°C until analysis. All experimental groups of chickens were monitored from the day of primary vaccination for any deviation from normal health status and the result was recorded [8].
Fourteen days after booster vaccination, all vaccinated and control groups were challenged with 1 ml of a virulent strain of P. multocida containing 1.67 × 108 cfu/ml by the intramuscular route according to previous studies. Chickens were monitored for 14 days post-challenge, during which any observed clinical signs were recorded.
Any dead chicken were autopsied and tissue samples (2 gm) from spleen, heart and liver was taken aseptically following standard protocols for re-isolation of P. multocida. Re-isolation and identification of P. multocida was done based on morphological study, staining properties, cultural and biochemical characteristics described previously. Molecular confirmation of the re-isolated bacteria was done using PCR targeting capsular biosynthesis gene (cap gene) of P. multocida.
Filed evaluation of the safety and immunogenicity of fowl cholera vaccine
Vaccination and sampling: In the field evaluation, 100 randomly selected layer chicken from a poultry farm were vaccinated with 0.5 ml of the vaccine intramuscularly twice at three weeks interval. Blood was collected just before vaccination, on day 21 and 35 post vaccination after which serum was recovered using similar method as in on-station study [9].
Determination of immune response: Sera samples were analyzed using Indirect Haemagglutination Test (IHA) as described by Sawada et al. Accordingly, sera of the immunized and control birds were collected and tested by IHA. Briefly, 90 μl of PBS was first poured in the first row and 50 μl each well up to 10th well of vertical row of 96 well microstate plate. 10 μl of test serum was added in the 1st well and tenfold dilutions of serum ranging from 1:10 to 1:1280 were made by transferring 50 μl of the mixture from the 1st well to 2nd well and thus continuing successively up to the 8th well from where an excess amount of 50 μl of the mixture was discarded. A volume of 50 μl of capsular antigen sensitized sheep RBC was taken in each of the ten wells. The content of the wells of the test system was mixed by gentle agitation of the micro titer plate and incubated at 37°C for 1 hour. The resulting haemagglutination that occurs due to coupling of the antigens to chemically modified erythrocytes that readily react with specific antibodies was observed and the results recorded [10].
Evaluation of the safety of fowl cholera vaccine at farm level: Evaluation of safety of the vaccine was done according to the OIE manual for vaccine safety. Vaccinated chickens were observed starting from time of vaccination up to 35 days on daily bases by professionals at the farm and any deviation from normal health such as depression, anorexia, ruffled feather and any reaction at the site of injection was recorded.
Data analysis
The data concerning safety, immunogenicity and challenge tests was entered in to Microsoft Excel spreadsheet and coded for analysis using Stata version-12.0. Descriptive statistics such as proportions and averages were used in summarizing quantitative data as required. The Analysis of Variance (ANOVA) was used to find out the differences in the mean antibody titers among immunized groups vaccinated with the different vaccine dosages and student’s t-test was used to compare the mean antibody titer within the groups. The desired level of precision and confidence level used for this study purpose was 5% and 95% respectively. Analysis of protective efficacy data from the challenge experiment was evaluated by determining Protective Index (PI) as described by.
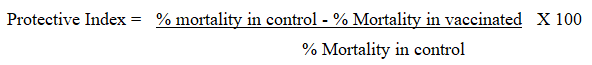
Results
Safety of the fowl cholera vaccine
Large scale produced fowl cholera vaccine using P. multocida production medium resulted in a vaccine with a titer of 2.5 × 108 cfu/ml. Observation of all the chickens vaccinated with the inactivated fowl cholera vaccine both in experimental study and field/farm site showed no abnormal reaction to the vaccine including at the site of injection indicating that the vaccine was 100% safe in both on-station and field condition.
Immunogenicity of the inactivated fowl cholera vaccine
Immunogenicity at on-station study: The pre vaccination mean of IHA titers of sera samples of all vaccinated and control chicken were less than two. After the primary vaccination, IHA antibody titers increased in both vaccinated groups. Booster vaccination (at day 21) resulted in rapid rise in IHA antibody titers in groups I and II. The mean IHA antibody titers in Group I were 1.6 ± 1.2, 211.3 ± 2.1, 244.5 ± 1.2, 319.8 ± 1.2 and 502.3 ± 1.2 at day 0, 14, 21, 28 and 35 post vaccination, respectively.
Similarly, in group II the mean IHA antibody titers at day 0, 14, 21, 28 and 35 post vaccination were 1.3 ± 1.2, 203.7± 3.0, 234.2.0 ± 1.2, 367.2 ± 1.2 and 452.9 ± 12 respectively. However, in group III the IHA antibody titers remained constant (Figure 1 and Table 1) [11].
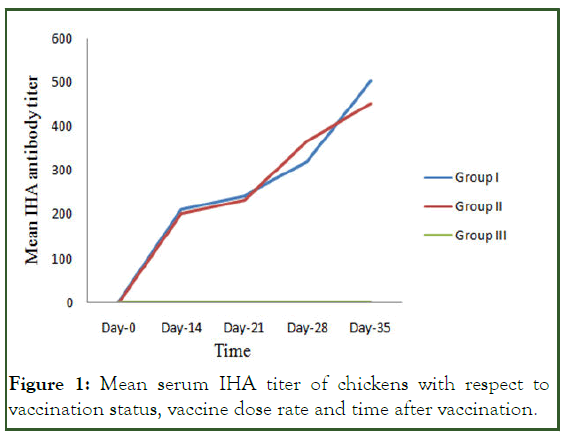
Figure 1: Mean serum IHA titer of chickens with respect to vaccination status, vaccine dose rate and time after vaccination.
| Time | Group (vaccination) | Mean ± SE | p-value |
|---|---|---|---|
| Day-0 | Group I | 1.6 ± 1.2 | 0.687 |
| Group II | 1.3 ± 1.2 | ||
| Group III | 1.4 ± 1.2 | ||
| Day-14 | Group I | 211.3 ± 2.1a | 0.0001 |
| Group II | 203.7 ± 3.0ac | ||
| Group III | 1.41 ± 2.0b | ||
| Day-21 | Group I | 244.5 ± 1.2a | 0.0001 |
| Group II | 234.2 ± 1.2ac | ||
| Group III | 1.41 ± 1.2b | ||
| Day-28 | Group I | 319.8 ± 1.2a | 0.0001 |
| Group II | 367.2 ± 1.2ac | ||
| Group III | 1.41 ± 1.2b | ||
| Day-35 | Group I | 502.3 ± 1.2a | 0.0001 |
| Group II | 452.9 ± 1.2ac | ||
| Group III | 1.4 ± 1.2b |
Table 1: Geometric (Mean ± SE) of IHA titer sera of chickens vaccinated with fowl cholera vaccine at two dose rates.
Immunogenicity in field evaluation
The results of mean IHA antibody titer of the field evaluation are presented in the Table 2. The pre vaccination means of IHA titers of sera samples of all vaccinated chickens were 4.2 ± 1.1. The primary vaccination improved the mean IHA antibody titers to 310.4 ± 1.0. Booster vaccination resulted a sharp increase in mean IHA antibody titers to 532.6+1.0. The mean IHA antibody titer of primary and booster vaccinations showed significant increase compared to the mean value of prevaccination titre (p<0.0001) (Table 2) [12].
| Time | Mean ± SE | p-value |
|---|---|---|
| Pre-vaccination(day 0) | 4.2 ± 1.1 | |
| Day-21 | 310.4 ± 1.0 | 0.0001® |
| Day-35 | 532.6 ± 1.0 |
Table 2: Geometric mean and standard error of IHA antibody titer at farm level.
Protective efficacy
In vaccination-challenge test, 90% of chicken survived in group I (no=20 vaccinated with 1mL) after challenge. Similar result was obtained in group II while only 20% survived from unvaccinated group (Table 3).
| Group | Chickens (#) | No of chicken survived (%) | No of chicken died (%) | Protective efficacy |
|---|---|---|---|---|
| I | 20 | 18 (90%) | 2 (10%) | 87.5% |
| II | 20 | 18 (90%) | 2 (10%) | 87.5% |
| III | 20 | 4 (20%) | 16 (80%) | - |
Table 3: Protective efficacy evaluation of inactivated fowl cholera vaccine.
The numbers of chickens showing clinical signs of fowl cholera were presented in Table 4. There is no significant difference in the number of chickens showing the clinical signs between vaccinated groups with only few chicken showing clinical signs (Table 4) while all the chickens in the control group had visible clinical signs consistent to fowl cholera. Death in unvaccinated control started as early as 3 days after challenge (Figure 2).
| Group and no of chickens | Dose | Greenish diarrhea | Lameness | Conjunctivitis | Ruffled feathers | Labor breathing | Total no of chickens showing any of the signs |
|---|---|---|---|---|---|---|---|
| I=20 | 1 ml | 2 | 3 | 2 | 2 | 2 | 3 |
| II=20 | 0.5 ml | 2 | 3 | 1 | 4 | 4 | 4 |
| III=20 | Control | 20 | 20 | 8 | 20 | 20 | 20 |
Table 4: Number of chicken showed clinical sign of fowl cholera, after post challenge dose.

Figure 2: Clinical sign of fowl cholera during challenge experiment in the control group.
Microbiological examination of chicken that died during the course of the study resulted the re-isolation of P. multocida from internal organs (liver, spleen and heart) which was subsequently identified both by phenotypic and PCR technique (Figure 3) [13].
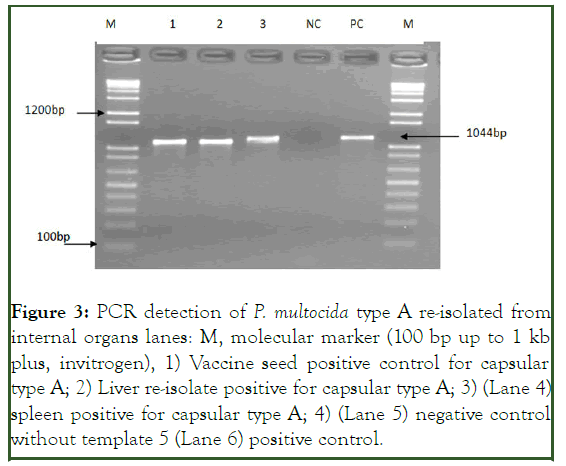
Figure 3: PCR detection of P. multocida type A re-isolated from internal organs lanes: M, molecular marker (100 bp up to 1 kb plus, invitrogen), 1) Vaccine seed positive control for capsular type A; 2) Liver re-isolate positive for capsular type A; 3) (Lane 4) spleen positive for capsular type A; 4) (Lane 5) negative control without template 5 (Lane 6) positive control.
Discussion
In this study, a higher antibody titer was observed in both groups vaccinated with different doses (1 ml and 0.5 ml) after vaccination, which is significantly different from the antibody titer on day 0. A similar result was reported by Islam et al. who reported that the IHA antibody titer in ducks immunized with fowl cholera vaccine was found to be significantly increased after vaccination. In group III (control), the IHA antibody titer value remained constant throughout the experiment, suggesting that the increase in antibody titer may be due to a specific antibody response to the vaccine (Table 1), although whether it is protective or not remains to be proven. The finding that a rapid significant rise in antibody titer after a booster dose up to 35 days with (P<0.001) compared to the rise from day 0 to day 21 is consistent with the findings of Modak et al., who also found statistically significant increase in antibody titer in chickens immunized with fowl cholera vaccine after secondary vaccination [14].
The absence of significant differences in IHA antibody titers between group I and group II (Table 1) indicates that vaccination using the 0.5 ml and 1 ml dose rates did not affect immunogenicity. Rather, the use of two doses (prime and booster) at different time intervals had a significant impact on the antibody response. According to immunized chickens with a formalin-killed fowl cholera vaccine with precipitated fowl cholera prepared with P. multocida and administered twice at a two-week interval and observed an increase in immunoglobulin levels in the doubly vaccinated groups of birds and similarly comparable results were observed in this study. Our finding is also consistent with who reported that two doses were required two to four weeks apart and in inactivated vaccines full immunity could not be achieved until approximately two weeks after the second dose of vaccine. primary vaccination. Similarly, in this study, a high immune response was observed after a booster dose, indicating the need for a booster dose in vaccination programs using inactivated fowl cholera vaccine [15].
In this study, the protective efficacy of the vaccine was further evaluated by exposing vaccinated and control chickens to a virulent strain of P. multocida isolated from a field outbreak. The immune responses of chickens vaccinated with 1 ml (group I) and 0.5 ml (group II) were similar in terms of protection against P. multocida, with 87.5% protective efficacy achieved in both vaccine doses, which is in accordance with IHA titers. The present result agreed with Islam et al. in which ducks immunized with fowl cholera vaccine showed a 90% survival rate after challenge infection within three weeks of vaccination.
The higher mortality recorded in the control group in this study was comparable to a previous study conducted in Ethiopia where 85% mortality was recorded in the control groups. Similarly, the clinical sign of fowl cholera in the control group observed during the exposure experiment agreed with previous studies. On the other hand, in the vaccinated groups, few chickens showed clinical signs of fowl cholera and mortality, indicating that the vaccine not only reduced mortality but also morbidity.
Similar findings in immune response and protective efficacy observed in groups of chickens vaccinated with different doses are in agreement with the reports of in which the immune responses of pigeons vaccinated with 0.4 ml/bird, 0.6 ml/bird, 0.8 ml/bird and 1 ml/bird were satisfactory in terms of the degree of protection against P. multocida. In addition, vaccination at a dose of 0.5 ml was observed to provide significant protection in terms of inducing an immune response as reported by where inoculation of 0.5 ml/bird. The fowl cholera vaccine induced significant serum antibody as determined by the indirect hemagglutination test. Considering the cost and use of the vaccine, vaccination with a lower dose of 0.5 ml was preferred for the field study.
Conclusion
The results of the immune response in the field study were similar to the station study, where the antibody titer increased significantly on day 21 after primary vaccination and with a sharp increase after booster (day 35) compared to the prevaccination titer. This indicates the need for a booster dose in vaccination programs using inactivated fowl cholera vaccine under field conditions. Similar findings were reported in a previous study in which vaccination with fowl cholera vaccine in poultry farms at doses of 0.5 ml produced higher levels of antibodies when a booster dose was given after the basic vaccination.
In conclusion, the current evaluation of a fowl cholera vaccine produced on a large scale at NVI from local isolates of P. multocida at the station and in the field showed that the vaccine is safe and immunogenic with a high level of protective efficacy (87.5%) against poultry. cholera in chicken. Use of the vaccine at a dose of 0.5 mL given IM twice at 3-week intervals provided an optimal immune response and protection, suggesting its potential use to prevent fowl cholera in Ethiopia.
References
- Akand MSI, Choudhury KA, Kabir SML, Sarkar SK, Amin KMR. Development of a cholera vaccine from washed poultry cells in Bangladesh. Int J Poul Sci. 2016;3:534-537.
- Akhtar M, Rahman MT, Ara MS, Rahman M, Nazir KH, Ahmed S, et al. Isolation of Pasteurella multocida from chickens, preparation of formalin-killed fowl cholera vaccine and determination of efficacy in experimental chickens. J Adv Veter Animal Res. 2016;3(2):45-50.
- Aye PP, Angrick EJ, Morishita TY, Harr BS. Prevalence and characteristics of Pasteurella multocida in commercial turkeys. Avian Dis. 2001;45(1):182-190.
- Bag MAS, Amin MM, Rahman MB, Arafat YA, Salim M, Rasel IH, et al. Immune response of fowl cholera vaccine produced at Bangladesh agricultural university at the farm level. Res Agr Live Fisher. 2002;2(1):13-107.
- Choudhury KA, Mondal SK, Rahman MM, Amin MM, Sarker AJ. Changes in leukocyte, total serum protein and immunoglobulin levels in chickens immunized against fowl cholera. Ban Veter J. 2006;2(5):27-30.
- Dana N, Dessie T, Vander WLH, van Arendonk JAM. Morphological traits of indigenous chicken populations of Ethiopia. Animal Gen Res. 2007;46(8):11-23.
- Dick JW, Avakian AP. Response of broiler chickens to fowl cholera vaccination at 1 to 6 weeks of age. Avian Dis. 1991;35 (3):761-766.
[Google Scholar] [PubMed]
- Islam MT, Ali1 MH, Chandra A, Saha S. Standardization of effective dose of fowl cholera vaccine in pigeons Bangladesh. J Vet Med. 2015;15(1):97-105.
- Islam MT, RahmanMk, RahmanMB, Siddiky ANM, Kafi Am, Ali MH, et al. Efficacy of formalin-killed fowl cholera vaccine in experimentally immunized Fayoumi chickens. Ban J Vet Med. 1991;2(4):23-25.
- Kardos G, Kiss I. Molecular epidemiological investigation of poultry outbreaks. J Clin Microbiol. 2006;43(1):2959-2961.
[Crossref] [Google Scholar] [PubMed]
- Marza AD, Abdullah FFJ, Ahmed IM, Chung ELT, Ibrahim HH, Zamri-Saad M, Omar AR, et al. Involvement of the 5 nervous system as a result of 2001 nervous system infection B:2. A review of clinicopathological and pathophysiological changes. J Adv Vet Animal Res. 2006;2(7):252-226.
- Modak M, Amin MM, Saha S, Hassan J. Immunogenicity of BAU-bivalent Salmonella and fowl cholera vaccine in Shuvra chickens. Micro Health. 2006;1(4):50-53.
- Hassen M. The Oromo of Ethiopia: A history, 1570-1860. Cambridge University Press, London, 1990.
- Murithi T. Practical peacemaking wisdom from Africa: Reflections on Ubuntu. J Pan Afr Stud. 2006;1(4):25-34.
- Bakwesegha CJ. The role of the organization of African unity in conflict prevention, management and resolution. Int J Ref. 1995;7(6):207.
Citation: Wolde ZT, Mamo G, Gelaye E, Abayneh T, Shuka J (2023) On-Station and Field Evaluation of Inactivated Fowl Cholera Vaccine Produced from Local Pasteurella multocida Isolates, Ethiopia. J Bacteriol Parasitol. 14:484.
Copyright: © 2023 Wolde ZT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

