Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Publons
- International committee of medical journals editors (ICMJE)
- Geneva Foundation for Medical Education and Research
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 26, Issue 7
Null Hypothesis Proven in Sebum Infectant to Immune Reflex through Sebaceous Immunobiology by COVID-19 Vaccine
Yang I. Pachankis*Received: 12-Jul-2023, Manuscript No. JOP-23-22184; Editor assigned: 14-Jul-2023, Pre QC No. JOP-23-22184 (PQ); Reviewed: 28-Jul-2023, QC No. JOP-23-22184; Revised: 04-Aug-2023, Manuscript No. JOP-23-22184 (R); Published: 11-Aug-2023, DOI: 10.35248/2378-5756.23.26.615
Abstract
Background: The completed interventional trial was conceived from the phenomena of COVID-19 (Coronavirus Disease 2019) post-vaccination adverse events. The accumulated evidence has proven the null hypothesis with significant results that falsify the predominant belief in the vaccination method. The alternative hypothesis is adjusted with proton equilibrium and sebaceous immunobiology’s correlations with immune reflex.
Methods: The sole-participant interventional trial compared the main medicines in myocarditis treatment from Nifedipine to Angiotensin-Converting Enzyme Inhibitor (ACEI) and Angiotensin Receptor-Neprilysin Inhibitor (ANRI) with increasing power level. T values and Z statistics are calculated for statistical analysis, and the introduction of proton-pump inhibitor is uncertain with the case’s neurodivergent conditions.
Result: Inter-ACEI comparison suggests the introduction of beta blockers regulated the immune reflex through heart rate with the blood-borne pathogen. ANRI superiority suggests S2 pathogens can be more severe without S1 constraints, and raises alerts on SARS-CoV-2 (Severe Acute Respiratory Syndrome-Coronavirus-2) mutational directions from Omicron. Historic data from the participant after the second COVID-19 vaccine shot recorded the viral entry through Low-Density Lipoprotein Cholesterol (LDL-C).
Discussion: The study protocol with data refers to SARS-CoV-2’s S2 infection concentration and viral characteristics in LDL-C in human host. It is highly probable that S2 pathogen starts with LDL-C in vaccine poisoning. It is possible that HDL-C levels are responsible for the cytokine storms in neurologically infected cases. The placebo effect is maximized by the vaccine mandates, and the mass psychological biases need time to be narrowed down.
Trial registration: The study protocol is registered on ClinicalTrials.gov with the identifier number NCT05711810. Following trial on the hypothalamic-pituitary-adrenal axis is registered with the identifier number NCT05930912.
Keywords
COVID-19; Immune deficiency; Fusogenicity; LDL-C; Natural immunity; Neurodiversity; Proton therapy; Vaccination poisoning; SARS-Cov-2; Sebum
Background
Vaccines used to be believed to be the most and only effective method in dealing with the public health emergencies against infectious diseases. The virological features of SARS-CoV-2 incentivized the vaccine developments to Spike 1 (S1) protein targeting in avoiding the HIV-1 (Human immunodeficiency virus) gp41 structured Spike 2 (S2) protein [1,2]. Against the Nuremberg Codes, Emergency use Authorization not only put COVID-19 vaccines in the markets, but also incentivized a global mandate of vaccination with the premise of restricting civil freedoms of the unvaccinated [3,4].
Practice from mandated vaccinations emerged numerous sudden deaths that reasonably raised doubts from the ethical medicare professionals. SARS-CoV series are still categorized as respiratory viruses despite of the contradictory evidences from its nuclear structure, pathogenesis etc. [5-7]. If SARS-CoV-2 vaccination was effective from its designs, why post-vaccination sudden deaths mainly symptomize from acute myocarditis; if they weren’t effective, why antibodies exist in post-vaccination myocarditis cases [8-10].
The interventional trial was conceived from the phenomena of post-vaccination adverse events, and retrospectively registered after the primary efficacy point due to the emergent intervention that started the trial. The aim of the interventional trial purposed to reduce the risks for post-vaccination sudden deaths and prevent the potentials for neurological infections based on the analysis [7]. The interventional strategies originated from the transmembrane domain’s relevance to the proton equilibrium in immune reflex [11-19].
Even though the primary purpose of the study is to salvage from the post-vaccination complexities, the alternative hypothesis’ corroborations to the null hypothesis have been furthering the causal inference from the initial study protocol. The null hypothesis of the research is that, SARS-CoV-2’s S1 and S2 proteins can independently induce pathogens in human hosts, and underlie the reasons on post-vaccination sudden deaths. The alternative hypothesis is that proton therapy can be delivered with Angiotensin-Converting Enzyme Inhibitor (ACEI) to reduce the S2 binding activities in the blood, and prevent potential neurological infections. The alternative hypothesis is currently adjusted with Angiotensin Receptor-Neprilysin Inhibitor (ARNI) in the follow-up studies, for its superiority in power level on the blood pressure and heart rate control for the discrete purpose of decreasing sudden death risks [20].
There’re few literatures on the correlations between Low- Density Lipoprotein Cholesterol (LDL-C) levels and SARSCoV- 2 pathogenicity, even though sebum levels and COVID-19 cases suggest a causal relationship. There is a knowledge gap in the biological functions of sebum in medical and biochemical researches. Recent literature started to draw the correlations between sebum and the nerve system with the concept of sebaceous immunobiology [21,22]. The conceptual framework is not only partially explainable to the power level superiority from receptor inhibition in the alternative hypothesis of the study design, but also shed new light on the readings of the participant’s historic data obtained after the second SARS-CoV-2 vaccine shot through muscle subcutaneous tissues [23]. Vaccination information is seen in Table 1.
| Dose | Date | Lot Number | Manufacturer | Site of injection | Adjuvants |
|---|---|---|---|---|---|
| 1 | Apr 14, 2021 | 2.02E+08 | Anhui Zhifei Longcom Biopharmaceutical co., Ltd. | Chongqing Bishan People’s Hospital (COVID-19 Temporary Vaccination Site) | Chinese hamster ovary cell |
| 2 | May 31, 2021 | A202105069 | |||
| 3 | Jul. 13, 2021 | B202106098 |
Table 1: Details of the participant’s vaccination (34, Han Chinese, male, 130 kg, smoking)
The significance of the work contributes to the more precise evidence on COVID-19 vaccination poisoning, and the finding’s potentials in furthering the discoveries for treatment plans both for the vaccinated and unvaccinated that are prone to infection [24]. In the cancer screening physical examinations from the participant’s historic records, the participant also tested for HIV with chemiluminescence method with the historic data seen in Table 2. The slight level of HIV chemiluminescence could have originated from the methodological bias with the structural similarities between SARS-CoV series’ S2 protein and HIV-1 gp41 (Glycoprotein 41), and the data interpretation is not without precedence [1,25]. For the clarities of the methods reading, where the initial baseline was set to the emergent intervention hematology, the renewed baseline is seen in Table 2 [26,27].
| Indicator | Baseline | Reference range | Unit | Relevant indicator | Baseline | Reference range | Unit |
|---|---|---|---|---|---|---|---|
| WBC | 6.51 | 3.5-9.5 | 10^9/L | ||||
| NEU# | 4.03 | 1.8-6.3 | 10^9/L | Neu% | 61.8 | 40-75 | % |
| LYM# | 1.68 | 1.1-3.2 | 10^9/L | Lym% | 25.9 | 20-50 | % |
| MONO# | 0.32 | 0.10-0.60 | 10^9/L | Mon% | 4.9 | 03-Oct | % |
| EOS# | 0.46 | 0.02-0.52 | 10^9/L | Eos% | 7.1 | 0.4-8.0 | % |
| BASO# | 0.02 | 0.05-0.10 | 10^9/L | Bas% | 0.3 | 0-1 | % |
| RBC | 5.18 | 4.3-5.8 | 10^12/L | ||||
| tHb | 160 | 130-175 | g/L | Hct. | 46.7 | 40-50 | % |
| MCV | 90.2 | 82-100 | fL | ||||
| MCH | 30.9 | 27-34 | pg | MCHC | 343 | 316-354 | g/L |
| RDW-CV | 15.3 | 11.0-16.0 | % | RDW-SD | 45.5 | 35-56 | fL |
| PLT | 207 | 125-350 | 10^9/L | PCT | 0.2 | 0.108-0.282 | % |
| PDW | 16.4 | 15-17 | fL | MPV | 9.4 | 07-Nov | fL |
| HIV | 0.08 | 0.00-1.00 | S/CO | Method | Chemiluminescence | ||
| ALT | 16 | Sep-50 | U/L | AST | 24 | Dec-37 | U/L |
| ALP | 85 | 45-125 | U/L | Γ-GGT | 19 | Oct-60 | U/L |
| TP | 75.7 | 65-85 | g/L | ALB | 47.4 | 40-55 | g/L |
| GLO | 28.3 | 20-40 | g/L | A/G | 1.67 | 1.2-2.4 | |
| TBil | 11.5 | 0-26 | umol/L | DBil | 1.8 | 0-8 | umol/L |
| UREA | 5.04 | 3.1-8.0 | mmol/L | CREA | 83 | 57-97 | umol/L |
| UA | 471 | 208-428 | umol/L | TC | 5.2 | 0-5.18 | mmol/L |
| TG | 1.92 | 0-1.7 | mmol/L | HDL-C | 1.2 | 1.16-1.42 | mmol/L |
| LDL-C | 3.08 | 0-3.37 | mmol/L | GLU | 5.18 | 3.9-6.1 | mmol/L |
| CEA | 1.38 | 0-5 | ng/ml | AFP | 2.51 | 0-7 | ng/ml |
| CA19-9 | 7.36 | 0-27 | U/ml | CA125 | 7.86 | 0-35 | U/ml |
| CA15-3 | 6.93 | 0-25 | U/ml | TPSA | 0.37 | 0-4 | ng/ml |
Table 2: The participant’s HIV test, haematology, and cancer screening test results not long after the second COVID-19 vaccine shot with slight acidification indication.
Methodology
The interventional trial aimed to reduce the risks of sudden death and neurological infections from COVID-19 vaccination. It started with an emergent intervention contributed by the participant’s suddenly increased risks from the pericarditis symptoms to acute myocarditis [27]. The sole participant of the trial, 35-year old Asian male, is confirmed to be autistic with relatively high possibilities in Attention-Deficit/Hyperactivity Disorder (ADHD) and Obsessive- Compulsive Disorder (OCD) side-symptoms [28,29].
The interventional design is two dimensional on the intervention timeline. The participant centered dimension treats with the aim in improving the participant’s health, and the time dimension from the process is dissected into trials for data collection in assessing the interventional medication efficacies and readjustments, with a placebo trial included for the secondary efficacy endpoint for the initial design’s neurotypical presumptions. The study schema is seen in Figure 1, and the study process strives to balance between the neurodivergent case and generalizability of the study.
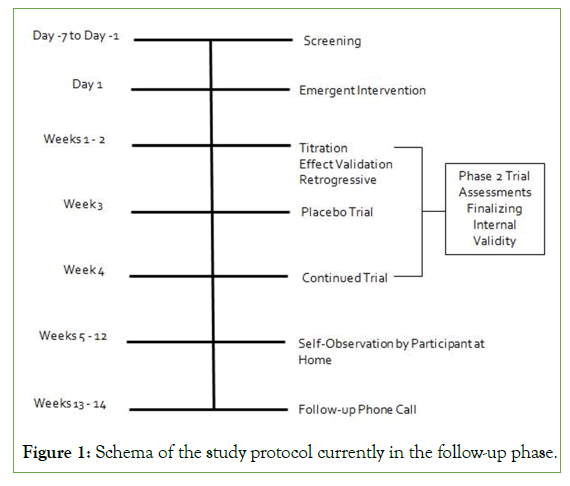
Figure 1: Schema of the study protocol currently in the follow-up phase.
The main interventional medicines tested in the study include Nifedipine (30 mg per day), ACEI (Enalapril and later Ramipril, from 5 mg to 20 mg per night and from 5 mg to 10 mg per night, respectively), and ARNI (Sacubitril Valsartan Sodium 100 mg separated in day and night time every day). Beta blocker (Metoprolol from 23.75 mg to 95 mg per day) is used for heart rate control along with the aforementioned comparison medicines, except for with Nifedipine, and dose-dependent effects have been seen in the intervention. Proton-Pump Inhibitor (PPI) is used from 15 mg to 30 mg per day with coordinated blood peak with ACEIs and ARNI. No apparent effects have been observed with or without the use of PPI in the combination. There is an ambiguity in the risks and benefits on the use of PPI in neurotypical persons and the neurodivergent case [19,30-35].
Apart from the qualitative hematology indicators in the continuity of the participant’s health, the main data obtained from the study design is blood pressure and heart rate. The power level of the indicators is evaluated horizontally as interrelated elements, and vertically on an individual basis for the immune-reflex-stasis indications from the effects of medicines. Table 3 shows the power level obtained from the main medicine comparisons without cut- off for sampling bias.
| Medicine | Sample size | Power Level | T-Value (one-sided) | |||||
|---|---|---|---|---|---|---|---|---|
| Overall | SBP | DBP | Heart rate | SBP | DBP | Heart rate | ||
| Placebo | 8 | 0% | 12.50% | 50% | 62.50% | 0.01—0.005 | 0.025—0.01 | 0.025—0.01 |
| Nifedipine | 10 | 0% | 10% | 60% | 40% | 0.01—0.005 | 0.025—0.01 | 0.01—0.005 |
| Enalapril | 58 | 36.21% | 43.10% | 51.72% | 84.48% | 0.005—0.0025 | 0.005—0.0025 | 0.01—0.005 |
| 34 | 58.82% | 64.71% | 91.18% | 97.06% | 0.005—0.0025 | 0.005— 0.0025 | 0.01—0.005 | |
| Ramipril | 16 | 50% | 81.25% | 93.75% | 62.50% | 0.005— 0.0025 | 0.025—0.01 | 0.025—0.01 |
| Sacubitril Valsartan | 27 | 77.78% | 100% | 100% | 77.78% | 0.01—0.005 | 0.025— 0.01 | 0.0025—0.001 |
Table 3: The superiority tests of medicine efficacy
Type I error rate is set at 5% with 80% power level. Power level is
defined as the sampled blood pressures within the normal healthy
range within 120/80/90 and above 90/60/60 to the total sampled
events within each time period. Mean variance of each period is
calculated, with sample variance 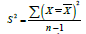 [36]. Z statistics is used to determine the single-participant p value:
[36]. Z statistics is used to determine the single-participant p value:
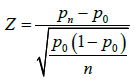
The primary efficacy point is used for the aggregated initial p-value
in evaluating inter-phase efficacy. The baseline from the emergent intervention is calculated 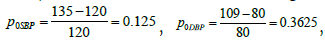
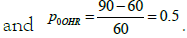 Therefore, it is calculated that p0 = 0.3621.
Therefore, it is calculated that p0 = 0.3621.
Statistic tests are conducted in every endpoint. It means that the statistical method uses interim analyses to test the alternative hypothesis with the statistical changes variable to intervention methods adopted. Therefore, Type I error and Type II error testing belong to different axes with the same set of data. Adverse events are seen in Table 4.
| Category | Type | Specificity | Affected | Related to intervention | # Events |
|---|---|---|---|---|---|
| All-cause morality | N | N | |||
| Serious Adverse Events | Cardiac Disorders | Acute Myocarditis | N | N | |
| Cardiomegaly | N | Y | |||
| Heart Failure | N | P | |||
| Infections and infestations | Partial Organ Failure | N | P | ||
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Cancer | N | N | ||
| Nervous system disorders | Neurological Infection | N | N | ||
| Epilepsy and Seizure | N | Y | |||
| Adverse Events | Blood and lymphatic system disorders | Leukocyte Disorder | Y | N | 1 |
| Cardiac Disorders | Irregular Heart Rhythms | Y | Y | 5 | |
| Pericarditis | Y | N | 5 | ||
| Endocrine disorders | Pancreas and Thyroid gland | Y | P | 8 | |
| Infections and infestations | Kidney Sore | Y | Y | 1 | |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Polyps | Y | N | 10 | |
| Psychiatric disorders | Migraine | Y | P | 5 | |
| Respiratory, thoracic and mediastinal disorders | Asthma and Coughing | Y | P | 10 | |
| Skin and subcutaneous tissue disorders | Arthritis | Y | N | 5 | |
| Vascular disorders | Y | N | 5 | ||
Table 4: Adverse events in the interventional trial
Results
The original design with ACEI and beta blocker has significantly decreased the mortality risk of the participant, while the effects of PPI remain uncertain. The study design is compatible with neurodivergent treatment medicines, and it is inferred that mild and moderate adverse events may occur less in neurotypical persons. Epilepsy and seizure risks mainly apply to neurodivergent persons. The introduction of PPI is highly plausible in the immune reflex regulation interventions if not the transmembrane fusogenicity treatment [19].
Inter-ACEI comparison suggests the introduction of beta blockers regulated the immune reflex through heart rate with the blood-borne pathogen. Even though Ramipril shows superiority over Enalapril on Diastolic Blood Pressure control, its inferior performance pharmaco-kinetically with beta blockers suggests: 1) The pathogens from the veins have not circulated past the blood-brain barrier; 2) Cardiac barrier is next to neurological infection without the effects of S1 protein’s targeting on angiotensin-converting enzyme; 3) The infections came directly from vaccine shots, indicated by asthma and coughing without effects from cytokine storm [37-39].
The superiority of ARNI in the combination suggests immune deficiency pathogen is enhanced without the S1 protein. The respiratory symptoms from COVID-19 are not the lethal pathogen of the virus. New animal experiment suggests pulmonary fibrosis is a result from neurological infection, and the side products from natural immune responses in the neuronal recovery process [39,40]. Therefore, the Omicron variant may pose more profound concerns in SARS-CoV-2’s mutation directions [18].
From the retrospective analyses on the participant’s historic data, not only is the null hypothesis evidenced, but also is the transmembrane fusogenicity of the pathogens in the case by vaccine poisoning. The reversal causality in the LDL-C indicator for prognosis of severe and critical COVID-19 risks results from the differences between infection paths of poisoning and natural infection, explaining the rapid acidification origination from the circulatory tract in the case seen in [4]. The historic data provides the internal validity in the PPI use in the study design [19,21,22,33,41-46].
Discussions and Conclusion
The study protocol with data refers to SARS-CoV-2’s S2 infection concentration and viral characteristics in LDL-C in human host. Dyslipidemia’s associations with the severity and mortality risks in COVID-19 imply the correlations between sebaceous immunobiology and Rhesus factor, more specifically the nuclear force dynamics between sebum and antigens that drive the immune reflexes’ functions against pathogens.
The proven null hypothesis indicates to the lipoprotein tract in SARS-CoV-2 S2 pathogens in the transmembrane domain and immune attacks in human host. Even though the causal inference between LDL-C and High-Density Lipoprotein Cholesterol (HDL-C) looks contradictory in different statistical cases, the study suggests it is highly probable that S2 pathogen starts with LDL-C with the historic data implications.
The HDL-C and LDL-C correlations in COVID-19 severity and mortality risks may be associated with the depths of immune attacks. Higher levels of LDL-C with lower levels of HDL-C may be a positive sign in initial infections, but negative in blood-borne pathogenicity. It is possible that HDL-C levels are responsible for the cytokine storms in neurologically infected cases.
Even though the power level has nearly been reached so far in the study design for the alternative hypothesis, the study design’s antiviral capacities are still uncertain. The discrete empiricist approach in the study protocol can be effective in reducing mortality in vaccine poisoning and possibly in early SARS-CoV-2 patients. The internal validity reached in the study protocol’s alternative hypothesis suggests SARS-CoV-2 treatment research and solution designs ought to concentrate on the defense for natural immunity and neurological regulations.
Without robust superiority studies between vaccination and natural immunity, the interventional trial implies that COVID-19 vaccination ought to be a choice instead of governmental mandates with a premise of coercion in restricting civil freedoms. The placebo effect is maximized by the vaccine mandates, and the mass psychological biases need time to be narrowed down.
Declarations
Ethics approval and consent to participate
The ethics committee Yang I. Pachankis assisted by Mingfang Cao approved the study designs and interventional trial. Consent was obtained from the participant.
Consent for publication
Consent for publication was obtained from the participant and the published data are deidentified.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the Open Science Framework repository with the DOI: 10.17605/OSF.IO/2MGJK.
Competing interests
The author receives royalties from Lambert Academic Publishing with the book Questions of COVID-19 cited.
Funding
There are no sources of funding to declare.
Authors’ contributions
YIP contributed to the conception and design of the work; the acquisition, analysis, and interpretation of data; and has drafted the work.
Acknowledgments
The author is thankful for Mingfang Cao’s valuable input that discretely prevented him from compulsive behaviors during the studies while respecting his individuality. The author is also thankful for the “Defeat the Mandates in D.C.” doctors that incentivized the study and actions of the author. Gratitude to the generous publication support from the Journal of Psychiatry.
References
- Zhang XW, Yap YL. Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. J Mol Struct Theochem. 2004;677(1-3):73-6.
- Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28.
- Ghooi RB. The Nuremberg code–a critique. Perspect Clin Res. 2011;2(2):72.
[Google Scholar] [PubMed]
- Fischer W, Giorgi EE, Chakraborty S, Nguyen K, Bhattacharya T, Theiler J, et al. HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe. 2021;29(7):1093-110.
- Morens DM, Taubenberger JK, Fauci AS. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host and Microbe. 2023;31(1):146-57.
- Pachankis Y. Questions of COVID-19: Institutional Derogations of Global Health. Lambert Academic Publishing. 2023.
- Haynes BF, Wiehe K, Borrow P, Saunders KO, Korber B, Wagh K, et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat Rev Immunol. 2022;23(3):142-58.
- Husby A, Gulseth HL, Hovi P, Hansen JV, Pihlström N, Gunnes N, et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNA vaccination in four Nordic countries: Population based cohort study. BMJ Medicine. 2023;2(1).
- Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, et al. Circulating spike protein detected in post–COVID-19 mRNA vaccine myocarditis. Circulation. 2023;147(11):867-76.
- Qiang W, Sun Y, Weliky DP. A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proc Natl Acad Sci. 2009;106(36):15314-9.
- Leaman DP, Zwick MB. Increased functional stability and homogeneity of viral envelope spikes through directed evolution. PLoS Pathogens. 2013;9(2):e1003184.
- Khan N, Chen X, Geiger JD. Role of endolysosomes in severe acute respiratory syndrome coronavirus-2 infection and coronavirus disease 2019 pathogenesis: implications for potential treatments. Front Pharmacol. 2020;11:595888.
- Petersen OH, Gerasimenko OV, Gerasimenko JV. Endocytic uptake of SARS-CoV-2: the critical roles of pH, Ca2+, and NAADP. Function. 2020;1(1):zqaa003.
- Ragia G, Manolopoulos VG. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur J Clin Pharmacol. 2020;76:1623-1630.
- Avolio E, Carrabba M, Milligan R, Kavanagh WM, Beltrami AP, Gupta K, e al. The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci. 2021;135(24):2667-89.
- Saito A, Irie T, Suzuki R, Maemura T, Nasser H, Uriu K, et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2021;602(7896):300-306.
- Meng B, Abdullahi A, Ferreira IA, Goonawardane N, Saito A, Kimura I, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706-14.
- Pachankis Y. Proton paths of cardiac immune reflex. Online J Cardiol Res Rep. 2023;7:1-3.
- Pachankis YI. The Pro-life ethics in palliative medicine. J Cri Res and Eme Med. 2023;2(1):1-2.
- Lovászi M, Szegedi A, Zouboulis CC, Törőcsik D. Sebaceous-immunobiology is orchestrated by sebum lipids. Derm Endocrinol. 2017;9(1):e1375636.
- Zouboulis CC, Coenye T, He L, Kabashima K, Kobayashi T, Niemann C, et al. Sebaceous immunobiology-skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front Immunol. 2022;13:1029818.
- Spick M, Longman K, Frampas C, Lewis H, Costa C, Walters DD, et al. Changes to the sebum lipidome upon COVID-19 infection observed via rapid sampling from the skin. EClinicalMedicine. 2021;33.
- Goswami D. K.(2022). Poison as discussed by susruta, the father of surgery. Biomed Sci Clin Res. 2022;1(1):18-20.
- Salih RQ, Salih GA, Abdulla BA, Ahmed AD, Mohammed HR, Kakamad FH, et al. False-positive HIV in a patient with SARS-CoV-2 infection; a case report. Ann Med Surg. 2021;71:103027.
- Kreutzberger AJ, Sanyal A, Saminathan A, Bloyet LM, Stumpf S, Liu Z, et al. SARS-CoV-2 requires acidic pH to infect cells. Proc Natl Acad Sci. 2022;119(38):e2209514119.
- Pachankis YI. Cardiac transfer of SARS-CoV-2 spike protein circulation techniques—medicine Induce d hemodialysis on “Vaccinated” immune attacks. Biomed Sci Clin Res. 2023 Feb 6;2(1):86-93.
- Parellada M, Penzol MJ, Pina L, Moreno C, González-Vioque E, Zalsman G, et al. The neurobiology of autism spectrum disorders. Eur Psychiatry. 2014;29(1):9-11.
- Seneff S, Kyriakopoulos AM, Nigh G, McCullough PA, Kyriakopoulos A. A potential role of the spike protein in neurodegenerative diseases: A narrative review. Cureus. 2023;15(2) :e34872.
- Pisliakov AV, Sharma PK, Chu ZT, Haranczyk M, Warshel A. Electrostatic basis for the unidirectionality of the primary proton transfer in cytochrome c oxidase. Proc Natl Acad Sci. 2008;105(22):7726-31.
- Tabares L, Betz B. Multiple functions of the vesicular proton pump in nerve terminals. Neuron. 2010;68(6):1020-2.
- Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-74.
- Anandakrishnan R, Zuckerman DM. Biophysical comparison of ATP-driven proton pumping mechanisms suggests a kinetic advantage for the rotary process depending on coupling ratio. PloS one. 2017;12(3):e0173500.
- Ortiz-Guerrero G, Amador-Muñoz D, Calderón-Ospina CA, López-Fuentes D, Nava Mesa MO. Proton pump inhibitors and dementia: Physiopathological mechanisms and clinical consequences. Neural Plast. 2018;2018.
- Lee JW. Electrostatically localized proton bioenergetics: Better understanding membrane potential. Heliyon. 2019;5(7).
- Bhandari P. How to calculate variance|calculator, analysis and examples. Scribbr. 2023
- Yang X, He Z, Hu R, Yan J, Zhang Q, Li B, et al. Dietary β-carotene on postpartum uterine recovery in mice: Crosstalk between gut microbiota and inflammation. Front Immunol. 2021;12:744425.
- Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; What we know so far. Front Immunol. 2020:1446.
- Naghynajadfard Jr M. The study of fibrotic scar at the long term spinal cord lesion rats. bioRxiv. 2023:2023-01.
- Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mikael TM, et al. Post COVID-19 pulmonary fibrosis; A meta-analysis study. Ann Med Surg. 2022;77:103590.
- Zhao M, Luo Z, He H, Shen B, Liang J, Zhang J, et al. Decreased low-density lipoprotein cholesterol level indicates poor prognosis of severe and critical COVID-19 patients: A retrospective, single-center study. Front Med. 2021;8:585851.
- Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V-ATPases. J Exp Biol. 2000;203(1):89-95.
- Zeng WZ, Xu TL. Proton production, regulation and pathophysiological roles in the mammalian brain. Neurosci Bull. 2012;28:1-3.
- DeCoursey TE. Voltage-gated proton channels: Molecular biology, physiology, and pathophysiology of the HV family. Physiol Rev. 2013;93(2):599-652.
- By S, Barry RL, Smith AK, Lyttle BD, Box BA, Bagnato FR, et al. Amide proton transfer CEST of the cervical spinal cord in multiple sclerosis patients at 3T. Magn Reson Med. 2018;79(2):806-14.
- Berry BJ, Trewin AJ, Amitrano AM, Kim M, Wojtovich AP. Use the protonmotive force: Mitochondrial uncoupling and reactive oxygen species. J Mol Biol. 2018;430(21):3873-91.
- Pachankis Y. Jeopardies in human security and politicization of COVID-19. 2022.
Citation: Pachankis YI (2023) Null Hypothesis Proven in Sebum Infectant to Immune Reflex through Sebaceous Immunobiology by COVID-19 Vaccine. J Psychia. 26:615.
Copyright: © 2023 Pachankis YI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.