Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2020) Volume 10, Issue 2
Membrane Bases Electrolysis
Natasha Watson*Received: 05-Jul-2020 Published: 24-Jul-2020, DOI: 10.35248/2155-9589.2020.10.208
Abstract
Nanoscale materials are of basic enthusiasm to the material researchers. The property of mass polymeric material which can change by atomic control in the nano scale locale rather than the basic alteration by substance response or physical methods could have incredible potential for cutting edge layer division applications.
Keywords
Membrane; Polymer; Technology
Introduction
The charge moves at the strong/fluid interfaces speak to either a wellspring of vitality or capacity of vitality. The two procedures are antipolar also, indistinguishable. The electrochemical frameworks, including the unique instances of bioelectrochemical frameworks, are shut electrical circuits wherevitality is taken from the framework or put away in the framework and Kirchoffs Law applies to shut circuits, electrochemical just as electrical. There is an anodic interface at which oxidation happens where electrons cause a valence change followed by the electrons going through an electronic way and arriving at the cathode where the interfacial procedure includes decrease. At last, the species delivered relocates through the arrangement (in any event, when a layer is available) to the cathode interface. Kirchhoff requires the pace of oxidation/ decrease be equivalent to the transport of charged particles through the arrangement and to the current in the outside all be equivalent. A layer between anodes doesn't have an impact upon the capability of the framework, yet can influence conduct of the framework. As an model, the save type auxiliary silver oxide/zinc battery has a ellulosic layer isolating the anodes. The actuation by presentation of electrolyte empowers the battery to be released quickly in light of the fact that the cellulosic layer separator becomes conductive despite the fact that the measures of electrolyte in every compartment may not be upgraded. Along these lines it is important to consider the vehicle of charge through the film just as through the electrolyte. Ohms Law unquestionably applies to electrochemical frameworks too as to electrical frameworks, however it must be perceived that the reason and impact connection between the potential and flow in electrical frameworks, just as the potential and ionic motion in electrochemical frameworks relies on the idea of the conductors between the terminals including the films. While the above contemplations seem self-evident, there are significant occurrences where they were not utilized. One is the film potential as utilized by Goldman and as second occurrence is the transference number as characterized by Hittorf. Goldman's suspicion of a expected circulation in the electrolyte has been examined beforehand The idea of transference number is an object of this present conversation. It is important to begin the thought of transference by examination of the fundamental work of Sir William Grove, the originator of what we currently perceive as a hydrogen/ oxygen energy unit battery. His work began with the electrolysis of a watery H2SO4 arrangement at platinum metal cathodes. The H2SO4 expands the conductivity of the electrolyte with the goal that electrolysis may continue at sensible rates in despite the fact that the SO4 particle isn't reducible under the conditions utilized. The continuous procedures in the electrolytic arrangement might be composed as follows:
H2SO4 ↔ 2H+ + SO4
2H+ ↔ H2O + 2H3O+
The portable ionic current transporter is the hydrated proton which is fit for response at the two cathodes, viz.:
Cathode electrolysis: 2H2O+ + 2e- → 2H2O + H2
Anode electrolysis: 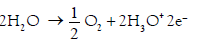
When composed this ay just a single animal groups is included at the terminals, and it is the sole bearer of charge from one terminal to the next. Forest had the option to arrangement three of the electrolyzed cells to make a battery competent causing the electrolysis response appeared above, in this manner procuring the title of "Father of the Fuel Cell" battery. The finding of the motion of the hydrated hydrogen particle is steady with the finding in other optional battery frameworks, for example, [1] silver oxide/ zinc, [2] nickel oxide/cadmium and [3] the normal lead corrosive batteries. Apparently a general guideline is that a solitary ionic species is answerable for the vehicle of charge in the arrangement stage of electrochemical frameworks. Consequently the "Hittorf transference number" is solidarity. In this manner, utilizing techniques to derive a "transference number measure something different, which presumably clarifies the contradictions detailed in the writing for transference numbers [4].
It is concluded that the procedure at the interfaces of strong/fluid can't be overlooked nor disregarded electrochemical frameworks even those respected as electro-active procedure, for example electrophoresis or spilling potential or the others distinguished in the IUPAQC Technical Report. Along these lines, finding the ionic conduction in every single electrochemical procedure to be solidarity clarifies the trouble in understanding between techniques to decide the Hittorf transference number. It is noticed that the pace of response among hydrogen and oxygen can run from and blast, when combined stoichiometrically what's more, lighted as in a Parr Bomb, to a fire where a separator for mass hydrogen is stripped away, as in the Hindenburg Zeppelin calamity (it took 36 seconds from first start to simply consuming of the cotton/cloth covering at the tail) or to a controlled response as in a Woods type power device. From the thermo compound information with a Parr Bomb there is a desire for 1.229 volts in an electrochemical cell, while a cutting edge power device utilizing hydrogen and oxygen about 1.10 volts are estimated. It may be estimated that the bomb has a response result of water and the power device has a hydrated proton which is shaped at one cathode and expended at the other [5]. In an electrophoretic cell, the circumstance is entangled by adsorption of particles by micelles and the charge conveyed as required by Kirchoff's Law, but the processes at the interface of solid/liquid are not considered. Yet it was reported that egg albumen behaves differently when pH of the medium is changed. Similar arguments arise in all bioelectrochemical systems including electrokinetic phenomenon. Identification of the charge transfer processes at the anode and cathode are necessary to interpretation of all parts including the ionic species flux as required by the conservative laws.
REFERENCES
- Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37-60.
- Seiger HN. A Note on Conduction in Aqueous Electrolytes. J Membrane Sci. 2014;4:1.
- Seiger HN. The confluence of Faraday's and Kirchoff's Laws in bioelectrochemical systems. Sci World J. 2012;4:85.
- Fritz HP, Kuhn A. Comparative determination of effective transport numbers in solid lithium electrolytes. J Power Sources. 1993;41:253-261.
- Delgado ÁV, González-Caballero F, Hunter RJ, Koopal LK, Lyklema J. Measurement and interpretation of electrokinetic phenomena. J Colloid Interface Sci. 2007;309:194-224.
Citation: Watson N (2020) Membrane Bases Electrolysis. J Membra Sci Technol 10:208. doi: 10.35248/2155-9589.2020.10.208
Copyright: © 2020 Watson N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

