Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 11, Issue 3
Mechanical Thrombectomy of Wall Adherant Thrombus and Post Thrombotic Syndrome Diagnosis: A comparison of PST free outcomes and the ATTRACT trial in literature review
Peter Stibbs*, Jeffrey Wells, Dileo Govantes and Lawrence WellerReceived: 22-Mar-2023, Manuscript No. JVMS-23-20232; Editor assigned: 27-Mar-2023, Pre QC No. JVMS-23-20232 (PQ); Reviewed: 14-Apr-2023, QC No. JVMS-23-20232; Revised: 21-Apr-2023, Manuscript No. JVMS-23-20232 (R); Published: 28-Apr-2023, DOI: 10.35248/2329-6925.23.11.516
Abstract
Background: Post Thrombotic Syndrome (PTS) occurs in 23%-60% of Deep Venous Thrombosis (DVT) cases, representing a significant clinical and economic burden. The NIH-funded ATTRACT trial showed no significant difference in PTS diagnosis rates between Catheter Directed Thrombolysis (CDT) and oral anticoagulation alone. However, newer mechanical thrombectomy devices designed to remove wall adherent thrombus have emerged, raising questions about their efficacy in comparison to traditional treatments.
Objective: This study aims to compare PTS diagnosis rates in patients receiving percutaneous mechanical thrombectomy with extirpation of thrombus versus traditional pharmacological lysing of thrombus with oral anticoagulation. Methods: A retrospective review of 62 peer-reviewed publications was conducted, ultimately narrowing the focus to 12 studies with significant procedural volumes and commonality in reporting PTS after initial treatment methodology. Two mechanical thrombectomy devices, the ClotTriever (Inari Medical) and the Cleaner TM (Argon Medical), were identified for their design targeting wall adherent thrombus.
Results: Both the ClotTriever and Cleaner TM thrombectomy devices showed lower rates of PTS diagnosis at six months compared to CDT with oral anticoagulation alone, as cited in the ATTRACT trial. The ClotTriever device had a PTS-free rate of 716%, while the Cleaner TM device demonstrated 72.4% and 72% PTS-free rates in separate studies.
Conclusion: Mechanical thrombectomy devices engaging wall adherent thrombus yielded lower rates of post thrombotic syndrome diagnosis at six months compared to catheter directed thrombolysis. However, this study is limited by its retrospective nature and varying inclusion/exclusion criteria across studies. Future prospective comparisons with controlled settings are recommended to evaluate the efficacy of these devices in relation to CDT and oral anticoagulation.
Keywords
Thrombectomy; Deep Vein Thrombosis (DVT); Thrombosis; Catheter Directed Thrombolysis (CDT); Adjunctive Catheter-Directed Thrombolysis (ATTRACT); INARI; ARGON; Post-Thrombotic Syndrome (PTS)
Introduction
Deep venous thrombosis is diagnosed in approximately 900,000 people in the US each year [1]. Aside from the painful and mobility limiting sequala associated with initial onset of deep venous thrombosis, there is the more debilitating associated post thrombotic syndrome that occurs even after the initial incident of in-situ Deep Venous Thrombosis (DVT) is treated. Post Thrombotic Syndrome (PTS) represents a significant clinical and economic burden on society with certain studies citing incident rates of diagnosis between 23% and 60% post initial incident of DVT [2]. To date, standard therapies such as catheter directed thrombolysis and/or oral anti coagulation have not been proven to mitigate the subsequent onset of post thrombotic syndrome as evidenced in the NIH funded ATTRACT trial which only demonstrated a reduction in the severity of symptomology when utilizing Catheter Directed Thrombolysis (CDT) in the iliofemoral area of the deep venous system [3]. There is common critique that trials like the ATTRACT trial did not represent the current standard of care with regards to utilizing mechanical or pharmacomechanical devices designed for percutaneous maceration or extirpation of thrombus. Specifically utilizing devices designed to remove thrombus and liberate the intimal wall of the vein with the hope of restoring vascular pliancy as well as restoring some function to the valves of the vein that the wall adherent thrombus may impinge, thus reducing intravenous pressure in the lower portions of the deep venous system [4].
Materials and Methods
Objective
This study is designed to look at the clinical data surrounding some of these newer devices in utilization for percutaneously performed, image guided thrombectomy procedures that remove thrombus directly from the wall of the venous intima and compare post thrombotic syndrome diagnosis rates in patients receiving thrombectomy utilizing these mechanical devices compared to traditional CDT and oral anticoagulation alone [5-7]. This will be a retrospective review of peer reviewed and published clinical literature. The goal of this research to determine if there is any clinical signaling in the data which may demonstrate a trend of possible lower rates of post thrombotic syndrome presentation in patients who receive percutaneous mechanical thrombectomy with extirpation of thrombus versus traditional pharmacological lysing of thrombus with oral anticoagulation.
Methodology
This study is a retrospective review of peer reviewed published research and as such it was determined to be exempt from the need of Institutional Review Board (IRB) approval [8].
A search for peer reviewed publications was performed utilizing clinical research databases, specifically; a public literature search was performed utilizing biomedical literature databases, Embase (www.embase.com) and PubMed (pubmed.ncbi.nlm.nih.gov). Key research terminology entered into the data base search engines include the words; thrombectomy, wall adherent thrombus, mechanical thrombectomy, Catheter-Directed Thrombolysis (CDT), post thrombotic syndrome, percutaneous thrombectomy, thrombus resolution [9]. Based on utilization of these keywords over 62 publications were reviewed for commonality in reporting post thrombotic syndrome after initial treatment methodology as well as inclusive of treatment parameters, as well as procedural volumes considered significant enough to demonstrate statistical significance. After reviewing for commonality parameters, approximately 12 studies were utilized and consideration to meeting the above-mentioned parameters for inclusion. These studies were reviewed even further to look at the methodology of treatment to compare devices that state they were utilized to thrombectomize wall adherent thrombus versus devices designed to channel through thrombus or dissolve thrombus via means of pharmacologic initiated lysis [10]. Based on this evaluation, initial procedural success was defined as 75% clearance of thrombus demonstrated on postprocedural venogram and scored using the Marder scoring system, as well as diagnosis rates of post thrombotic syndrome within six months of interventional percutaneous thrombectomy procedure completion were utilized for comparison and analysis using basic statistical computation.
Results
In comparative study and utilizing the parameter of mechanical thrombectomy devices designed specifically for removal of thrombus from the venous intimal wall, two specific devices stand out with regards to indication for use in peripheral thrombectomy, as well as IFU and/or marketing claims with regards to engaging thrombus found in adhesion to the intimal venous wall. The ClotTriever mechanical thrombectomy system from Inari Medical (Irvine, California, USA) and the Cleaner TM thrombectomy system from Argon Medical Devices Inc. (Plano, Texas, USA) [11]. Both of these devices appear in clinical literature with descriptive claims for utilization for wall adherent thrombus, and both devices tout the ability to be self-sizing to the wall of the blood vessel. Review and analysis of the clinical results of these devices in peer reviewed literature in comparison to standard CDT with oral anticoagulation based on the results of the prospective, randomized, National Institute of Health (NIH) funded ATTRACT trial demonstrate initial statistical significance with regards to lower rates of post thrombotic syndrome diagnosed six months after subsequent treatment in comparison to the use of standard CDT in oral anticoagulation alone.Discussion
There is controversy surrounding treatment methodologies with regards to deep venous thrombosis. Anecdotal demonstration of palliative effect with regards to reduction in immediate symptomology severity has never been in question, but whether performing interventional procedures which entails some inherent risk of complication is justified in retrospect for trying to mitigate, or lessen the risk of the associated and often debilitating effects of post thrombotic syndrome which becomes a chronic state of venous insufficiency with varying degree of severity from basic lower extremity edema, to chronic venous ulceration and skin integrity breakdown which can require surgical intervention [12]. To date, the only prospective randomized trial looking to evaluate weather performance of interventional procedures for patients diagnosed with deep venous thrombosis had any effect in mitigating the associated sequala from post thrombotic syndrome was the NIH funded ATTRACT trial by Vendatham et al. As the only, prospective, multi arm, randomized comparative study evaluating catheter directed thrombolysis with oral anticoagulation versus standard oral anticoagulation alone, this trial has become the benchmark arguably for comparative data looking at percutaneous intervention versus standard oral anticoagulation alone and its effect on diagnosis rates of post thrombotic syndrome. In reviewing the overall results from the ATTRACT trial there was no statistically significant difference between both groups with regards to subsequent diagnosis of post thrombotic syndrome. 49% Percutaneous Catheter Directed Thrombolysis (PCDT) with oral anticoagulation vs. 51% oral anticoagulation alone [3]. There was clinical signaling though that patients who were treated with PCDT and had obstructive thrombosis in the iliofemoral section of the deep venous system experienced less severity of post thrombotic syndrome symptomology in comparison to the study arm that only received oral anticoagulation alone. The outcomes of this study are considered controversial in that many physicians feel that the standard algorithm of treatment changed throughout the course of the long enrollment period and study follow up, and that newer devices that mechanically breakdown the thrombus or remove the thrombus from the vein were already being widely employed over CDT by the time the study was published so that the results of the ATTRACT trial may not represent current clinical outcomes experienced by patients receiving treatment with these newer generation device methodologies.
Two different devices stood out in clinical data with regards to mechanical thrombectomy devices designed for removal of thrombus from the wall of the vein, the ClotTriever by Inari Medical and the Cleaner thrombectomy device by Argon Medical Devices Inc.
ClotTriever is a single-use; percutaneous device approved by the US FDA in 2017, indicated for the removal of thrombi and emboli in the peripheral vasculature. The device contains a catheter with a nitinol coring element and a mesh collection bag, in addition to a sheath to remove thrombus (ClotTriever IFU, Inari Medical). The ClotTriever device is designed to have wall opposition against the intima of the vein and to remove thrombus directly from the wall of the blood vessel as well as to remove thrombus impinging the functionality of venous valves. In review of the clinical literature surrounding this device, The CLOUT registry cites a post thrombotic syndromes free rate of 76% at 6 months in comparison to the ATTRACT trials 51% PTS free diagnosis rate which represents a large statistical difference comparatively.
In peer reviewed literature, specifically Koksoy et al, and Elezoy et al, cite a post thrombotic syndrome free rate of 72.4% and 72% at six months respectively. This is in comparison to 51% as cited in the ATTRACT trial for CDT in combination with oral anticoagulation (Figure 1).
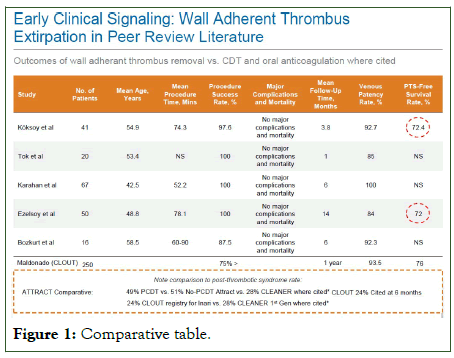
Figure 1: Comparative table.
The Cleaner TM thrombectomy system from Argon Medical Devices Inc. is a sinusoidal wave maceration catheter that spins at 4000 RPM’s and is advanced within the thrombus burden, macerating the thrombus while self-sizing to the wall of the blood vessel, allowing for dissolution of thrombus with subsequent aspiration of the thrombus through a selected sheath of the clinician’s choice (Figures 2 and 3).
Figure 2:ClotTriever (Inari Medical, Irvine, CA).
Figure 3:The cleaner TM (Argon Medical, Plano, TX).
Conclusion
In retrospective analysis of peer reviewed literature regarding rates of post thrombotic syndrome diagnosed at six months, mechanical thrombectomy devices like the ClotTriever (Inari Medical) and the CleanerTM thrombectomy system (Argon Medical) which engage thrombus that is adherent to the wall of the vein yielded lower rates of post thrombotic syndrome diagnosis at 6 months then catheter directed thrombolysis, as based on the results of the NIH funded ATTRACT trial for comparison.
This study is limited by being a retrospective comparative analysis of peer reviewed literature with varying inclusion and exclusion criteria. Recommendation for future prospective comparison may be warranted following parameters as set forth with the ATTRACT trial to limit inherent bias and evaluate the results of each therapeutic device in comparison to catheter directed thrombolysis with oral anticoagulation in a controlled setting.
References
- Reimold F, Tariq R, Gadey G. Flowtriever aspiration thrombectomy for Deep Vein Thrombosis (DVT). J Am Coll Cardiol. 2020;753375.
- Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28:465-476.
[Crossref] [Google Scholar] [PubMed]
- Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165(4),523-530.
[Crossref] [Google Scholar] [PubMed]
- Rodoplu O, Yildiz CE, Oztas DM, Beyaz MO, Ulukan MO, Unal O, et al. The efficacy of rotational pharmaco-mechanical thrombectomy in patients with acute iliofemoral deep vein thrombosis: Is the standard treatment of deep vein thrombosis changing? Phlebology. 2021;36(2):119-126.
[Crossref] [Google Scholar] [PubMed]
- Yuksel A, Tuydes O. Midterm outcomes of pharmacomechanical thrombectomy in the treatment of lower extremity deep vein thrombosis with a rotational thrombectomy device. Vasc Endovascular Surg. 2017;51(5):301-306.
[Crossref] [Google Scholar] [PubMed]
- Karahan O, Kutas HB, Gurbuz O, Tezcan O, Caliskan A, Yavuz C, et al. Pharmacomechanical thrombolysis with a rotator thrombolysis device in iliofemoral deep venous thrombosis. Vascular. 2016;24(5):481-486.
[Crossref] [Google Scholar] [PubMed]
- Bozkurt A, Kirbas I, Kösehan D, Demirçelik B, Nazli Y. Pharmacomechanical thrombectomy in the management of deep vein thrombosis using the cleaner device: an initial single-center experience. Ann Vasc Surg. 2015;29(4):670-674.
[Crossref] [Google Scholar] [PubMed]
- Köksoy C, Yilmaz MF, Basbug HS, �alik ES, Erkut B, Kaygin MA, etal. Pharmacomechanical thrombolysis of symptomatic acute and subacute deep vein thrombosis with a rotational thrombectomy device. J Vasc Interv Radiol. 2014;25(12):1895-1900.
[Crossref] [Google Scholar] [PubMed]
- Rabinovich A, Kahn SR. How I treat the postthrombotic syndrome. Blood. 2018;131(20):2215-2222.
[Crossref] [Google Scholar] [PubMed]
- Kahn SR, Ducruet T, Lamping DL, Arsenault L, Miron MJ, Roussin A, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Archives of Internal Medicine. 2005;165(10):1173-1178.
[Crossref] [Google Scholar] [PubMed]
- Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28:465-476.
[Crossref] [Google Scholar] [PubMed]
- Kurz X, Kahn SR, Abenhaim L, Clement D. Chronic venous disorders of the leg: Epidemiology, outcomes, diagnosis and management: Summary of an evidence-based report on the VEINES task force. Int Angiol. 1999;18(2):83.
[Crossref] [Google Scholar] [PubMed]
Citation: Stibbs P, Wells J, Govantes D, Weller L (2023) Mechanical Thrombectomy of Wall Adherant Thrombus and Post Thrombotic Syndrome Diagnosis: A Comparison of PST Free Outcomes and the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) Trial in Literature Review. J Vasc Surg. 11:516.
Copyright: © 2023 Stibbs P, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

